Abstract
Excess fatty acids and carbohydrates have both been implicated in the pathogenesis of type 2 diabetes, and both can reproduce essential features of the disease including insulin resistance and beta cell failure. It has been proposed that both nutrients may regulate metabolism through a common fuel sensing mechanism, namely hexosamine synthesis. We have previously shown that transgenic over-expression of the rate-limiting enzyme for hexosamine synthesis, glutamine:fructose-6-phosphate amidotransferase (GFA), targeted to muscle and fat, leads to insulin resistance mediated by increased O-linked glycosylation of nuclear and cytosolic proteins. We report here that hexosamine-induced insulin resistance is not additive with that induced by high fat feeding. In control mice fed a high fat diet, glucose disposal rates during euglycemic hyperinsulinemia were decreased by 37% (p < 0.02) compared to mice on a low fat diet. Transgenic mice overexpressing GFA and fed a low fat diet exhibited a 51% decrease in glucose disposal compared to controls on a low fat diet (p < 0.001), but no further decrease was evident in the transgenic mice fed a high fat diet. Decreased glucose disposal rates were mirrored by increases in skeletal muscle levels of the principal end product of the hexosamine pathway, UDP-N-acetyl glucosamine. Serum leptin levels, which are modulated both by feeding and hexosamine flux, also show no additivity in their stimulation by GFA overexpression and high fat feeding. These data are consistent with a shared nutrient sensing pathway for high fat and carbohydrate fluxes and a common pathway by which glucose and lipids induce insulin resistance.
Keywords: Insulin resistance, Hexosamine synthesis, N-Acetylglucosamine, O-Linked glycosylation
Introduction
Aspects of type 2 diabetes and the metabolic syndrome can be reproduced through the feeding of a high fat diet. In a variety of animal and in vitro models, high fat feeding is associated with obesity, hyperlipidemia, and insulin resistance resulting from decreased translocation of the glucose transporter GLUT4 (Boden 1997; McGarry 1998; Ruderman and Dean 1998; Bergman and Ader 2000). The mechanism by which elevated dietary fat results in insulin resistance is not completely understood, although roles have been demonstrated for the activation of inflammatory pathways, ectopic fat deposition, deleterious effects of products of fat metabolism, and others (Muoio et al. 2002; Hotamisligil 2006; Holland et al. 2007).
A large body of evidence also supports a role for the hexosamine biosynthesis pathway (HBP) in causing insulin resistance. The involvement of the HBP in metabolic regulation was originally demonstrated by its mediation in adipocytes of glucose-induced insulin resistance (Marshall et al. 1991), so-called “glucose toxicity” (Rossetti et al. 1990). Since then, numerous studies have shown that increased hexosamine flux can induce insulin resistance in cultured cells and whole animals (reviewed in McClain and Crook 1996; Rossetti 2000). Numerous studies of cell culture or infusion models have demonstrated that excess HBP flux results in insulin resistance (Robinson et al. 1993; Baron et al. 1995; Giaccari et al. 1995; Rossetti et al. 1995; Hawkins et al. 1997; Wang et al. 1998). Results of chronic but physiologic changes in hexosamine flux induced by tissue-specific over expression of the rate-limiting enzyme in the HBP, glutamine:fructose-6-P amidotransferase (GFA) have shown that the HBP serves a nutrient sensing function and affects metabolism in a wide-ranging manner in liver, muscle, fat, and beta cells (Hebert et al. 1996; Cooksey et al. 1999; McClain et al. 2000; Tang et al. 2000; Veerababu et al. 2000; Hazel et al. 2003). The insulin resistance induced by HBP flux mimics that of type 2 diabetes in being characterized by decreased recruitment of GLUT4 to the plasma membrane and reversibility by the antidiabetic drug troglitazone (Baron et al. 1995; Cooksey et al. 1999).
Acute infusion of free fatty acids into rats also leads to insulin resistance and this effect may also be mediated via increased hexosamine flux (Hawkins et al. 1997). Namely, free fatty acid infusions result in increased UDP-GlcNAc levels and the insulin resistance induced by that and other manipulations occurs in proportion to the increase in UDP-GlcNAc. To test whether the HBP might contribute to insulin resistance induced by chronic high fat feeding, we have fed control and transgenic mice with high fat diets. We report that the insulin resistance induced by high fat feeding is accompanied by increases in tissue UDP-GlcNAc levels and is not additive with that caused by increased hexosamine flux. These data are supportive of the hypothesis that insulin resistance resulting from either carbohydrate or lipid excess may share aspects of their mechanisms in both having a contribution from increased hexosamine flux.
Materials and methods
Animals
Mice with GFA overexpression targeted to adipose tissue and cardiac and skeletal muscle with the GLUT4 promoter have been previously described (Hebert et al. 1996; Cooksey et al. 1999). We use the highest GFA overexpressing founder line (8-3) (Hebert et al. 1996). The transgenic animals have been bred against a C57BL6 background for >10 generations. Heterozygous transgenic mice and control nontransgenic animals from the same litters were used in experiments that were approved by the Laboratory Animal Use Committees at the University of Utah Medical Center and the Salt Lake City Veterans Affairs Medical Center.
Diets
Two-month-old male mice (22.4 ± 0.3 g) were separated into groups (7–8/group) and fed a high carbohydrate diet containing 4 kcal% fat (Checkers Prolab RMH 2500, St. louis, MO) or a high fat diet containing 45 kcal% fat (Research Diets D12451 New Brunswick, NJ) for a period of 120 days.
Glucose tolerance and euglycemic clamp technique
All experiments were performed in weight-matched non-sedated transgenic and littermate control mice. After a 12-h fast, a glucose load of 1 mg/g body wt was administered intraperitoneally. Tail vein blood was sampled for blood glucose determination (Miles Elite glucometer) before and 5, 15, 30, 60, 90, 120 min after glucose administration. Insulin sensitivity for glucose disposal was assessed using the hyperinsulinemic–euglycemic clamp technique previously described (Cooksey et al. 1999). Catheters were implanted into the right internal jugular vein. The animals were allowed to recover from surgery for 3 days and then fasted overnight before the experiment. Animals were infused with recombinant human insulin (HumulinR, Eli Lilly & Co., Indianapolis, IN) at a rate of 20 mU/kg/min while 50% dextrose was infused by a variable infusion pump (Harvard Apparatus Inc., South Natick, MA). Whole blood samples (3 μl) were collected every 5–10 min from tail bleeds and measured by glucometer.
Tissue UDP-GlcNAc levels and serum hormone and chemistry assays
Glucose and triglycerides were assayed using diagnostic kits (Sigma Chemical Co., St. Louis, MO). Insulin concentrations were measured by using the Linco rat insulin radioimmunoassay (RIA) kit (Linco Research Inc., St. Louis, MO). Levels of UDP-N-acetyl-hexosamines were assayed by HPLC as described (Cooksey et al. 1999).
Results
Weights and serum nutrient and hormone levels
Male transgenic and control mice were placed on diets deriving either 4% (low fat) or 45% (high fat) of their calories from fat. The diets were begun at 2 months of age and continued for four additional months. At the end of that period, the animals on the high fat diet had gained more weight than those on the low fat diet, but there was no difference between the control and transgenic animals in either the high fat or low fat groups (Table 1). Neither serum glucose nor triglyceride levels differed between the groups in the fasting or the random-fed states. In the control animals, leptin levels were higher in the fed than the fasted state (p < 0.05 for all fed vs. all fasted mice). Consistent with previous results (McClain et al. 2000) the fasting leptin levels in the transgenic mice were higher than controls (p < 0.05) but did not increase further with feeding. Fasting insulin levels did not differ between groups.
Table 1.
Weights and serum levels of glucose, triglycerides, insulin, and leptin in control and transgenic animals on low and high fat diets
| Mouse strain | Control mice (C57BL6) | GFA transgenic mice | ||
|---|---|---|---|---|
| Low fat | High fat | Low fat | High fat | |
| Weight (g) | 25.8 ± 0.6 | 29.0 ± 0.6* | 24.9 ± 0.5 | 29.9 ± 1.2* |
| Fasting glucose (mg/dl) | 124 ± 7 | 129 ± 1 | 133 ± 5 | 120 ± 6 |
| Random-fed glucose (mg/dl) | 150 ± 5 | 161 ± 6 | 155 ± 5 | 160 ± 4 |
| Fasting triglycerides (ng/ml) | 36 ± 5 | 46 ± 8 | 29 ± 2 | 33 ± 2 |
| Random-fed triglycerides (ng/ml) | 54 ± 5 | 52 ± 6 | 79 ± 13 | 74 ± 11 |
| Fasting leptin (ng/ml) | 2.4 ± 0.1 | 3.8 ± 0.4 | 5.3 ± 0.9‡ | 4.3 ± 0.6 |
| Random-fed leptin (ng/ml) | 4.6 ± 0.9§ | 6.0 ± 0.9§ | 4.0 ± 0.5 | 5.4 ± 0.4 |
| Fasting insulin (ng/ml) | 0.19 ± 0.03 | 0.30 ± 0.03 | 0.21 ± 0.07 | 0.22 ± 0.08 |
Results are the means of 5–12 determinations per group, comparing transgenic animals to littermate controls
p < 0.05 high fat versus low fat diet in control and transgenic mice
p < 0.05 versus control mice fed low fat
p < 0.05, fed versus fasted control mice
We have previously reported that the insulin resistant mice overexpressing GFA have normal glucose tolerance compared to normal littermate controls; that is, the insulin resistance in these animals is well-compensated (Hebert et al. 1996). That finding was replicated in the current cohort of mice. Animals fed the high fat diet tended to have higher glycemic excursions but these differences were not statistically significant. Area under the glucose curve (AUC) for the low fat groups wt versus tg were 31,890 ± 718 and 30,171 ± 1,661, respectively. The AUC for the high fat fed groups wt versus tg were 33,802 ± 2,119 and 31,589 ± 2,084, respectively.
Insulin sensitivity assessed by the euglycemic clamp technique
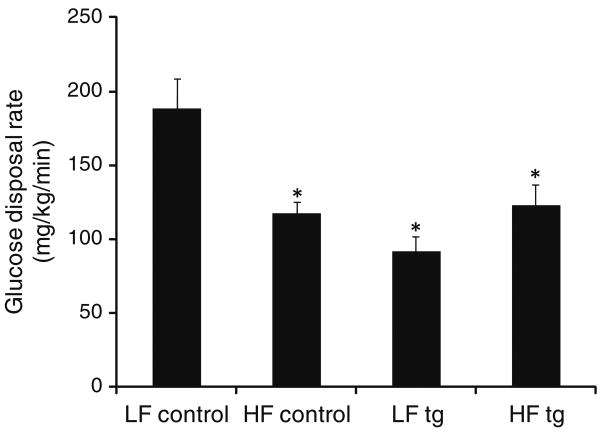
To assess the effects of diet on insulin sensitivity in the control and transgenic mice, we performed euglycemic clamp studies. Consistent with previous results, the transgenic animals on the low fat diet were insulin resistant compared to controls on the low fat diet, exhibiting a 51% decrease in their glucose disposal rates with maximal insulin (Fig. 1, p = 0.015). The high fat diet led to a 37% decrease in the glucose disposal rate of controls (p = 0.001) but had no effect on the transgenic animals. Thus, the high fat-fed control and transgenic animals were equally insulin resistant, with glucose disposal rates 118 ± 7 and 116 ± 25 mg/kg/min, respectively (p = 0.76).
Fig. 1.
Glucose disposal rates in vivo at maximal insulin in control and GFA transgenic (tg) mice fed low (LF) and high fat (HF) diets for 4 months. Results are the means ± SE, N = 6 for low fat control, 11 for high fat control, 3 for low fat transgenic, 4 for high fat transgenic. (p = 0.001 for low fat control vs. high fat control, p = 0.015 for low fat control vs. low fat transgenic, and p = 0.04 for low fat control vs. high fat transgenic)
Tissue UDP-GlcNAc levels
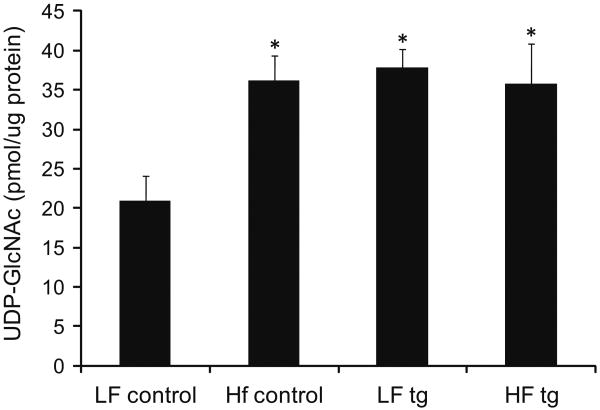
The changes in insulin sensitivity were next compared to the tissue levels of the end product of the hexosamine pathway, UDP-GlcNAc (Fig. 2). Transgenic animals had a 81% increase in UDP-GlcNAc in skeletal muscle compared to controls (p < 0.05), consistent with previous data (Cooksey et al. 1999). Control animals fed a high fat diet had 73% higher levels of UDP-GlcNAc than controls on the low fat diet (p < 0.05), but levels between high fat-fed control and transgenic mice were indistinguishable (p = 0.95).
Fig. 2.
UDP-GlcNAc levels in skeletal muscle after four months of low or high fat diet in control and GFA transgenic mice. (N = 5/group, p < 0.05 for control low fat vs. other groups)
Discussion
There is an accumulating evidence that products of the HBP may mediate insulin resistance induced by high carbohydrate flux. In several models including acute infusions of glucose and glucosamine as well as chronic overexpression of GFA (the rate-limiting enzyme for hexosamine synthesis) the degree of insulin resistance is well correlated with the accumulation of the principal end product of the pathway, UDP-GlcNAc (Robinson et al. 1993; Baron et al. 1995; Giaccari et al. 1995; Rossetti et al. 1995; Hebert et al. 1996; Hawkins et al. 1997; Wang et al. 1998; Cooksey et al. 1999; McClain et al. 2000; Tang et al. 2000; Veerababu et al. 2000; Hazel et al. 2003). This has led to the hypothesis that the HBP is a “nutrient sensor” that can lead to regulation of metabolism based on fuel availability.
The mediator of the effects of HBP flux is felt, in most cases, to be protein modification by O-linked N-acetyl-glucosamine (O-GlcNAc). Discovered ∼20 years ago (Holt and Hart 1986; Hanover et al. 1987), modification of proteins by O-GlcNAc is common and widespread (Zachara and Hart 2004). The enzyme responsible for O-linked glycosylation (O-glycosyltransferase, OGT) and proteins so modified have been found in all multicellular organisms examined, and over 500 nuclear and cytosolic proteins are modified by O-GlcNAc. O-glycosylation displays important features consistent with a role in signal transduction and nutrient sensing: the modification is inducible, reversible and dynamic. Finally, in cases of normal-to-excess glucose flux rates, it is substrate-limited by glucose availability for hexosamine synthesis and by the resulting levels of the substrate UDP-GlcNAc (Marshall et al. 1991; McClain and Crook 1996; Rossetti 2000). The pathway plays a broad role in regulating normal metabolism, growth, and development as well as in pathologic states, mediating some of the adverse consequences of hyperglycemia in diabetes. Although all of the effects of increased HBP flux have not been causally linked to changes in O-glycosylation, our group has demonstrated that the effects of increasing HBP flux in muscle and fat of transgenic mice (Hebert et al. 1996) are almost precisely replicated when OGT activity is increased in those same tissues (McClain et al. 2002). The mechanism of the insulin resistance was decreased GLUT4 translocation without changes in GLUT4 levels, and the insulin resistance so induced was reversible with thiazolidinedione treatment (Cooksey et al. 1999). The enzymology and biochemical aspects of the pathway have been reviewed recently (Copeland et al. 2008).
A role for the HBP in nutrient sensing for fuels other than carbohydrates was suggested by the finding of Rossetti's laboratory that acute infusion of free fatty acids in rats also led to insulin resistance in proportion to the ability to increase UDP-GlcNAc levels in those animals' tissues (Hawkins et al. 1997). This was hypothesized to occur via increased acetyl-CoA leading to inhibition of pyruvate dehydrogenase, leading in turn to increased fructose-6-phosphate availability for hexosamine synthesis (Rossetti 2000). Other methods of increasing UDP-GlcNAc levels such as infusion of uridine also led to insulin resistance (Hawkins et al. 1997). However, other workers have obtained conflicting results. Prolonged (5.5 h) infusions of Intralipid were able to induce insulin resistance without increasing levels of hexosamine products (Choi et al. 2001). One possible explanation of the discordance in these studies is that hexosamine flux rates should reflect activity of the sensing pathway better than steady state levels of UDP-GlcNAc. Furthermore, most UDP-GlcNAc is compartmentalized for N-linked glycosylation reactions so the size of the cytoplasmic pool available for cytosolic O-glycosylation is not necessarily reflected in total cellular levels. As pointed out in “Introduction”, it is also not unlikely that there are multiple routes to insulin resistance.
We therefore tested the hypothesis that the HBP acts as a more general nutrient sensing pathway in a chronic model of transgenic hexosamine excess combined with high fat feeding. We show that the degree of insulin resistance induced by high fat feeding is not additive with that induced by increased hexosamine flux. Leptin levels, which have been shown to be modulated by hexosamines (Wang et al. 1998; McClain et al. 2000), were also not additively stimulated by GFA overexpression and high fat feeding. These changes are well correlated with changes in tissue UDP-GlcNAc levels. UDP-GlcNAc levels are higher in transgenic mice than controls, but high fat feeding does not lead to further increases in the transgenic tissue. High fat feeding does lead to increased levels of UDP-GlcNAc in control muscle tissue, to levels comparable to those seen in the transgenic mice. Thus, overall there is a close correlation between the level of UDP-GlcNAc and insulin resistance (Fig. 1 compared to Fig. 2). The lack of additivity of high fat feeding and GFA overexpression in inducing insulin resistance is consistent with our observation that mice homozygous for the GFA transgene are not more insulin resistant than the heterozygotes used in this study (data not shown). Thus, it appears that the signal through the HBP is already maximal with heterozygous GFA overexpression and cannot be augmented by either increasing GFA copy number or by high fat feeding. The levels of UDP-GlcNAc may also be limited by the known feedback inhibition of GFA by UDP-GlcNAc (Kornfeld 1967).
Insulin resistance can be seen as one adaptive aspect of adjustment to overfeeding wherein muscle autoregulates glucose uptake in the face of excess glucose availability, allowing the excess to be stored ultimately as fat. The idea that insulin resistance can be adaptive is supported by the multiplicity of evolutionarily conserved pathways that result in insulin resistance, including those related to inflammation and nutrient sensing (see, for example Muoio et al. 2002; Hotamisligil 2006; Holland et al. 2007). More recent work has also suggested an adaptive role for insulin resistance in responding to oxidative stress (Hoehn et al. 2009). Therefore the current data, while supporting a role for the HBP in nutrient-induced insulin resistance, neither indicate an exclusive role, nor a role in all cell or animal models, for the HBP in insulin resistance. Consistent with this, there is evidence that insulin resistance can be induced independent of increased HBP flux in 3T3-L1 adipocytes (Robinson et al. 2007), and conversely elevations in HBP flux can occur in the same cells without insulin resistance (Macauley et al. 2008).
In summary, a large body of emerging evidence now suggests that nutrient signaling through the HBP serves an important role in cellular adaptation to fluctuations in nutrient availability even at physiological glucose concentrations (Soesanto et al. 2008). In addition to the functions of glucose uptake and hormone responsiveness examined in this study, a multiplicity of other pathways in metabolism, cell growth, and development are also modulated via the HBP and O-GlcNAc pathways (reviewed in Wells and Hart 2003; Zachara and Hart 2004; Love and Hanover 2005). These results support the hypothesis that the HBP and O-GlcNAc pathways serve as one integrator of nutritional status whose signaling results in global changes in cellular programs in response to those signals. The current data support expansion of that integrative function of the HBP to report nutrient status in terms of both carbohydrate and lipid intake and further underline the importance of the pathway in cellular signaling.
Acknowledgments
This work was supported by the National Institutes of Health (DK43526) and the Research Service of the Veterans Administration.
References
- Baron AD, Zhu JS, Zhu JH, Weldon H, Maianu L, Garvey WT. Glucosamine induces insulin resistance in vivo by affecting GLUT4 translocation in skeletal muscle. Implications for glucose toxicity. J Clin Invest. 1995;96:2792–2801. doi: 10.1172/JCI118349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351–356. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- Choi CS, Lee FN, Youn JH. Free fatty acids induce peripheral insulin resistance without increasing muscle hexosamine pathway product levels in rats. Diabetes. 2001;50:418–424. doi: 10.2337/diabetes.50.2.418. [DOI] [PubMed] [Google Scholar]
- Cooksey RC, Hebert LF, Jr, Zhu JH, Wofford P, Garvey WT, McClain DA. Mechanism of hexosamine-induced insulin resistance in transgenic mice overexpressing glutamine: fructose-6-phosphate amidotransferase: decreased glucose transporter GLUT4 translocation and reversal by treatment with thiazolidinedione. Endocrinology. 1999;140:1151–1157. doi: 10.1210/endo.140.3.6563. [DOI] [PubMed] [Google Scholar]
- Copeland RJ, Bullen JW, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. Am J Physiol Endocrinol Metab. 2008;295:E17–E28. doi: 10.1152/ajpendo.90281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccari A, Morviducci L, Zorretta D, Sbraccia P, Leonetti F, Caiola S, Buongiorno A, Bonadonna RC, Tamburrano G. In vivo effects of glucosamine on insulin secretion and insulin sensitivity in the rat: possible relevance to the maladaptive responses to chronic hyperglycaemia. Diabetologia. 1995;38:518–524. doi: 10.1007/BF00400719. [DOI] [PubMed] [Google Scholar]
- Hanover JA, Cohen CK, Willingham MC, Park MK. O-linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J Biol Chem. 1987;262:9887–9894. [PubMed] [Google Scholar]
- Hawkins M, Barzilai N, Liu R, Hu M, Chen W, Rossetti L. Role of the glucosamine pathway in fat-induced insulin resistance. J Clin Invest. 1997;99:2173–2182. doi: 10.1172/JCI119390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel M, Cooksey RC, Jones D, Parker G, Neidigh JL, Witherbee B, Gulve EA, McClain DA. Activation of the hexosamine signaling pathway in adipose tissue results in decreased serum adiponectin and skeletal muscle insulin resistance. Endocrinology. 2003;145(5):2118–2128. doi: 10.1210/en.2003-0812. [DOI] [PubMed] [Google Scholar]
- Hebert LF, Jr, Daniels MC, Zhou J, Crook ED, Turner RL, Simmons ST, Neidigh JL, Zhu JS, Baron AD, McClain DA. Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Invest. 1996;98:930–936. doi: 10.1172/JCI118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stocker R, Van Remmen H, Kraegen EW, Cooney GJ, Richardson AR, James DE. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA. 2009;106:17787–17792. doi: 10.1073/pnas.0902380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Holt GD, Hart GW. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986;261:8049–8057. [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Kornfeld R. Studies on l-glutamine d-fructose 6-phosphate amidotransferase. I. Feedback inhibition by uridine diphosphate-N-acetylglucosamine. J Biol Chem. 1967;242:3135–3141. [PubMed] [Google Scholar]
- Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE. 2005;2005:re13. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- Macauley MS, Bubb AK, Martinez-Fleites C, Davies GJ, Vocadlo DJ. Elevation of global O-GlcNAc levels in 3T3–L1 adipocytes by selective inhibition of O-GlcNAcase does not induce insulin resistance. J Biol Chem. 2008;283:34687–34695. doi: 10.1074/jbc.M804525200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–4712. [PubMed] [Google Scholar]
- McClain DA, Crook ED. Hexosamines and insulin resistance. Diabetes. 1996;45:1003–1009. doi: 10.2337/diab.45.8.1003. [DOI] [PubMed] [Google Scholar]
- McClain DA, Alexander T, Cooksey RC, Considine RV. Hexosamines stimulate leptin production in transgenic mice. Endocrinology. 2000;141:1999–2002. doi: 10.1210/endo.141.6.7532. [DOI] [PubMed] [Google Scholar]
- McClain DA, Lubas WA, Cooksey RC, Hazel M, Parker GJ, Love DC, Hanover JA. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci USA. 2002;99:10695–10699. doi: 10.1073/pnas.152346899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD. Glucose-fatty acid interactions in health and disease. Am J Clin Nutr. 1998;67:500S–504S. doi: 10.1093/ajcn/67.3.500S. [DOI] [PubMed] [Google Scholar]
- Muoio DM, MacLean PS, Lang DB, Li S, Houmard JA, Way JM, Winegar DA, Corton JC, Dohm GL, Kraus WE. Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) alpha knock-out mice. Evidence for compensatory regulation by PPAR delta. J Biol Chem. 2002;277:26089–26097. doi: 10.1074/jbc.M203997200. [DOI] [PubMed] [Google Scholar]
- Robinson KA, Sens DA, Buse MG. Pre-exposure to glucosamine induces insulin resistance of glucose transport and glycogen synthesis in isolated rat skeletal muscles. Study of mechanisms in muscle and in rat-1 fibroblasts overexpressing the human insulin receptor. Diabetes. 1993;42:1333–1346. doi: 10.2337/diab.42.9.1333. [DOI] [PubMed] [Google Scholar]
- Robinson KA, Ball LE, Buse MG. Reduction of O-GlcNAc protein modification does not prevent insulin resistance in 3T3–L1 adipocytes. Am J Physiol Endocrinol Metab. 2007;292:E884–E890. doi: 10.1152/ajpendo.00569.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L. Perspective: hexosamines and nutrient sensing. Endocrinology. 2000;141:1922–1925. doi: 10.1210/endo.141.6.7566. [DOI] [PubMed] [Google Scholar]
- Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13:610–630. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- Rossetti L, Hawkins M, Chen W, Gindi J, Barzilai N. In vivo glucosamine infusion induces insulin resistance in normoglycemic but not in hyperglycemic conscious rats. J Clin Invest. 1995;96:132–140. doi: 10.1172/JCI118013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman NB, Dean D. Malonyl-CoA, long chain fatty acyl-CoA and insulin resistance in skeletal muscle. J Basic Clin Physiol Pharmacol. 1998;9:295–308. doi: 10.1515/jbcpp.1998.9.2-4.295. [DOI] [PubMed] [Google Scholar]
- Soesanto YA, Luo B, Jones D, Taylor R, Gabrielsen JS, Parker G, McClain DA. Regulation of Akt signaling by O-GlcNAc in euglycemia. Am J Physiol Endocrinol Metab. 2008;295:E974–E980. doi: 10.1152/ajpendo.90366.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Neidigh JL, Cooksey RC, McClain DA. Transgenic mice with increased hexosamine flux specifically targeted to beta-cells exhibit hyperinsulinemia and peripheral insulin resistance. Diabetes. 2000;49:1492–1499. doi: 10.2337/diabetes.49.9.1492. [DOI] [PubMed] [Google Scholar]
- Veerababu G, Tang J, Hoffman RT, Daniels MC, Hebert LF, Jr, Crook ED, Cooksey RC, McClain DA. Overexpression of glutamine: fructose-6-phosphate amidotransferase in the liver of transgenic mice results in enhanced glycogen storage, hyperlipidemia, obesity, and impaired glucose tolerance. Diabetes. 2000;49:2070–2078. doi: 10.2337/diabetes.49.12.2070. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- Wells L, Hart GW. O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar. FEBS Lett. 2003;546:154–158. doi: 10.1016/s0014-5793(03)00641-0. [DOI] [PubMed] [Google Scholar]
- Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]