Abstract
Background
Increased activity of the habenula has been implicated in the etiology of major depressive disorder (MDD), in which reductions in habenula volume are present at postmortem. We conducted the first MRI analysis of habenula volume in MDD and bipolar disorder (BD).
Methods
High-resolution images (resolution≈0.4mm3) were acquired using a 3T scanner, and a pulse sequence optimized for tissue contrast resolution. The habenula was manually segmented by one rater blind to diagnosis. Seventy-four healthy controls (HC) were compared to both medicated (lithium/divalproex, n=15) and unmedicated, depressed BD (n=22) patients, unmedicated, depressed MDD patients (n=28), and unmedicated MDD patients in remission (RD, n=32).
Results
The unmedicated BD patients displayed significantly smaller absolute (p<0.01) and normalized (p<0.05) habenula volumes than the HC subjects. In post hoc assessments analyzing males and females separately, the currently-depressed females with MDD had smaller absolute (p<0.05) habenula volumes than the healthy control females. None of the other psychiatric groups differed significantly from the HC group.
Conclusions
We provide further evidence for the involvement of the habenula in affective illness, but suggest that a reduction in volume may be more pronounced in unmedicated, depressed BD subjects and female, currently depressed MDD subjects. The habenula plays major roles in the long-term modification of monoamine transmission and behavioral responses to stress, and in the suppression of dopamine cell activity following the absence of an expected reward. A reduction in habenula volume may thus have functional consequences which contribute to the risk for developing affective disease.
Keywords: major depressive disorder, bipolar disorder, habenula, magnetic resonance imaging, high resolution, lithium
Background
The habenula is an epithalamic structure that serves as a point of convergence for striatal and limbic input, and provides forebrain control over serotonergic and dopaminergic transmission from the midbrain. The major afferent projections to the habenula originate from inter alia, the lateral hypothalamus, central amygdala, globus pallidus, nucleus accumbens, and prefrontal cortex, while the habenula’s efferent projections target nuclei such as the dorsal raphe nucleus, the ventral tegmental area (VTA), and the mesopontine rostromedial tegmental nucleus(1–4). Efferent neurotransmission from the habenula to these latter structures results in inhibition of the tonic firing activity of serotonergic and dopaminergic neurons(1–4). The habenula is thus uniquely positioned to integrate information received from the cerebral and limbic cortex, and to regulate the activity of ascending monoaminergic projections from the brainstem(5). Preclinical studies support this conclusion, by providing evidence that the habenula plays a role in the long-term modification of monoamine transmission and behavioral responses to stress(6, 7), as well as the suppression of dopamine cell activity following the absence of an expected reward(8, 9).
Consistent with the monoaminergic hypothesis of depression, increased metabolism of the habenula has been observed in animal models of stress(10) and depression(11, 12). Moreover, antidepressant medication attenuated the metabolic response to experimental manipulations that induced behavioral analogs of depression(11) and lesions to the habenula ameliorated the behavioral response to repeated stress(6, 13). In humans, Roiser et al.(14) showed that acute tryptophan depletion (ATD) was associated with greater blood flow in the left habenula of remitted patients with MDD but not healthy controls. These data are consistent with an earlier study which reported a positive correlation between depressive symptoms and habenula metabolism during ATD(15), and a report that deep brain stimulation of the lateral habenula induced remission of symptoms in a patient with treatment refractory MDD(16). Further, a recent postmortem study reported a 24% reduction in volume of the right medial habenular nucleus (p<0.05; a 20% reduction on the left showed a trend toward significance) in a mixed sample of 6 MDD and 8 BD patients versus controls. In addition, the MDD and BD patients displayed a significant reduction in volume (20%) of the right lateral habenular nucleus, and a significant reduction (∼30–40%) in neuronal numbers and neuronal cell area in the medial habenular nucleus bilaterally(17).
Here, using high-resolution imaging (volumetric resolution≈0.4mm3), we conduct the first MRI study of habenula volume in MDD, and the first imaging study of the habenula in BD. Given the above-mentioned postmortem findings(17), we hypothesized that the habenula volume would be decreased in both the MDD and currently depressed BD samples. Further, based on the results of our recent analysis of amygdala volume in BD, in which we reported decreased amygdala volume in unmedicated BD patients but increased amygdala volume in medicated BD patients compared with HC(18), we hypothesized that mood-stabilizing medication would attenuate habenula volume loss in our medicated BD sample. Finally, we also included a sample of unmedicated, fully-remitted patients with MDD (RD) in order to test the effects of depressive symptomatology on habenula volume. We remained agnostic regarding habenula volume loss in patients with RD, however, since in addition to their currently euthymic mood state they showed the capacity to remain in remission while untreated, introducing a potential selection bias that may influence the presence of neuropathological changes.
Methods
Subjects gave written informed consent to participate, as approved by the NIMH IRB. Participants met DSM-IV criteria for BD, most recent episode depressed, MDD in a current major depressive episode, MDD in full remission (RD) based on the structured clinical interview for the DSM-IV (SCID-IV)(19) and an unstructured clinical interview with a psychiatrist. The following exclusion criteria applied: significant medical or neurological disorders, past head injury with loss of consciousness, significant risk of suicide, meeting DSM-IV criteria for substance abuse within the previous 6 months or substance dependence within the previous 5 years, pregnancy, general MRI exclusion criteria or electrolyte disturbance, anemia, or positive illicit drug or HIV screen on laboratory testing. Healthy control (HC) subjects (n=74) met the same exclusion criteria, had no lifetime history of a psychiatric disorder, and no first degree relative with a mood or anxiety disorder, as established using the Family Interview for Genetic Studies (FIGS)(20).
The unmedicated BD group consisted of 22 subjects who had not been exposed to psychotropic medications at least 2 months prior to scanning. Of these patients, 8 were naïve to psychotropic drugs and the remaining 14 were unmedicated for an average of 86±103 (range 10 to 345) weeks. Unmedicated patients with BD had the following comorbid conditions: post-traumatic stress disorder (PTSD) and social phobia (n=2); generalized anxiety disorder (GAD) (n=2); social phobia and panic disorder (n=2); social phobia and bulimia nervosa (n=1), and social phobia and obsessive compulsive disorder (OCD) (n=1). Five unmedicated BD patients met criteria for alcohol abuse in the remote past. One unmedicated individual with BD had a remote history of cannabis dependence, and 1 individual had a remote history of alcohol dependence.
The medicated group (n=15) consisted of 8 cases taking lithium, 6 cases taking divalproex, and 1 case taking chlorpromazine (lithium and divalproex therapeutic blood levels documented within several days of scanning). The following comorbid conditions were recorded: panic disorder (n=1); OCD (n=1); PTSD and GAD (n=1), social phobia and PTSD (n=1); panic disorder, social phobia, and PTSD (n=2); bulimia nervosa (n=2); and social phobia and PTSD (n=1). Six medicated subjects with BD had a past history of alcohol abuse, and 1 patient had abused both cannabis and phencyclidine (PCP). Two medicated BD patients had a remote history of alcohol dependence.
All MDD (n=28) and RD (n=32) patients were either medication-naïve or were unmedicated for at least 4 weeks (6 for fluoxetine). One MDD patient had co-occurring OCD, and 3 had a history of alcohol abuse. Three patients with RD had a past history of alcohol abuse.
High-resolution anatomical images were acquired using a GE 3T MRI scanner; a standard head radiofrequency coil; and a magnetization-prepared, rapid gradient echo (MP-RAGE) pulse sequence: (echo time [TE]=2.1 msec, repetition time [TR]=7.8msec, prep time = 725 msec, delay time=1400 msec, flip angle=6°). One hundred twenty-four axial slices (slice thickness=0.6 mm) were acquired with a 14 cm field-of-view and in-plane resolution of 224×224 voxels, resampled to 256×256x124 voxels for reconstruction, resulting in a displayed resolution of 0.55×0.55×0.6 mm. Three to four 13 minute scans were consecutively acquired, coregistered, and summed to increase signal-to-noise ratio. To enhance the accuract of manual segmentation, prior to analysis the signal-to-noise ratio was increased by summing each two consecutive coronal planes to enhance the accuracy of manual segmentation.
A second MP-RAGE image of the entire brain also was acquired to measure whole brain volume (WBV) (TE=4.94msec; TR=11.6 msec, prep time=725 msec; delay time=1400msec, voxel size=0.85 × 0.85 × 1.2 mm).
The habenula was segmented by one rater (WB) blind to diagnosis, in each coronal plane in which this structure was seen bulging into the third ventricle along the ventromedial aspect of the thalamus or lying ventral and medial to the stria medullaris of the thalamus (figure 1; Mai et al. (21)). The medial boundary was formed by the cerebrospinal fluid of the third ventricle, and the ventral boundary by the white matter of the posterior commissure. The dorsal and lateral borders were defined by the white matter of the stria medullaris of the thalamus in anterior planes or the mediodorsal thalamic nucleus, limitans nucleus or pretectal area in posterior planes (figure 1,(21)). The inter-rater reliability scores for the segmentation of 10 images was assessed using an interrater correlation coefficient.
Figure 1.
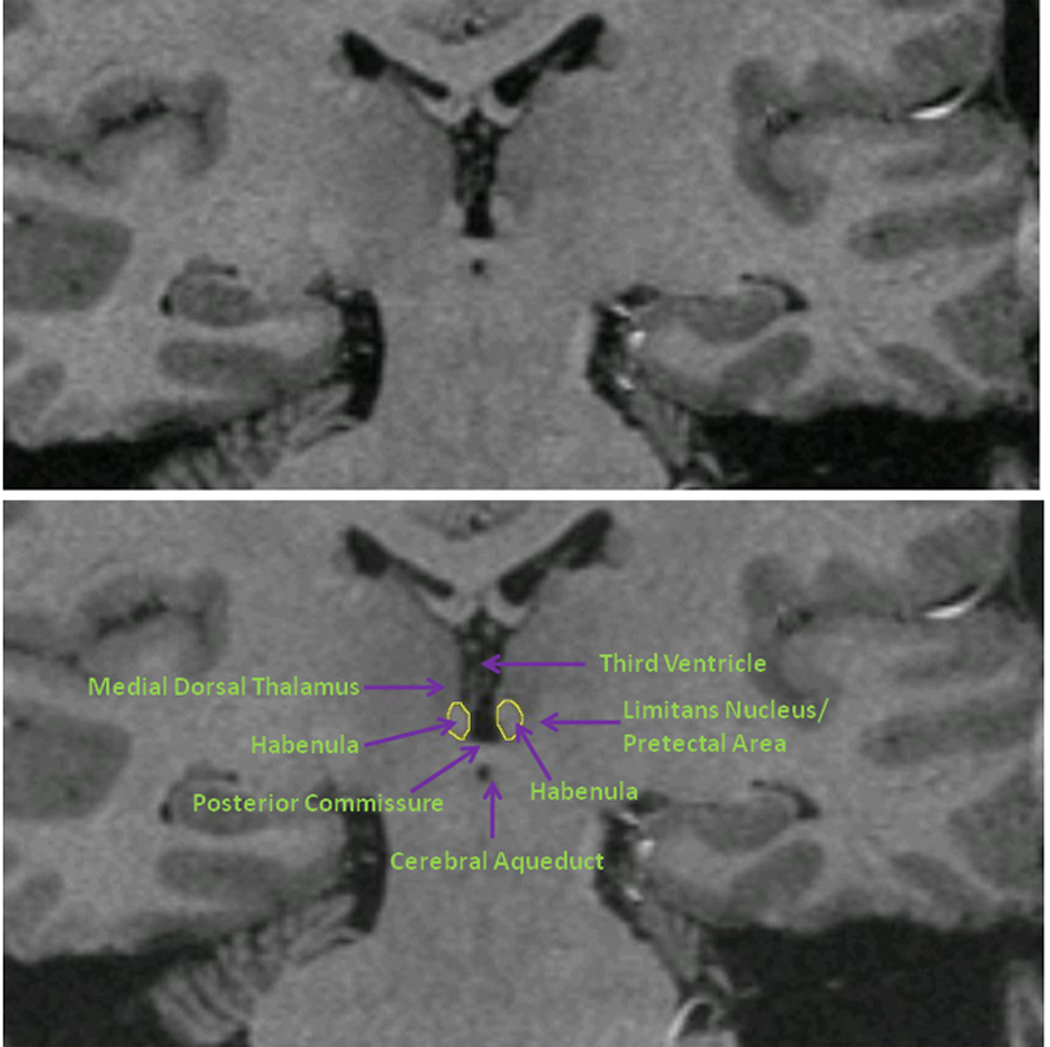
Coronal MRI sections showing the habenula and the local anatomical landmarks which enabled its segmentation. Because the habenular nuclei contain relatively dense white matter plexuses they can be collectively delimited from the gray matter of the adjacent thalamus dorsolaterally and of the limitans nucleus and pretectal area ventrolaterally (Mai et al. 2004). Moreover, in posterior planes the habenula is clearly evident as a pyramidal-shaped structure that bulges into the third ventricle along the ventromedial aspect of the thalamus, whereas in anterior planes it can be delimited ventrally and medially from the thalamus by the stria medullaris of thalamus (the white matter track that delimits the ventromedial aspect of the medial thalamus). In the image shown the habenular location shows sufficient asymmetry that the typical view of the posterior aspect is illustrated by the habenular nuclear complex located on the reader’s left, while the latter case is illustrated by the habenular complex on the reader’s right. Finally, the habenular nuclei are delimited ventrally by the white matter of the posterior commissure. The medial and lateral habenular nuclei could not be resolved specifically, so were combined within a single habenular volume-of-interest. The upper and lower panels consist of the identical image. The tracing of the habenula is shown in yellow in the lower panel.
Whole brain volume (WBV) was measured using an automated technique, as described previously(18). Briefly, the FSL tool, FAST, was used to segment the whole brain image into gray matter, white matter and CSF images, after correcting for intensity nonuniformity using the minc tool, N3. The gray and white matter components then were summed to generate the WBV.
The a priori hypothesis that the habenula volume would be decreased bilaterally in depressed MDD subjects and unmedicated, depressed BD subjects versus healthy controls was tested by comparing absolute and normalized (absolute habenula volume ÷ WBV) volumes across groups. First, repeated measures ANOVAs with left versus right hemisphere as the within subjects factor was used to test for absolute and normalized habenula volume differences between the 3 groups (MDD, unmedicated BD and HC). Thereafter, independent sample t-tests were used to test the individual group contrasts except where demographic or clinical variables differed between diagnostic groups, in which case a general linear model with the appropriate covariates was used instead. In post hoc analyses aimed at addressing the specificity of the findings from these comparisons, the habenula volume of the medicated BD group was compared to the HC group using a general linear model, controlling for handedness and age. The RD group did not differ from the HC group in any demographic variable, and therefore the difference in habenula volumes between these 2 groups was compared using an independent sample t-test.
Finally, exploratory analyses were performed post hoc to assess sex effects on the habenular volumes within the mood disorder samples, and to evaluate potential associations between habenular volumes and clinical parameters of age-at illness-onset, illness duration, time spent medication free, symptom severity, bipolar subtype (I versus II). BD I is characterized by episodes of major depression and mania while patients with BD II suffer from episodes of major depression and sub-threshold symptoms of mania, that is, hypomania(22).
Results
Results are shown in tables 1 and 2, and figures 2 through 4, as well as in Supplement 1.
Table 1.
Comparison of Demographic and Habenula Volume Data Across the Diagnostic Groups
| Unmed.BD (n=22) |
Med.BD (n=15) |
MDD (n=28) |
RD (n=32) |
HC (n=74) |
Med.BD VS Unmed.BD |
Unmed.BD VS HC |
Med.BD VS HC |
MDD VS HC | RD VS HC |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Age at Scan | 34.4±6.7 | 45.1±9.1 | 43.9±11.5 | 41.3±13.3 | 37.1±11.9 | Med>Unmed t=3.7 df=39 p=0.001 |
NS t=0.6 df=101 p=0.527 |
Med>HC t=2.6 df=94 p=0.010 |
MDD>HC t=2.6 df=100 p=0.010 |
NS t=1.6 df=104 p=0.113 |
| Sex (M/F) | 7/15 | 5/10 | 13/15 | 8/24 | 29/45 | NS t=0.4 df=39 p=0.687 |
NS t=0.9 df=101 p=0.375 |
NS t=0.3 df=94 p=0.765 |
NS t=0.9 df=100 p=0.394 |
NS t=1.5 df=104 p=0.128 |
| Right/Left Hand | 17/5 | 10/5 | 24/4 | 31/1 | 71/3 | NS t=0.6 df=39 p=0.551 |
Unmed>HC Lefthanders t=2.9 df=80 p=0.005 |
Med>HC lefthanders t=4.2 df=73 p<0.001 |
MDD>HC Lefthanders t=2.4 df=79 p=0.020 |
NS t=1.2 df=104 p=0.252 |
| BDI/BDII | 6/16 | 4/11 | NA | NA | NA | NS t=0.1, df=39 p=0.917 |
NA | NA | NA | NA |
| Family History (yes/no/adopted) |
21/0/1 | 15/0/0 | 23/5/0 | 28/4/0 | NA | NS t=0.0 df=39 p=1.00 |
NA | NA | NA | NA |
| Age at Illness Onset (years) |
19±7 | 19±10 | 24±12 | 26±11 | NA | NS t=0.4 df=36 p=0.707 |
NA | NA | NA | NA |
| Duration of illness (years) | 15±9 | 26±3 | 19±13 | 17±12 | NA | Med>Unmed t=3.1 df=36 p=0.004 |
NA | NA | NA | NA |
| Weeks Unmedicated | 86±103 | NA | 72±105 | - | NA | NA | NA | NA | NA | NA |
| MADRS | 21±13 | 21±12 | 27±11 | 0±0 | 0.2±0.8 | NS t=0.2 df=32 p=0.866 |
Unmed>HC t=12.0 df=66 p<0.001 |
Med>HC t=13.4 df=60 p<0.001 |
MDD>HC t=16.3 df=66 p<0.001 |
NS t=0.6 df=48 p=0.552 |
| YMRS | 5.9±5.7 | 4.6±3.6 | 4.5±4.8 | 0.2±0.2 | 0.4±0.9 | NS t=0.7 df=30 p=0.451 |
Unmed>HC t=5.0 df=66 p<0.001 |
Med>HC t=5.9 df=60 p=0.001 |
MDD>HC t=6.8 df=66 p<0.001 |
NS t=0.4 df=48 p=0.719 |
| Absolute Left Habenula Volume (mm3) |
16.3±3.2 | 19.4±5.9 | 19.1±4.6 | 19.3±4.5 | 19.5±5.2 | α NS F=0.2 df=1 p=0.652 |
β Unmed<HC F=7.9 df=1 p=0.006 |
Ω NS F=0.2 df=1 p=0.883 |
Ω NS F=0.1 df=1 p=0.910 |
µ NS t=0.2 df=104 p=0.827 |
| Normalized Left Habenula Volume |
1.45×10−5 ±3.63×10−6 |
1.80×10 5± 5.41 ×10−6 |
1.70×10−5 ±4.90×10 6 |
1.71×10−5 ±3.76×10 6 |
1.69 ×10−5 ± 5.17×10−6 |
α NS F=0.01 df=1 p=1.0 |
β Unmed<HC F=4.6 df=1 p=0.034 |
Ω NS F=0.01 df=1 p=1.0 |
Ω NS F=0.01 df=1 p=1.0 |
µ NS t=0.2 df=104 p=0.845 |
| Absolute Right Habenula Volume (mm3) |
14.1±3.0 | 16.8±5.0 | 15.6±4.8 | 16.7±4.2 | 17.0±4.7 | α NS F=1.1 df=1 p=0.306 |
β Unmed<HC F=7.3 df=1 p=0.008 |
Ω NS F=0.2 df=1 p=0.895 |
Ω NS F=1.7 df=1 p=0.189 |
µ NS t=0.3 df=104 p=0.768 |
| Normalized Right Habenula Volume |
1.25×10−5 ±3.20×10−6 |
1.56×10−5 ±4.60×10 6 |
1.38×10−5 ±4.54×10 6 |
1.49×10−5 ±3.66×10 6 |
1.47 ×10−5 ±4.44×10 6 |
α NS F=1.6 df=1 p=0.218 |
β Unmed<HC F=4.7 df=1 p=0.033 |
Ω NS F=0.01 df=1 p=1.0 |
Ω NS F=1.0 df=1 p=0.324 |
µ NS t=0.2 df=104 p=0.823 |
| Total Habenula Volume (mm3) |
30.4±4.3 | 36.2±9.2 | 34.7±7.4 | 36.0±8.0 | 36.5±8.7 | α NS F=1.0 df=1 p=0.324 |
β Unmed<HC F=9.8 df=1 p=0.002 |
Ω NS F=0.3 df=1 p=0.853 |
Ω NS F=0.7 df=1 p=0.418 |
µ NS t=0.3 df=104 p=0.774 |
| Normalized Total Habenula Volume |
2.70 ×10−5 ±5.60×10−6 |
3.35 ×10−5 ±8.58×10 6 |
3.07 ×10−5 ±7.89×10 6 |
3.16 ×10−5 ±8.64×10 6 |
3.20 ×10−5 ±8.64×10 6 |
α NS F=1.4 df=1 p=0.244 |
β Unmed<HC F=5.4 df=1 p=0.022 |
Ω NS F=0.01 df=1 p=1.0 |
Ω NS F=0.01 df=1 p=0.999 |
µ NS t=0.2 df=104 p=0.818 |
| WBV (mm3) | 1179815.0 ±119631.4 |
1107793.4 ±92368.5 |
1185300.0 ±124943.3 |
1163612.0 ±137540.3 |
1174544.2 ±118902.6 |
α NS F=1.3 df=1 p=0.270 |
β NS F=0.1 df=1 p=0.726 |
Ω NS F=3.5 df=1 p=0.066 |
Ω NS F=0.01 df=1 p=0.997 |
µ NS t=1.0 df=104 p=0.322 |
Blue shading = demographic and clinical variables. Light blue shading = volumetric data. Pink shading = demographic and clinical data for each diagnostic group. Green shading = statistical comparisons.
AD=antidepressant medication, BDI=Bipolar Disorder Type I, BDII=Bipolar Disorder Type II, MADRS=Montgomery-Asberg Rating Scale for Depression, Med=currently medicated, NA=not applicable, NS=not significant, Unmed=currently unmedicated, WBV=Whole Brain Volume, YMRS=Young Mania Rating Scale.
α = F-Test controlling for age at scan and duration of illness.
β= F-Test controlling for handedness.
Ω=F-Test controlling for handedness and age at scan.
µ=t-test (no covariates).
Table 2.
Correlations between clinical variables and habenula volumes in the unmedicated (top panel) and medicated (bottom panel) BD samples.
| Variable | Absolute Left Habenula Volume |
Normalized Left Habenula Volume |
Absolute Right Habenula Volume |
Normalized Right Habenula Volume |
Absolute Total Habenula Volume |
Normalized Total Habenula Volume |
|---|---|---|---|---|---|---|
| Unmedicated BD Sample (n=22) | ||||||
|
Duration of Illness |
r=0.27, p=0.253 | r=0.29, p=0.212 | r=0.02, p=0.919 | r=0.04, p=0.871 | r=0.17, p=0.464 | r=0.21, p=0.379 |
|
Age at Onset of Illness |
r=0.02, p=0.923 | r=−0.17, p=0.481 | r=−0.21, p=0.371 | r=−0.31, p=0.191 | r=−0.16, p=0.497 | r=−0.28, p=0.231 |
|
Weeks Medication Free |
r=−0.06, r=0.846 | r=0.05, p=0.847 | r=0.02, p=0.931 | r=−0.02, p=0.952 | r=0.04, p=0.881 | r=0.03, p=0.900 |
| MADRS | r=−0.31, p=0.211 | r=−0.23, p=0.357 | r=0.08, p=0.761 | r=0.08, p=0.766 | r=−0.19, p=0.446 | r=−0.11, p=0.673 |
| YMRS | r=0.04, p=0.885 | r=−0.20, p=0.458 | r=−0.10, p=0.715 | r=−0.24, p=0.362 | r=−0.1, p=0.723 | r=−0.26, p=0.339 |
| Medicated BD Sample (n=15) | ||||||
|
Duration of Illness |
r=0.54, p=0.046* | r=0.52, p=0.052 | r=0.56, p=0.038* | r=0.55, p=0.041* | r=0.65, p=0.013* | r=0.63, p=0.016* |
|
Age at Onset of Illness |
r=−0.40, p=0.162 | r=−0.35, p=0.217 | r=−0.49, p=0.077 | r=−0.44, p=0.117 | r=−0.52, p=0.059 | r=−0.46, p=0.101 |
|
Weeks Medication Free |
NA | NA | NA | NA | NA | NA |
| MADRS | r=0.50, p=0.097 | r=0.52, p=0.081 | r=0.03, p=0.927 | r=0.10, p=0.757 | r=0.29, p=0.359 | r=0.36, p=0.244 |
| YMRS | r=0.16, p=0.622 | r=0.09, p=0.784 | r=−0.19, p=0.552 | r=−0.23, p=0.225 | r=−0.04, p=0.905 | r=−0.09, p=0.788 |
p<0.05, MADRS=Montgomery-Asberg Depression Rating Scale, YMRS=Young Mania Rating Scale
Figure 2.
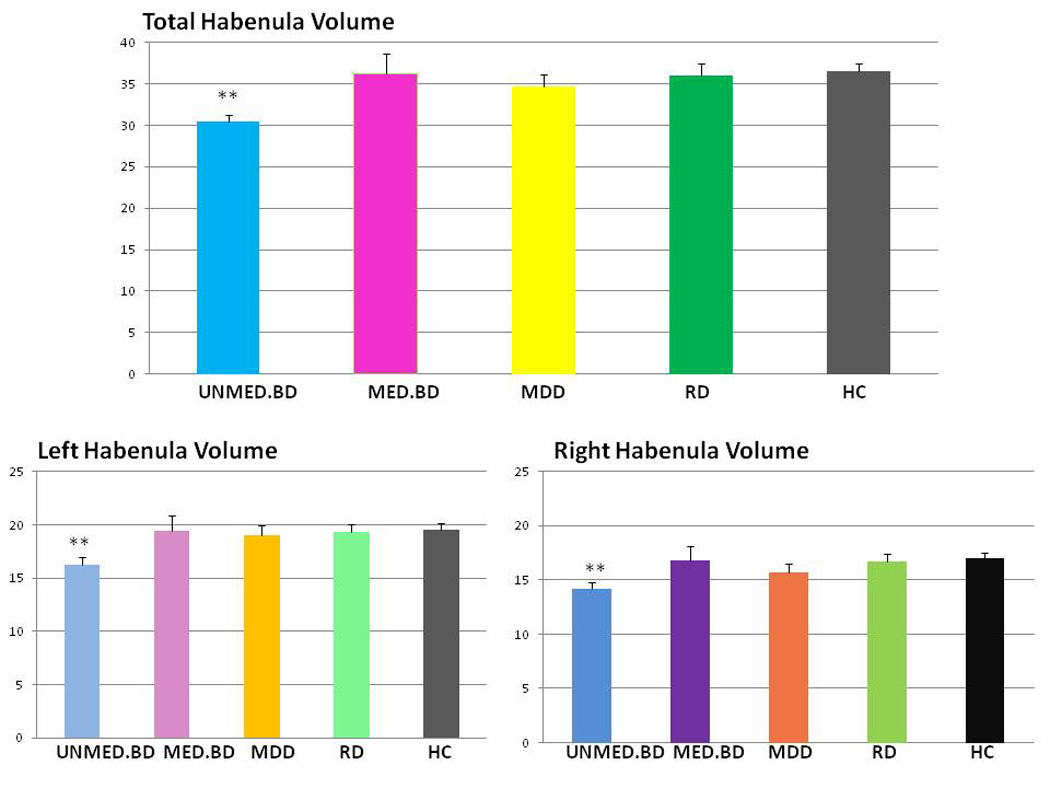
Bar chart showing a comparison of absolute left and right habenula volume across the diagnostic groups. Absolute total habenula volumes (y-axis: scale = 0–40ml) are shown in the top panel, and absolute left and right habenula volumes (y-axis: scale 0–25ml) are shown in the bottom panel. Unmed.BD refers to the unmedicated BD sample, and Med.BD refers to the medicated BD sample. The standard error of the mean (SEM) is displayed on the top of each bar. The symbol, ** is indicative of a statistically significant (p<0.01) difference in habenula volume compared with the healthy control group.
Figure 4.
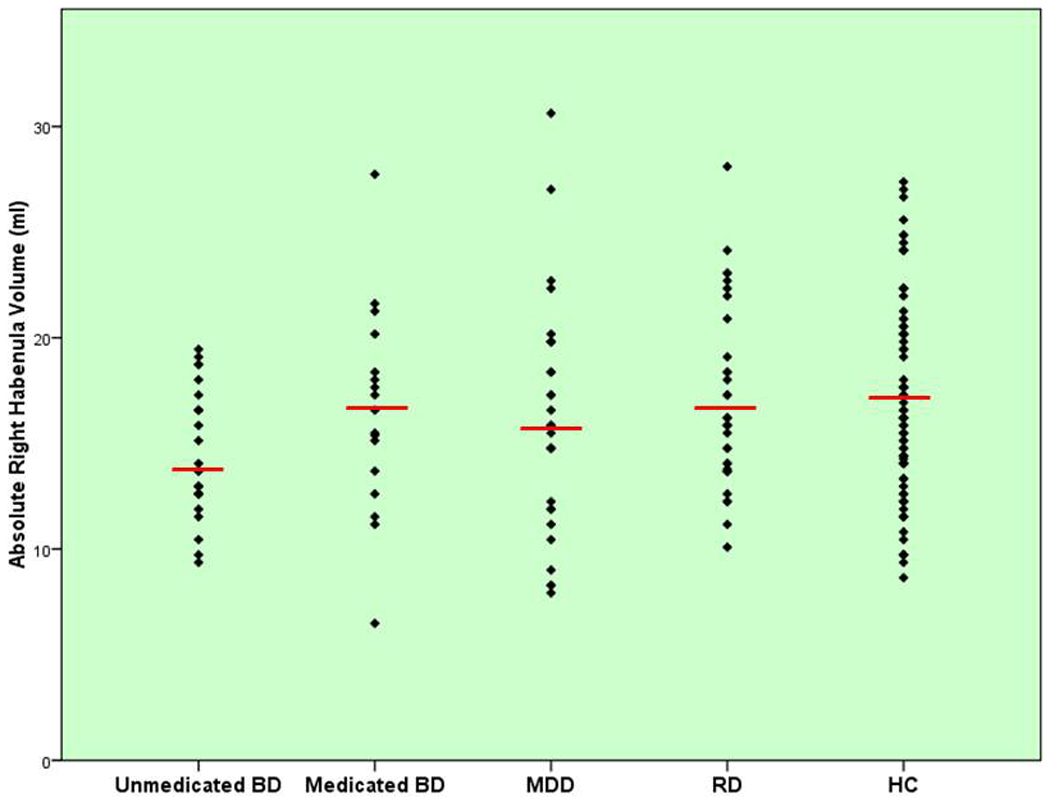
Scatterplot of absolute right habenula volumes (y-axis) for the medicated BD, unmedicated BD, MDD, HC, and RD samples, respectively. The red horizontal bar shows the mean volume of each group.
The interrater reliability coefficient was 0.970 for the left habenula and 0.945 for the right habenula.
None of the subject groups differed from each other in WBV.
A priori hypotheses
The omnibus ANOVA was indicative of a significant difference between the MDD, unmedicated BD and HC groups in habenula volume (F=5.1, p=0.008). The within-subjects factor, hemisphere, was significant (F=5.9, p=0.017) but there was no significant interaction between hemisphere and diagnosis (F=1.3, p=0.278), indicating that the left habenula was larger than the right habenula across all three diagnostic groups. In addition, a trend towards a significant difference between the MDD, unmedicated BD and HC groups in normalized habenula volume was observed (F=2.7, p=0.074). For the normalized volumes, no significant effects were found for the within-subjects factor, hemisphere (F=2.4, p=0.123), and there was no significant interaction between hemisphere and diagnosis (F=0.5, p=0.616). The total habenula of the MDD group was 5% smaller than that of the habenula of the HC group (left habenula 2%, right habenula 8%). This volumetric difference was not statistically significant (p>0.15). Nevertheless, after analyzing males and females separately, we found that the female MDD group had a smaller absolute total habenula volume (F=5.4, p=0.025) than the female HC group. The effect was driven by a decrease in right habenula volume (right: F=6.1, p=0.017; left: F=2.0, p=0.165) By contrast, the male MDD group did not differ from the male HC group in total, left or right habenula volume (p-values>0.7). The MDD group did not differ from the HC in total normalized habenula volume (F=0.4, p=0.540) but the female MDD patients showed a trend towards having smaller total normalized habenula volumes than the female HC group (F=3.2, p=0.081). A difference in normalized habenula volumes between the female MDD and the female HC groups was found for the right (F=4.6, p=0.036) but not left (F=1.1, p=0.310) habenula.The unmedicated BD patients displayed significantly smaller absolute total (17%, F=9.8, p=0.002), habenula volumes than the HC subjects. The unmedicated BD patients also had smaller normalized habenula volumes than HC subjects (F=5.4, p=0.022) (table 1, and Figures S1 – S3 in Supplement 1). Both male and female unmedicated BD patients had smaller habenula volumes than male and female healthy controls. The unmedicated BD group also showed a trend toward having smaller absolute total habenula volumes than the MDD group (12%, F=3.9, p=0.054). The effect was primarily driven by a decrease in left habenula volumes in the unmedicated BD group compared with the MDD group (left: 15%, F=4.7, p=0.035; right: 10%, F=0.8, p=0.371). The unmedicated BD group also showed a trend towards smaller normalized habenula volumes than the MDD group (F=3.7, p=0.061). The statistical trend was the result of a left (F=4.8, p=0.033), but not right-sided (F=1.2, p=0.281) habenula volume decrease in the unmedicated BD group compared with the MDD group.
In order to ensure that the reduction in habenula volume in the unmedicated BD group was not an artifact of the increased frequency of sinistrality in that group, we re-analyzed the data with right-handed subjects, only. Similar results were obtained (Table S1 and Figures S4 and S5 in Supplement 1).
Post-Hoc hypotheses
No significant difference in absolute or normalized habenula volume was found between the medicated BD sample and the HC sample or between a combined medicated and unmedicated BD group and the HC sample (p-values>0.2). The absolute habenula volume of the unmedicated BD sample was 16% smaller than the absolute habenula volume of the medicated BD sample (table 1, figures 2 through 4). However, this difference in volume did not reach statistical significance (F=1.0, p=0.324). Both males and females within the unmedicated BD group showed a nominal reduction in habenula volume versus males and females of the medicated BD group, although the results of the gender-stratified analyses did not reach significance. In addition, no significant difference in normalized habenula volume was found between the unmedicated and medicated BD patients (F=1.4, p=0.244) (Figures S1 – S3 in Supplement 1).
In both the unmedicated and medicated BD sample there was no correlation between age at onset, number of weeks medication free, Montgomery-Asberg Depression Rating Scale (MADRS) or Young Mania Rating Scale (YMRS) scores and absolute or normalized habenula volume. In the medicated, but not the unmedicated BD sample, illness duration correlated positively with absolute (r=0.65, p=0.013) and normalized (r=0.63, p=0.016) habenula volume (table 2).
The unmedicated BDI (n=6) and BDII (n=16) patients did not differ significantly from each other in absolute or normalized habenula volume. The medicated BDI (n=4) and BDII (n=11) patients also did not differ significantly from each other in absolute or normalized habenula volume. Medicated BD patients treated with lithium did not differ from BD patients treated with divalproex in absolute or normalized habenula volume (p-values>0.6).
The RD group did not differ significantly from the HC sample in absolute or normalized habenula volume (all p-values>0.7) (figures 2 through 4 and A through C). The unmedicated BD group had significantly smaller total absolute habenula volumes than the RD group (F=12.5, p=0.001). The unmedicated BD group also had smaller total normalized habenula volumes than the RD group (F=12.3, p=0.001).
Conclusions
On the basis of a recent postmortem study(17), which reported a reduction in neuron numbers and density of the habenula in a mixed sample of patients with BD and MDD, we expected to find volumetric decreases in our currently depressed MDD and unmedicated BD subjects compared with HC subjects. We also scanned a medicated BD sample in order to evaluate the effects of mood stabilizer treatment with lithium or valproate on habenula volume, and a group of subjects with RD in order to evaluate the effects of mood state on habenular volume.
Our main finding was that BD subjects who were medication-naïve or had been unmedicated for at least two months displayed smaller habenula volumes than the HC group. Conversely, the medicated BD subjects did not differ significantly in habenula volume from the HC group. These data echo our recent report of an amygdala volume reduction in unmedicated BD subjects but an amygdala volume increase in medicated BD patients compared with HC(18). Our result also appears broadly consistent with evidence for increased whole brain, ACC(23, 24), and hippocampal volumes(25) in longitudinal studies of lithium-treated BD patients, although habenula volumes did not differ significantly between the unmedicated and medicated BD samples assessed cross-sectionally in the current study.
The cause of the reduction in habenula volume in our unmedicated BD sample remains unclear. In the hippocampus, medial prefrontal cortex, and some amygdala nuclei, the elevated adrenal steroid secretion associated with repeated stress has been shown to facilitate dendritic atrophy in rodents by increasing N-methyl-d-aspartate (NMDA) receptor signaling through the modulation of NMDA and GABA-A receptor gene expression, the activation of voltage-gated calcium channels, and a reduction in the expression of the glutamate transporter(26). Conceivably, stress-induced excitotoxicity in the habenula may be correlated with previously reported increases in habenula activity in MDD patients, and dendritic atrophy may explain the previously reported decrease in habenula volume in MDD subjects studied post mortem(17) as well as the decrease in habenula volume of unmedicated BD patients presented here. Moreover, the significant reduction (∼30–40%) in neuronal counts and cell area of the medial habenula reported in the Ranft et al.(17) postmortem study, raises the possibility that the reduction in habenula volume in our unmedicated BD group may reflect more than dendritic remodeling – perhaps a neurotoxic process leading to neuronal death.
As we have pointed out elsewhere(27) developmental factors may also account for the volumetric changes observed in some regions of the brain in affective disorders. Conceivably, the reduction in habenula volume observed in our unmedicated BD group may be due to a congenital abnormality that increases the risk for the subsequent development of mood disorders.
Animal studies suggest that lithium and divalproex increase the mRNA expression of several genes that enhance synaptic plasticity, facilitate neurogenesis, and promote cellular resilience to physiological stress(28), perhaps explaining why our medicated BD sample did not differ in habenula volume from our HC sample. The positive correlation between duration of illness and habenula volume, which was only present in the medicated BD sample, could conceivably reflect the extent of lifetime medication use. However, because of the confounding effects of medication class, dose, and treatment compliance, as well as the difficulty in obtaining accurate information from patients about the duration of their past medication use, we were unable to test this hypothesis.
The absence of habenula volume differences between the MDD and HC groups may be a consequence of reduced statistical power. The absolute right habenula volume was 8% smaller in the MDD versus the HC group (figure 2 and figure 4) which taken together with the Ranft et al.(17) finding of reduced right habenula volume in MDD and BD subjects studied post mortem, raises the possibility that in a larger sample the MRI-based volumetric difference in MDD may become statistically significant. Nevertheless, because the Ranft et al.(17) sample was composed of both BD and MDD patients, it is possible that the reduction in habenula volume reported by the authors was primarily driven by the BD subsample. In addition, there may be a gender effect such that females with MDD are more vulnerable to developmental abnormalities of the habenula or more likely to suffer from excitotoxic damage than males with MDD. Nevertheless, the Ranft et al.(17) postmortem sample consisted of approximately equal numbers of males and females and no gender differences were reported by the authors.
Several additional limitations of our study design merit comment. Even in high-resolution images, it remains difficult to accurately segment the habenula from adjacent tissues, particularly at the anterior and posterior aspects. However, there is no reason to expect that the extent of any errors would differ systematically between unmedicated BD patients and our other diagnostic groups since segmentation was performed blind to subject identity/diagnosis. Further, the limits of current MRI technology do not allow the lateral and medial habenula to be distinguished from each other, which may have accounted for the absence of differences between our entire (i.e. both males and females) MDD and HC samples: in the Ranft et al.(17) sample, neuronal loss was found in the medial habenular nuclei but could not be assessed in the lateral habenula. Thirdly, our mood disorder patients were selected naturalistically, raising the possibility that selection bias was introduced with respect to illness severity or chronicity. Although there was no difference between the unmedicated and medicated BD groups in severity of depression at the time of scanning, the medicated BD had been ill for longer than the unmedicated BD group. Conceivably, the reduction in habenula volume in the unmedicated BD group may reflect a non-habenular-related trait such as clinical course that biased the likelihood of the BD subjects being unmedicated. Finally, our results should be treated with caution given the small sample sizes of certain diagnostic subgroups which may have led to type II error.
In summary, we identified significant volume reduction in the habenula in unmedicated, but not medicated BD patients. Habenula structure and function merit further study given this structure’s central role in adaptation to stressful events(6, 7, 10) and negative feedback during reward processing(8). A reduction in habenula volume may have functional consequences contributing to the risk for developing affective disease.
Supplementary Material
Figure 3.
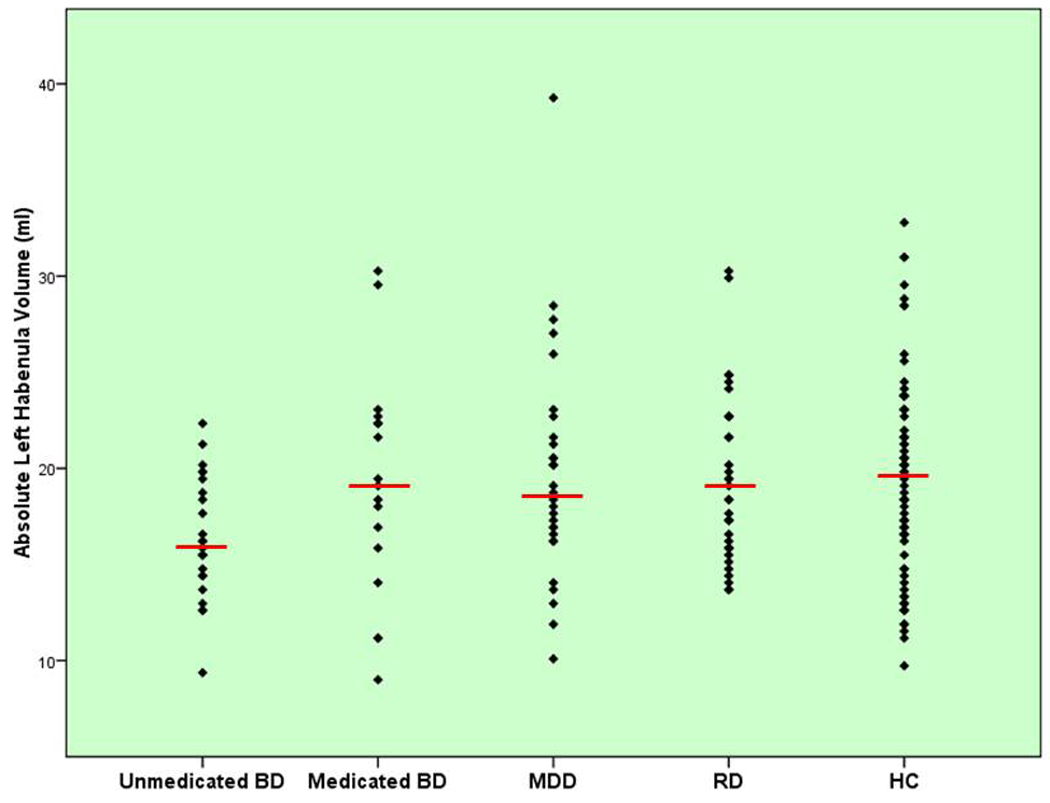
Scatterplot of absolute left habenula volumes (y-axis) for the medicated BD, unmedicated BD, MDD, HC, and RD samples, respectively. The red horizontal bar shows the mean volume of each group.
Acknowledgements
The authors would like to thank Ghedem Solomon and Niara Wright for administrative support. This study was funded by the intramural research program at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Interest
Earl Bain, MD is currently an employee of Abbott Laboratories. Husseini Manji, MD is currently an employee of Johnson and Johnson, Inc. In 2006 and 2007, Dennis Charney, MD consulted for Astra Zeneca, Bristol Myers Squibb Company, Cyberonics, Neurogen, Neuroscience Education Institute, Novartis Pharmaceuticals Corporation, Orexin, and Unilever UK Central Resources Limited. Dr. Charney has a patent pending for the use of ketamine in the treatment of depression. Wayne Drevets, MD consulted for Pfizer Pharmaceuticals. Dr. Zarate is listed as co-inventor on a patent for the use of ketamine in major depression. Dr. Zarate has assigned his patent rights on ketamine to the U.S. government. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008 Nov 12;28(46):11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianco IH, Wilson SW. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2009 Apr 12;364(1519):1005–1020. doi: 10.1098/rstb.2008.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009 Oct;30(7):1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010 Jan;35(1):27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geisler S, Trimble M. The lateral habenula: no longer neglected. CNS Spectr. 2008 Jun;13(6):484–489. doi: 10.1017/s1092852900016710. [DOI] [PubMed] [Google Scholar]
- 6.Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001 Oct 26;917(1):118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- 7.Thornton EW, Bradbury GE. Effort and stress influence the effect of lesion of the habenula complex in one-way active avoidance learning. Physiol Behav. 1989 May;45(5):929–935. doi: 10.1016/0031-9384(89)90217-5. [DOI] [PubMed] [Google Scholar]
- 8.Shepard PD, Holcomb HH, Gold JM. Schizophrenia in translation: the presence of absence: habenular regulation of dopamine neurons and the encoding of negative outcomes. Schizophr Bull. 2006 Jul;32(3):417–421. doi: 10.1093/schbul/sbj083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci. 2003 May 15;23(10):4308–4314. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wirtshafter D, Asin KE, Pitzer MR. Dopamine agonists and stress produce different patterns of Fos-like immunoreactivity in the lateral habenula. Brain Res. 1994 Jan 7;633(1–2):21–26. doi: 10.1016/0006-8993(94)91517-2. [DOI] [PubMed] [Google Scholar]
- 11.Caldecott-Hazard S, Mazziotta J, Phelps M. Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. J Neurosci. 1988 Jun;8(6):1951–1961. doi: 10.1523/JNEUROSCI.08-06-01951.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003 Feb 14;963(1–2):274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- 13.Yang LM, Hu B, Xia YH, Zhang BL, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res. 2008 Mar 17;188(1):84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Roiser JP, Levy J, Fromm SJ, Nugent AC, Talagala SL, Hasler G, et al. The effects of tryptophan depletion on neural responses to emotional words in remitted depression. Biol Psychiatry. 2009 Sep 1;66(5):441–450. doi: 10.1016/j.biopsych.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999 Aug;10(2):163–172. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- 16.Sartorius A, Kiening KL, Kirsch P, Gall CC, Haberkorn U, Unterberg AW, et al. Remission of Major Depression Under Deep Brain Stimulation of the Lateral Habenula in a Therapy-Refractory Patient. Biol Psychiatry. 2009 Oct 19; doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Ranft K, Dobrowolny H, Krell D, Bielau H, Bogerts B, Bernstein HG. Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychol Med. 2009 Aug 12;:1–11. doi: 10.1017/S0033291709990821. [DOI] [PubMed] [Google Scholar]
- 18.Savitz J, Nugent AC, Bogers W, Liu A, Sills R, Luckenbaugh DA, et al. Amygdala volume in depressed patients with bipolar disorder assessed using high resolution 3T MRI: The impact of medication. Neuroimage. 2010 Feb 15;49(4):2966–2976. doi: 10.1016/j.neuroimage.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press, Inc; 1996. [Google Scholar]
- 20.Maxwell M. Family Interview for Genetic Studies (FIGS): Manual for FIGS. Bethesda, MD: Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health; 1992. [Google Scholar]
- 21.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. 2nd ed. Amsterdam: Elsevier; 2004. [Google Scholar]
- 22.APA. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Washington, D.C.: APA Press; 1994. [Google Scholar]
- 23.Moore GJ, Cortese BM, Glitz DA, Zajac-Benitez C, Quiroz JA, Uhde TW, et al. A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. J Clin Psychiatry. 2009 May;70(5):699–705. doi: 10.4088/JCP.07m03745. [DOI] [PubMed] [Google Scholar]
- 24.Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000 Oct 7;356(9237):1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- 25.Yucel K, Taylor VH, McKinnon MC, Macdonald K, Alda M, Young LT, et al. Bilateral hippocampal volume increase in patients with bipolar disorder and short-term lithium treatment. Neuropsychopharmacology. 2008 Jan;33(2):361–367. doi: 10.1038/sj.npp.1301405. [DOI] [PubMed] [Google Scholar]
- 26.McEwen BS, Magarinos AM, Reagan LP. Structural plasticity and tianeptine: cellular and molecular targets. Eur Psychiatry. 2002 Jul;17 Suppl 3:318–330. doi: 10.1016/s0924-9338(02)00650-8. [DOI] [PubMed] [Google Scholar]
- 27.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009 May;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manji HK, Moore GJ, Chen G. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biol Psychiatry. 2000 Oct 15;48(8):740–754. doi: 10.1016/s0006-3223(00)00979-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


