Abstract
Background and objectives
Patients treated by percutaneous coronary intervention (PCI) receive aspirin and P2Y12 ADP receptor inhibitors to reduce thrombotic complications. The choice of methodology for monitoring the effects of treatment and assessing its efficacy is still a topic of debate. We evaluated how decreased P2Y12 function influences platelet aggregate (thrombus) size measured ex vivo.
Methods and Results
We used confocal videomicroscopy to measure in real time the volume of platelet thrombi forming upon blood perfusion over fibrillar collagen type I at the wall shear rate of 1,500 s−1. The average volume was significantly smaller in 31 patients receiving aspirin and clopidogrel (19) or ticlopidine (12) than 21 controls, but individual values were above the lower limit of the normal distribution, albeit mostly within the lower quartile, in 61.3% of cases. Disaggregation of platelet thrombi at later perfusion times occurred frequently in the patients. Vasodilator-stimulated phosphoprotein (VASP) phosphorylation, reflecting P2Y12 inhibition, was also decreased in the patient group and only 22.6% of individual values were above the lower normal limit. We found no correlation between thrombus volume formed onto collagen fibrils and level of P2Y12 inhibition, suggesting that additional and individually variable factors can influence the inhibitory effect of treatment on platelet function.
Conclusions
Measuring platelet thrombus formation in flowing blood reflects the consequences of anti-platelet therapy in a manner that is not proportional to P2Y12 inhibition. Combining the results of the two assays may improve the assessment of thrombotic risk.
Keywords: Aspirin, platelet receptor blockers, thienopyridines, thrombosis
Platelet-mediated arterial thrombosis leads to acute coronary syndromes (ACS)[1], thus patients undergoing percutaneous coronary intervention (PCI) receive anti-platelet therapy [2, 3]. Aspirin (acetylsalicylic acid, ASA) and thienopyridines (ticlopidine or clopidogrel), which inhibit thromboxane A2 synthesis and P2Y12 adenosine diphosphate (ADP) receptor respectively, are administered in combination for long-term treatment [4, 5]. Anti-platelet drugs reduce adverse events after PCI, and resistance to therapy may result in thrombotic complications [6, 7]. The latter are rare (incidence 0.5–2%) but altogether relevant because PCI procedures are performed frequently. So called “resistance” to aspirin and clopidogrel has been reported [8, 9]. Pharmacological resistance to aspirin may be rare, yet individual responses vary considerably with respect to platelet inhibition [10]. In the case of clopidogrel, reduced intestinal absorption, abnormal metabolism and reduced interaction with the target receptor have been associated with therapy failure [11—13]. The next generation of P2Y12 inhibitors may overcome these problems, yet the need for platelet testing to identify thrombotic risk will remain as uniform dosing may not fit all patients [14]. There is, however, no established approach on how to gauge platelet inhibition in relation to clinical outcome [15] and results obtained with different methods have been discordant [9, 16–18].
Here, we have evaluated platelet function using confocal videomicroscopy with three-dimensional resolution of thrombus formation in real time (four-dimensional analysis) [19, 20]. Unlike light transmittance aggregometry (LTA) - tested sequentially with individual agonists in a poorly defined fluid dynamic environment and independently of surface interactions – platelet thrombus formation under arterial flow conditions is more directly related to mechanisms considered of physiopathological relevance [19, 20]. These include activation following tethering to a collagen surface mediated by bound plasma-derived von Willebrand factor (VWF), the influence of shear stress-dependent biomechanical stimuli [21, 22] and release of platelet α-granule content, including ADP [23]. We found that the volume of platelet aggregates formed in flowing blood is not proportional to the level of P2Y12 inhibition in individual patients. Results of the test may help improve understanding of pathogenetic mechanisms and assessment of thrombotic risk.
Materials and Methods
Blood samples and patient population
Blood from 21 medication-free healthy controls and 31 patients was drawn from an antecubital vein with informed consent according to the Declaration of Helsinki and with the approval of an Institutional Review Board. Four-ml blood aliquots were collected into tubes with 68 USP units of lithium heparin or other anticoagulant when indicated (BD Vacutainer, Buccinasco, Milan, Italy); the first aliquot was discarded. After treatment for the acute episode and PCI, according to standard protocols that included a 300 mg loading dose of clopidogrel per os, all patients received aspirin, 100 mg daily indefinitely, and clopidogrel, 75 mg daily for variable periods of time. During the course of treatment, some patients were switched to ticlopidine (250 mg twice daily) for medical reasons or as required by national health insurance policy. Patients were tested after 30–365 days of treatment. For monitoring of compliance to treatment, patients were instructed to count pills on a weekly basis and questioned on the issue at the time of routine check-up visits. Relevant characteristics of the patient population are reported in Table 1.
Table 1.
Clinical characteristics of the patient population.
| Characteristic | n=31 |
|---|---|
| Age (SD) | 62 (14) |
| Male/female gender | 22/9 |
| Hypertension (%) | 18 (58.1) |
| Diabetes (%) | 8 (25.8) |
| Hyperlipidemia (%) | 18 (58.1) |
| Family history of CAD (%) | 7 (22.5) |
| Smoker (%) | 21 (67.7) |
| Number of vessel diseased (%) | |
| 1 | 11 (35.5) |
| 2 | 9 (29) |
| 3 | 11 (35.5) |
| Previous MI (%) | 5 (16.1) |
| Previous CABG (%) | 2 (6.5) |
| Drug-eluting stent (%) | 22 (71) |
| Bare-metal stent (%) | 9 (29) |
| Chronic renal failure (%) | 4 (12.9) |
| Ejection Fraction (SD) | 50(9) |
| Indication for PCI (%) | |
| STEMI | 26 (83.8) |
| NSTEMI | 5 (16.2) |
CAD = coronary artery disease; MI = myocardial infarction; CABG = coronary artery bypass graft; STEMI = myocardial infarction with ST elevation; NSTEMI = myocardial infarction without ST elevation.
Measurement of vasodilator-stimulated phosphoprotein (VASP) platelet reactivity index (PRI)
Blood for this assay was collected into 0.011 M trisodium citrate and stored at room temperature (18–25 °C) before testing within 24 h. VASP phosphorylation at Ser239 in response to prostaglandin (PG) E1 with or without concomitant ADP stimulation was determined with a specific monoclonal antibody by flow cytometry (Epics XL, Beckman Coulter, Cassina de’ Pecchi, Milan, Italy) using a commercial kit (BioCytex, Marseille, France) according to Schwarz et al [24]. PRI was expressed as:
where MFI is mean fluorescence intensity.
Thrombus formation ex vivo and volume measurement
As described previously [19] in detail, a glass coverslip was coated with acid-insoluble type I collagen (Sigma Chemical Co. St. Louis, MO) and assembled at the bottom of a rectangular flow chamber. This was placed on the stage of an inverted fluorescence microscope equipped with a confocal module (TCS SP5, Leica, Milan, Italy). Platelets in whole blood containing lithium heparin (17 USP U/ml) were rendered fluorescent with 10 µM quinacrine hydrochloride (mepacrine) added before perfusion. Constant flow yielding a wall shear rate of 1,500 s−1 was controlled with a syringe pump (New Era Pump System NE-1000, KF Technology, Rome, Italy) aspirating from the chamber outlet. In selected experiments, we evaluated the effect on platelets thrombus formation and VASP phosphorylation of adding to control blood varying concentrations of the P2Y12 inhibitor, 2-methylthioadenosine 5′-monophosphate triethylammonium salt (2-MeSAMP; Sigma), or ASA (lysine acetylsalicylate; Sanofi-Aventis, Milan, Italy), alone or in combination. Images obtained through a HP PL APO 20x/0.70 objective (Leica) were digitized in real time with a video camera (QiCam Cooled Mono, Q-Imaging, Surrey, BC, Canada). Series of confocal sections were collected at defined time points. The area of the field of view seen through the 20x objective and the videocamera was 207,025 µm2. Total thrombus volume per field of view, reflecting the number of aggregated platelets, was calculated as the sum of the surface area covered by platelets in each individual confocal section multiplied by the z-axis interval (0.94 µm) between adjacent sections, as reported [19]. For measurements in treated patients and controls, all z planes were included in the calculation; for measurements after exogenous addition of inhibitors to normal blood, the values of z planes corresponding to the layer of collagen-adherent platelets were excluded from the calculation. The latter were identified as the plane with the greatest total platelet area plus twice the values of the planes below it. In a plot of platelet surface coverage versus distance from the collagen surface, these values define a symmetric peak around the one with greatest surface coverage value, arbitrarily taken to represent the volume of a monolayer of collagen adherent platelets. The threshold between signal and background was established using an unbiased algorithm. Image analysis was performed with ImageJ (http://rsbweb.nih.gov/ij/). Movies were edited with Final Cut Express (version 4.0.1; Apple Inc., Cupertino, CA). It is important to note that certain preparations of heparin have an inhibitory effect on platelet thrombus formation measured under the conditions described here. For these studies, we verified that the volume of platelet thrombi formed in control samples was within the range previously observed with blood containing 40–80 µM D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK) as anticoagulant. However, we also found that, as compared to PPACK-containing blood, certain heparin preparations may inhibit platelet thrombus volume on collagen by >50–70%. The effect of heparin, which interacts with the VWF A1 domain [25], is likely the result of interference with VWF binding to GPIb and/or collagen, but we cannot explain why certain preparations of the anticoagulant have a greater effect than others in this regard. Of note, heparin has previously been used as anticoagulant for perfusion studies similar to the ones we present here; thrombus formation was found to be similar in heparin- or PPACK-containing blood, but unexplained effects of the anticoagulant used on the results obtained with different inhibitors of thrombus formation have also been reported [26].
Statistical analysis
Continuous variables are presented as number and percentage or mean and standard deviation (SD), as appropriate. Since the distribution of values was not uniformly normal and sample groups were small and unequal in size, differences were evaluated with nonparametric tests. For more than two groups we used the Kruskal-Wallis test followed by the Dunn’s test to evaluate differences between selected pairs; for two groups we used the Mann-Whitney test. Correlation between independent measurements was evaluated with the Spearman test. The power of an assay for discriminating between control individuals or treated patients was evaluated with receiver-operating characteristic (ROC) curves. Experiments performed by exogenous addition of inhibitors to normal blood were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. Statistical significance was set at a P value <0.05, and all tests were two-sided. Statistical analyses were performed with GraphPad Prism version 5.0c (GraphPad Software, San Diego, CA).
All authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Effects of a P2Y12 inhibitor or aspirin added exogenously to normal blood
We first evaluated the extent to which 2-MeSAMP, a selective inhibitor of the ADP P2Y12 receptor [27], or ASA, added alone or in combination to normal donor blood ex vivo, interfered with the process of platelet thrombus formation under the conditions used for this study. Stable surface-adherent platelet aggregates (thrombi) developed in blood without inhibitors during perfusion over fibrillar collagen type I at the wall shear rate of 1,500 s−1 (Fig. 1A). Total perfusion time was 8 minutes and measurements were performed in real time during flow at 6 minutes, when platelet aggregates on the surface had reached a steady volume (see below). The flow rate was constant and the flow path was never occluded by thrombi during experiments. Addition of 2-MeSAMP (Fig. 1B) or ASA (Fig. 1C) reduced the size of platelet aggregates. At the highest 2MeSAMP concentration used (340 µM), a layer of platelets loosely attached to one another adhered to the collagen fibrils, but aggregation above the surface was markedly impaired as compared to that seen in untreated blood (Fig. 1B, D). In samples containing 300 µM ASA, platelet cohesion into aggregates was more similar to normal than in the presence of 2-MeSAMP, but the number of thrombi that grew above the surface was decreased (Fig. 1C).
Figure 1. Effect of 2-MeSAMP and ASA on platelet aggregate formation over fibrillar collagen type I exposed to flowing blood.
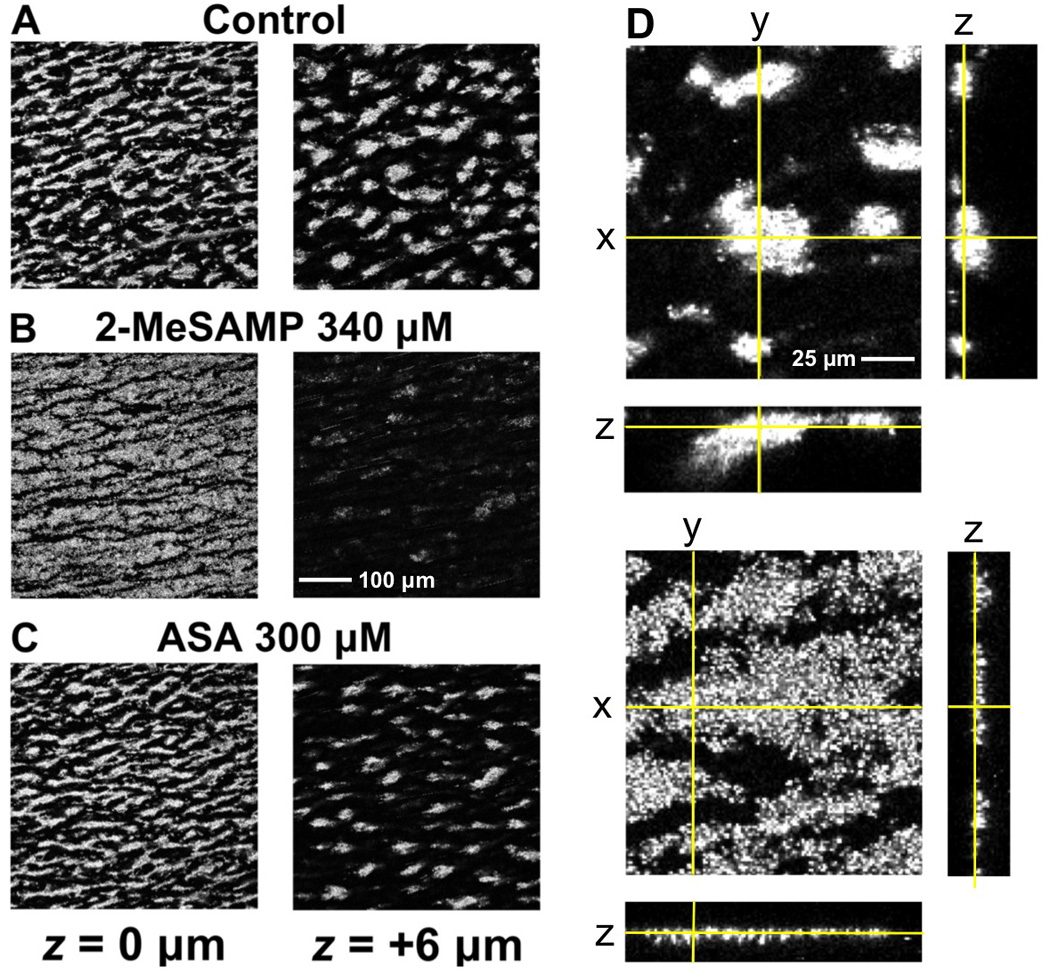
A–C. Pairs of single confocal images taken at the collagen surface (z position = 0 µm) and 6 µm above it, as indicated, after 6 min of perfusing blood at the wall shear rate of 1,500 s−1. D. Orthogonal projections of thrombi formed in control (top images) and 2-MeSAMP-treated blood (bottom images). The position in z of the x, y planes is indicated by yellow lines in the x, z and y, z projections, which were constructed with a series of confocal sections taken at 0.94 µm interval in the z axis. Note the effect of 2-MeSAMP preventing the tight clustering of platelets into aggregates growing above the surface exposed to flowing blood, but not the recruitment of the first layer of platelets adherent to the collagen fibrils. In contrast, aggregation still occurs in the presence of ASA but the number of thrombi is decreased. Different dimension bars in A–C and D are shown.
Measurement of total platelet surface coverage in each z-plane of a series of confocal sections confirmed that addition of 2-MeSAMP, and less so of ASA, prevented aggregate growth in height while the first layer of platelets contacting collagen was actually more extended than in untreated blood (Fig. 2A, B). The effect of 2-MeSAMP on platelet aggregate volume measured in the planes above the layer of collagen-adherent platelets was dose-dependent (Fig. 2C) and maximal at a concentration (~30 µM) that had essentially no influence on VASP phosphorylation (Fig. 2D). Addition to blood of ASA alone resulted in a limited reduction of platelet aggregate growth that was maximal at ~150 µM (Fig. 2E). As compared to control, concurrent addition of ASA and 2-MeSAMP resulted in a more pronounced effect than seen with each inhibitor alone, but the difference between combined or individual addition of inhibitors was not significant (Fig. 2F). Of note, ASA did not change the effect of 2-MeSAMP on VASP phosphorylation.
Figure 2. Quantitative determination of the effect of 2-MeSAMP and ASA on platelet thrombus formation in blood flowing over collagen.
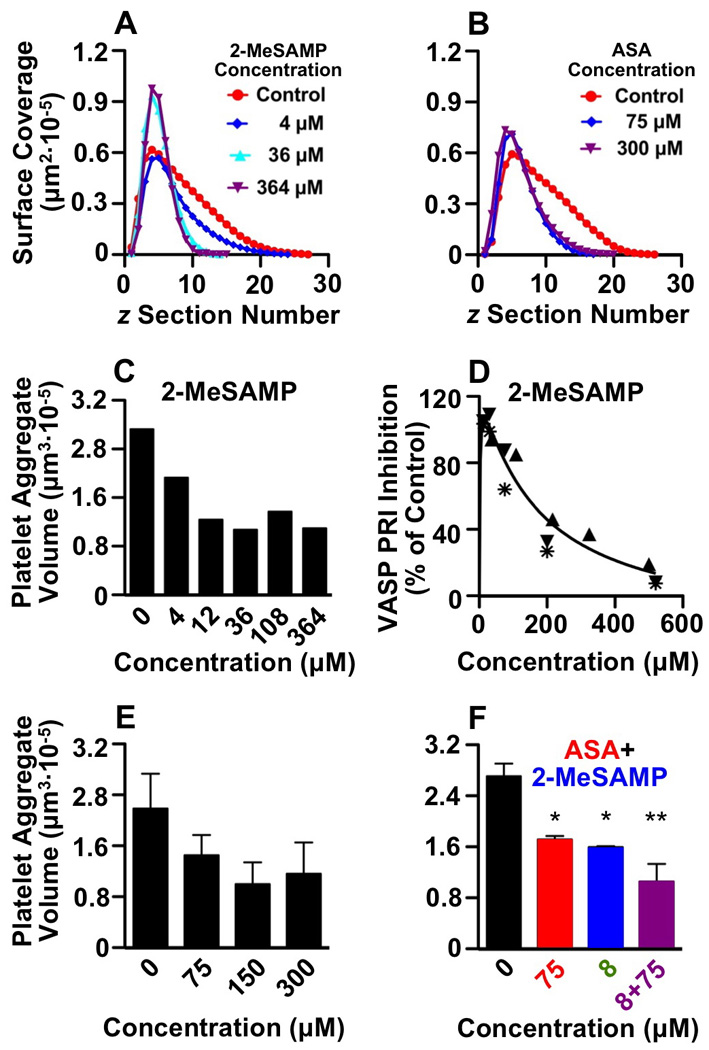
A, B. Total area of adhering and aggregated platelets in individual planes of confocal z-sections plotted against the z position, measured from the collagen surface (0) to the top of thrombi. The results shown are in blood treated with increasing concentrations of 2-MeSAMP (A) or ASA (B) after 6 min of perfusion over fibrillar collagen type I at the wall shear rate of 1,500 s−1. The peak surface area value corresponds to the section through the middle of the first layer of platelets in contact with collagen, and is actually greater in the presence of higher inhibitor concentrations than control. In contrast, as compared to control, the value decreases markedly in planes above the surface and more so in the presence of higher 2-MeSAMP than ASA concentrations, indicating inhibition of platelet aggregation into thrombi. C. Effect of increasing concentrations of 2-MeSAMP on platelet aggregate volume above the layer of adhering platelets. One representative experiments is shown of three that gave comparable results. D. VASP phosphorylation measured in the presence of increasing concentrations of 2-MeSAMP expressed as PRI relative to the value in untreated blood. The results of three separate experiments are shown and different individuals tested are identified by distinct symbols. E. Effect of different ASA concentrations on platelet aggregate volume above the layer of adhering platelets. The average of three different experiments is shown F. Effect of the indicated concentrations of ASA and 2-MeSAMP, individually or in combination, on platelet aggregate volume above the layer of adhering platelets. The average of three different experiments is shown. Error bars in E and F indicate the standard error of the mean. Significance of comparison with control: *, P <0.05; **, P <0.01.
Platelet thrombus formation in patients treated with antiplatelet therapy
The development of surface-adherent platelet aggregates during blood perfusion over fibrillar collagen type I was time-dependent, and total thrombus volume (including the first layer of collagen-adherent platelets) reached the maximum value at 5 to 7 minutes in controls (Fig. 3A). The difference between the extremes of the normal distribution of values was >3-fold (Fig. 3A, Supplemental Movie 1). In the group of treated patients, platelet adhesion and aggregation were normal during the initial 3 minutes of perfusion, and 71% of individual values were within the normal range at this time. The progression of thrombus growth, however, was impaired in a majority of treated patients, and in 14 of them (45.2%) disaggregation of formed thrombi occurred at later time points. This event, resulting in thrombus volume at 7 minutes being ≤ volume at 3 minutes, was a distinctive consequence of anti-platelet treatment; it was never observed in controls and was a discriminating feature between patients responsive and resistant to therapy (Fig. 4, Supplemental Movie 2). In the context of this study, a patient was considered resistant to therapy when the average volume of platelet aggregates formed between 5 and 7 minutes of blood perfusion over fibrillar collagen was within the range of control values. Because of the variable time of onset of disaggregation in treated patients, we used the average volume of aggregates measured at 5 to 7 minutes of perfusion at three different positions in the chamber to evaluate the effects of anti-platelet treatment. As a consequence of decreased growth rate and disaggregation, thrombus size was significantly smaller in the patient than control group at time points between 5 to 7 minutes from the beginning of perfusion (Fig. 3B). Of note, the average thrombus volume at these later time points was essentially the same in the 19 patients treated with aspirin and clopidogrel (mean±SD: 437271±151257 µm3) and 12 treated with aspirin and ticlopidine (mean±SD: 493178±83020 µm3), indicating an equivalent pharmacological effect of the two drugs at the dosage used. For comparison, the thrombus volume in patients on chronic aspirin treatment was within the normal range in 8 out of 10 samples tested (Fig. 3B); the corresponding mean value was lower in this as compared to the control group (mean±SD: 630358±184493 vs. 829152±295723 µm3) but the difference was not significant, as was that with the clopidogrel and ticlopidine treatment groups.
Figure 3. Time-course of platelet thrombus formation in normal controls and treated patients.
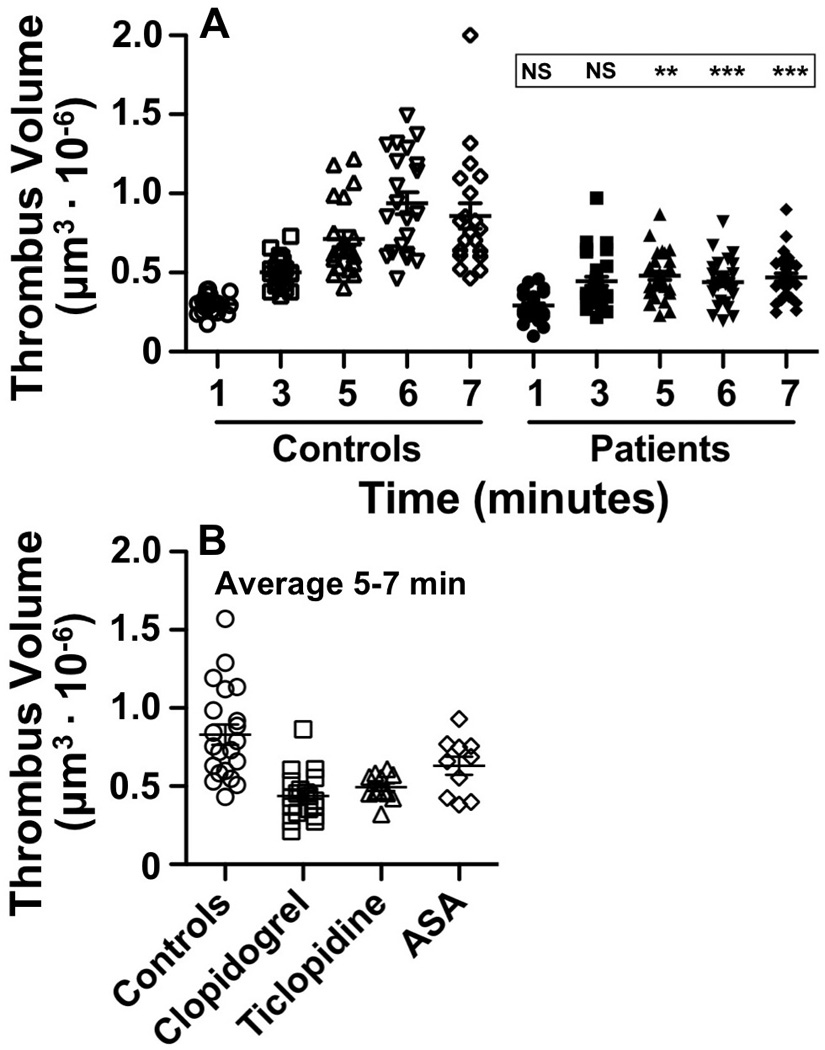
Heparinized blood (17 USP heparin U/ml) containing mepacrine is perfused over fibrillar collagen type I at the shear rate of 1,500 s−1 for 8 min at 37 °C. Confocal z sections obtained at the indicated time points are used to calculate the volume of platelet aggregates formed onto the surface of the field of view (207,025 µm2). A. Positions (P) at different distance from the chamber flow inlet are visualized at different time points: P1, 1.5 mm (1 and 6 min); P2, 2.5 mm (3 min); P3, 5 mm (5 min); P4, 10 mm (7 min). Results of 21 normal controls and 31 patients are shown along with the significance of thrombus volume differences (NS, P >0.05; **, P <0.01; ***, P <0.001). B. Average of thrombus volumes measured after 5–7 min at three different positions in the flow chamber, shown separately for patients treated with aspirin and clopidogrel or aspirin and ticlopidine as well as controls. There is no difference between patients treated with the two drugs and both groups are significantly different (P<0.001) from controls. A group of 10 patients on chronic aspirin treatment (100 mg daily) is shown for comparison; the 5–7 min average thrombus volume in this group is not significantly different from control, but also not significantly different from patients receiving a P2Y12 inhibitor.
Figure 4. Different thrombus growth in patients resistant or responsive to anti-platelet treatment.
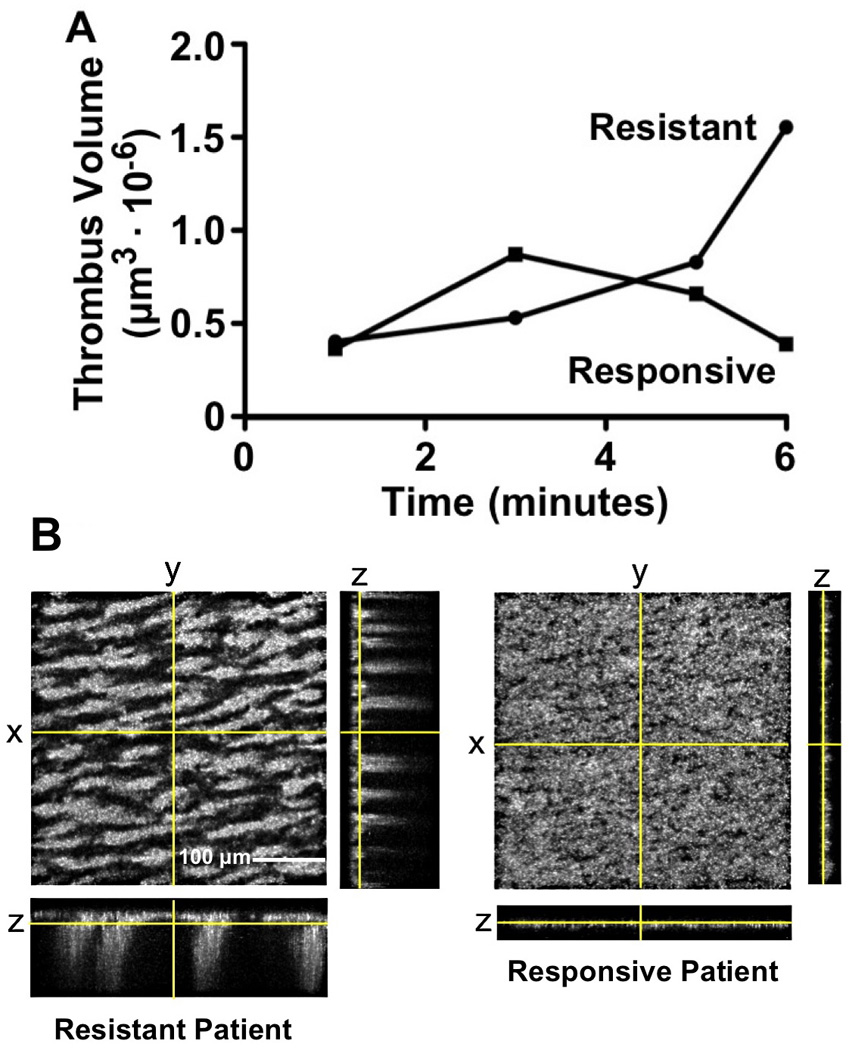
A. The total volume of platelet aggregates forming on fibrillar collagen in a patient resistant to ASA-clopidogrel treatment more than doubles between 3 and 6 minutes of blood perfusion, but decreases to about half in a typical responsive patient, indicating thrombus instability and disaggregation. B. Orthogonal projections of the corresponding platelet aggregates on the collagen surface, calculated from confocal z-sections obtained after 6 min of blood perfusion. See legend to Fig. 1 for details on how these projections are generated. Note the similarity between the extensive platelet surface coverage but lack of aggregate growth in height resulting from clopidogrel treatment in a responsive patient and the effect of the direct P2Y12 inhibitor, 2-MeSAMP, added exogenously to normal blood (see Fig. 1D).
Effect of P2Y12 inhibition on VASP PRI and platelet thrombus volume
The VASP PRI was equally and significantly lower in patients treated with aspirin plus clopidogrel (mean±SD: 51.2±21.6) or ticlopidine (mean±SD: 48.1±25.7) than in controls (mean±SD: 91.4±7.4%; P <0.001 for both comparisons; Fig. 5A). Since there was no difference between the two patient groups, they were treated as one in subsequent evaluations. By ROC curve analysis, the 90% specificity cut-off value, i.e. the value (PRI =81.9%) smaller than in 90% of normal controls, corresponded to 100% sensitivity, i.e. all treated patient had a smaller value (Fig. 5B); only 7 patients (22.6%) had PRI above the lower limit of the normal distribution (PRI =74.2%). Similar ROC curve analysis of platelet thrombus volume results (shown in Fig. 3B), considering clopidogrel- and ticlopidine-treated patients as one group, revealed that the 90% specificity cut-off value (518,334 µm3) corresponded to 67.7% sensitivity (Fig. 5C); 15 patients (48.4%) had thrombus volume above the lower limit of the normal distribution, but only 1 was above the first quartile limit.
Figure 5. ROC curve analysis of VASP PRI and thrombus volume measurements.
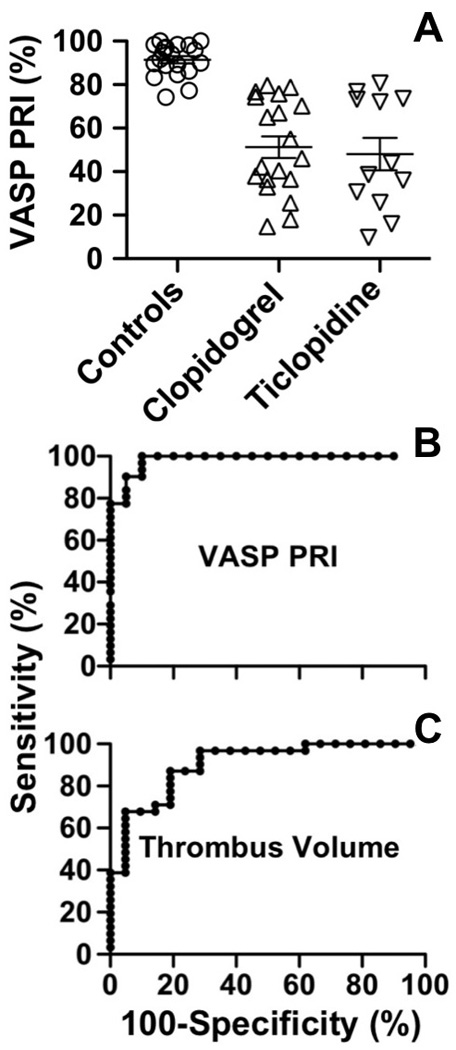
A. VASP PRI is significantly lower (p<0.001) in patients treated with clopidogrel or ticlopidine as compared to controls and there is no difference between the two groups of patients. B. ROC curve analysis of the VASP PRI results. C. ROC curve analysis of the thrombus volume measurements shown in Fig. 3B. As the results in patients treated with clopidogrel or ticlopidine are not significantly different, they are considered as a single group. The percentage of normal samples above (specificity) and patient samples below (sensitivity) each measured value is calculated and plotted as sensitivity vs. 100-specificity. The area under the ROC curve is calculated and expressed as a fraction of 1 with 95% confidence intervals (CI), and the significance of the difference with the null hypothesis (0.5) is then expressed with a P value. VASP PRI: area =0.984, CI 0.957–1.011, P<0.0001. Thrombus volume: area =0.903, CI 0.820–0.987, P<0.0001.
VASP PRI and thrombus volume
There was no correlation between PRI and average thrombus volume measured in patients treated with dual anti-platelet therapy, regardless of whether they were receiving clopidogrel or ticlopidine (Fig. 6). PRI below 69–48% has been associated with better clinical outcome post-PCI [28–30], but platelet thrombus volume was not different in patients with PRI above or below these limits. In our study, 20 patients (64.5%) had PRI below 69%, and 17 of these (54.8%) were below 48%; 12 of 20 (or 11 of 17) had average platelet aggregate volume above the lower normal limit, and 7 of 20 (or 6 of 17) were above the 90% specificity cut-off (Fig. 6). Of note, 4 of the 7, all with PRI <48%, showed thrombus disaggregation between 3 and 7 min of perfusion, thus the platelet aggregates formed were unstable in spite of the total volume of platelets deposited on the surface being normal. Such findings suggest that evaluating platelet thrombus stability in flowing blood, in addition to measuring volume, may provide relevant information on the effects of P2Y12 inhibition. Considering our results from a different perspective, 10 of 14 patients with PRI above 48% (or 8 of 11 considering the 69% cut-off) had platelet aggregate volume below the 90% specificity cut-off; 6 (or 4) of these were below the lower limit of the normal distribution (Fig. 6). In fact, 3 of 7 patients with PRI within the normal range had platelet thrombus volume below the lower limit of normal. Therefore, significant effects of anti-platelet therapy on platelet function can be demonstrated also in patients with P2Y12 inhibition considered sub-optimal.
Figure 6. Lack of correlation between VASP PRI and platelet thrombus volume.
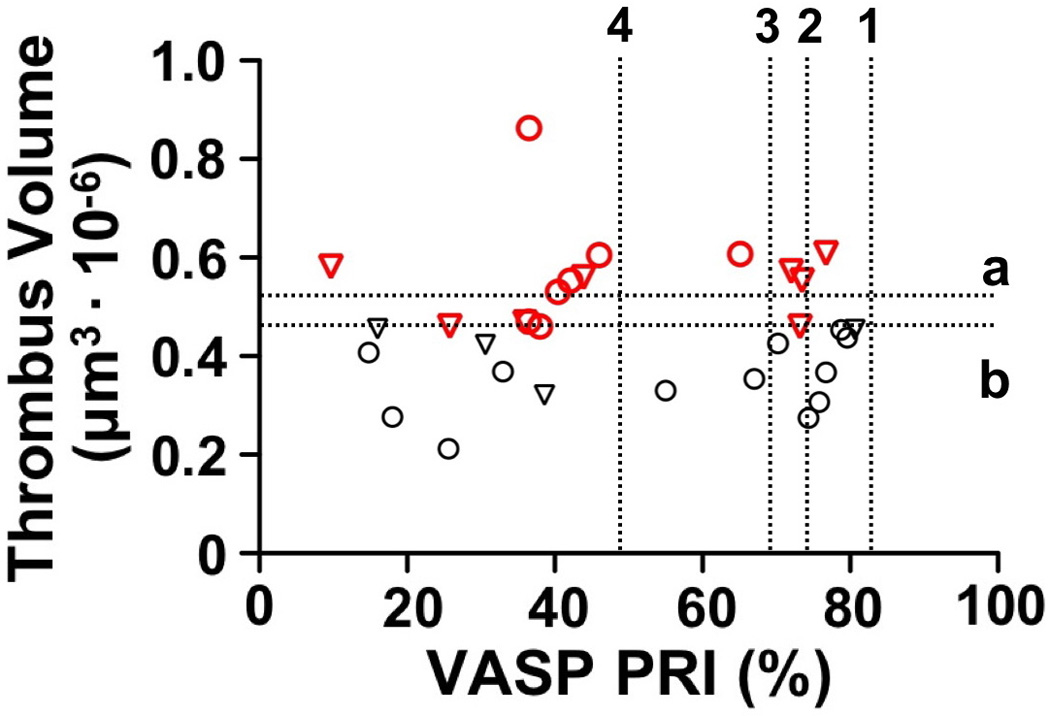
The two assays, done with the same blood specimen but mixed with different anticoagulants, were performed in 31 patients treated for 30–365 days with ASA and clopidogrel (circles) or ASA and ticlopidine (triangles), considered as a single group (see Figs. 3 and 5). Thrombus volume is the average of 3 measurements at different positions in the flow chamber after 5–7 min of perfusion (see Fig. 3B). Red symbols identify patients with platelet thrombus volume above the lower limit of the normal distribution. There is no correlation between the results of the two assays (rs =0.0145; P =0.938). The horizontal dotted lines indicate (a) the thrombus volume cut-off value (518,334 µm3) with 90% specificity; and (b) the lower limit of the normal distribution (431,578 µm3). The vertical dotted lines show (1) the 90% specificity PRI cut-off value (81.9%); (2) the lower limit of the normal distribution (74.2%); and the two PRI values of 69% (3) and 48% (4) previously shown [29, 30] to separate patients with higher and lower thrombotic risk post PCI.
Discussion
The beneficial effect of aspirin and clopidogrel treatment in ACS patients [31] is thought to result from decreased platelet reactivity [15]. Thienopyridines such as clopidogrel and ticlopidine target the Gi-coupled P2Y12 ADP receptor pathway, which contributes to platelet aggregate stability [23, 32, 33]. With the support of results from prospective clinical trials, VASP PRI, which specifically measures P2Y12 function, is considered a good indicator of receptor blockade and, thus, thrombotic risk [28–30]. However, we present here evidence indicating that the level of P2Y12 inhibition fails to reflect the impairment of platelet function as assessed with a blood flow-based method for measuring in real time the volume of platelet aggregates forming onto fibrillar collagen. Indeed, comparable P2Y12 inhibition in different individuals, as evidenced by similar VASP phosphorylation levels, was associated with a range of thrombus volumes from markedly decreased to normal. Of note, we found that in several patients with PRI values below suggested therapeutic cut-off limits, thus judged to have an effective response to treatment, the total volume of platelets deposited onto a collagen surface under flow was within normal limits, although in most cases in the lower quartile of the normal distribution. However, platelet aggregates were unstable in many of these patients, an indication that anti-platelet drugs were altering platelet reactivity [32]. Our results in treated patients and in ex vivo studies with exogenous addition of inhibitors to blood, show that P2Y12 blockade has no effect on initial platelet adhesion to collagen and is compatible with extensive surface deposition of adherent platelets, albeit not aggregated into thrombi. For this reason, measuring the total volume of surface deposited platelets may underestimate the effects of anti-platelet treatment on the formation of stable aggregates growing into the flow path, more likely to represent potentially occluding thrombi. The introduction of corrective parameters, such as aggregate shape (height), is likely to make the measurement of thrombus formation under flow more concordant with the lowering of VASP PRI values within therapeutic limits.
On the other hand, we observed significantly decreased platelet thrombus formation in patients whose P2Y12 inhibition was considered sub-optimal, a more relevant issue as it might lead to considering therapeutic changes in spite of a good reduction in platelet reactivity. In our study, 35 to 45% of patients receiving dual anti-platelet therapy at dosage in accordance with standard guidelines had PRI above the designated cut-off values for antithrombotic efficacy. Such a preponderance of cases with an apparent unsatisfactory response to treatment appears high in view of the relative low frequency of thrombotic complications, suggesting the possibility of therapeutic effects not reflected by the levels of VASP phosphorylation. Indeed, others have already noted that the response to administration of clopidogrel is individually variable [15], with results of VASP PRI in one series ranging from 6.6 to 85.8% [34]. In our study, over 70% of patients with an apparently unsatisfactory response to anti-platelet treatment had reduced thrombus formation on collagen under flow, with total volumes below or near the lower limit of the normal distribution. The results of ex vivo addition of a direct P2Y12 inhibitor, 2-MeSAMP, to normal blood may explain this discrepancy, as the concentration required for maximal inhibition of aggregate formation on collagen (~30 µM) essentially did not change the level of VASP phosphorylation measured in the same samples. Accordingly, >50% VASP PRI reduction required concentrations of 2-MeSAMP (~200 µM) that had no additional influence on platelet function. It appears, therefore, that patients may be protected from the risk of thrombosis even with a relatively poor inhibition of P2Y12, suggesting that a global platelet function test may help achieve a better stratification with respect to thrombotic risk than by using only assays more specific for P2Y12 function.
Evidence has bee presented that direct P2Y12 inhibitors, such as 2-MeSAMP used here and ARC69931MX (Cangrelor), may act as antiplatelet agents by raising intracellular cAMP levels independently of P2Y12 or Gi coupling [35]. The possibility of pharmacological effects beyond those linked to regulation of VASP phosphorylation is supported by the substantial difference we observed between concentrations of 2-MeSAMP (higher) required for altering the VASP PRI response and those (lower) required to inhibit platelet function. Whether the same conclusion applies also to a different class of compounds such as the thienopyridines is unknown at present. It is intriguing to note that exogenous addition to blood of 2-MeSAMP and in vivo administration of clopidogrel and ticlopidine had remarkably similar and distinctive consequences on the process of thrombus formation in flowing blood. In either case, platelet adhesion to collagen was normal and initial thrombus growth minimally affected. The key pharmacological effect was the occurrence of disaggregation of formed thrombi eventually leading to a collagen surface almost entirely covered by single platelets or small aggregates, essentially none of which protruded into the flowing blood. The similar influence on a complex biological process may indicate a common pharmacological action, indeed suggesting that VASP PRI may not reflect all mechanisms of platelet inhibition exerted by drugs considered to target P2Y12.
These considerations are relevant in evaluating whether anti-platelet therapy should be monitored and how. Clinical trials have linked resistance to clopidogrel and risk of thrombotic complications after PCI [36]. In this respect, directly probing the drug target by measuring VASP phosphorylation can indicate whether the expected pharmacodynamic effect has been achieved. As shown by our results, however, a test of this kind may not be fully informative on the effects that treatment has on platelet function. Since platelet aggregation is associated to the risk of thrombosis, a more global rather than pathway-specific test may be a valuable addition for assessing the efficacy of anti-platelet therapy. Such an approach has the additional advantage of evaluating the contribution of aspirin, included in all anti-platelet regimens for ACS patients, to the pharmacologic inhibition of platelet function. As shown here, adding aspirin to blood ex vivo causes a trend towards reduced thrombus height on collagen fibrils under flow and, although without effect on aggregate stability, potentiates the reduction of thrombus formation associated with inhibiting P2Y12. Although patients receiving aspirin therapy alone are not significantly different from normal with respect to total thrombus volume formed on collagen fibrils, it seem logical to assume that a test sensitive to both components of the standard anti-platelet regimen for ACS patients should be valuable for predicting the risk of thrombotic complications. In this regard, a limitation of our study is that we did not evaluate thromboxane A2-associated parameters to monitor compliance to aspirin treatment, which could have influenced the functional testing of platelet aggregate formation. Nonetheless, one major observation we made was decreased platelet reactivity in patients with modestly decreased or normal VASP-PRI, a finding that cannot be reversed by the lack of compliance to aspirin treatment.
Previous reports have highlighted the importance of methodology in evaluating platelet function and, thus, its inhibition [9]. LTA is considered a standard in this respect, but its value for predicting thrombotic complications after PCI is debated [37]. A problem associated with this test is the choice of agonist used since different ones – such as ADP, collagen, α-thrombin and arachidonic acid probe partly distinct pathways of platelet activation. Because thienopyridines target the P2Y12 ADP receptor, it is not surprising that VASP PRI and ADP-induced aggregation have shown correlation [28, 29], although not necessarily concordance in predicting thrombotic risk [30]. Testing platelet activation with the combined use of multiple agonists may be more informative [38] as compared to measuring specifically P2Y12 inhibition, but in many published studies only the response to ADP has been evaluated [18, 39, 40]. Assays that involve the effects of hydrodynamic force are considered relevant in assessing mechanisms of arterial thrombosis [41] and are influenced by P2Y12 inhibition [23, 33, 42]. Yet, results obtained with a flow-based point-of-care instrument have been contradictory and the approach deemed unsatisfactory for monitoring the effects of P2Y12 inhibitors [43–46]. However, as shown here, there is a disconnection between the levels of P2Y12 blockade that influence VASP phosphorylation as compared to platelet thrombus formation under flow. Thus, the lack of correlation between assays probing processes differentially sensitive to P2Y12 function is not necessarily surprising. This notwithstanding, our study suggests that adequate platelet functional testing may have a role in assessing the efficacy of anti-platelet therapy and managing thrombotic risk. Ultimately, the unresolved problem is the correspondence between laboratory evaluation and clinical efficacy of a given pharmacologic treatment, a question that can only be resolved by trials of the size required to link test results and clinical outcome.
Supplementary Material
Acknowledgments
Supported by National Institute of Health grants HL-31950, HL-42846 and HL-98542 (to Z.M.R.).
Abbreviations used
- ACS
acute coronary syndrome
- PCI
percutaneous coronary intervention
- GP
platelet membrane glycoprotein
- ASA
acetylsalicylic acid (aspirin)
- ADP
adenosine diphosphate
- LTA
light transmittance aggregometry
- VWF
von Willebrand factor
- VASP
vasodilator-stimulated phosphoprotein
- PRI
platelet reactivity index
- PG
prostaglandin
- MFI
mean fluorescence intensity
- PBS
phosphate-buffered saline
- CI
confidence intervals of the mean
Footnotes
Addendum. Statement of authors’ contribution: GLM planned and executed research, analyzed data and contributed to writing the manuscript; DZ contributed to research planning and provided patient material; MB executed research and analyzed data; EC and MM provided patient material; CL, PP and LR contributed to data analysis; ZMR planned research, contributed to data analysis and wrote the manuscript. The authors declare that they have no actual or potential conflict of interest capable of influencing judgment on the part of any author, and that all have seen and approved the data presented.
References
- 1.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 2.Schoemig A, Neumann FJ, Kastrati A, SchÅhlen H, Blasini R, Hadamitzky M, Walter H, Zitzmann-Roth EM, Richardt G, Eckhard A, Scmitt C, Ulm K. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. New Engl J Med. 1996;334:1084–1089. doi: 10.1056/NEJM199604253341702. [DOI] [PubMed] [Google Scholar]
- 3.Raju NC, Eikelboom JW, Hirsh J. Platelet ADP-receptor antagonists for cardiovascular disease: past, present and future. Nat Clin Pract Cardiovasc Med. 2008;5:766–780. doi: 10.1038/ncpcardio1372. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. New Engl J Med. 2001;345:464–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 5.Steinhubl S, Roe MT. Optimizing platelet P2Y12 inhibition for patients undergoing PCI. Cardiovasc Drug Rev. 2007;25:188–203. doi: 10.1111/j.1527-3466.2007.00013.x. [DOI] [PubMed] [Google Scholar]
- 6.Wenaweser P, Dorffler-Melly J, Imboden K, Windecker S, Togni M, Meier B, Haeberli A, Hess OM. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45:1748–1752. doi: 10.1016/j.jacc.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 7.Blindel KP, Di Chiara J, Gurbel PA. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? J Am Coll Cardiol. 2007;49:657–666. doi: 10.1016/j.jacc.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 8.Gori AM, Marcucci R, Migliorini A, Valenti R, Moschi G, Paniccia R, Buonamici P, Gensini GF, Vergara R, Abbate R, Antoniucci D. Incidence and clinical impact of dual nonresponsiveness to aspirin and clopidogrel in patients with drug-eluting stents. J Am Coll Cardiol. 2008;52:734–739. doi: 10.1016/j.jacc.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Dor I, Kleiman NS, Lev E. Assessment, mechanisms, and clinical implication of variability in platelet response to aspirin and clopidogrel therapy. Am J Cardiol. 2009;104:227–233. doi: 10.1016/j.amjcard.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Santilli F, Rocca B, De Cristofaro R, Lattanzio S, Pietrangelo L, Habib A, Pettinella C, Recchiuti A, Ferrante E, Ciabattoni G, Davi G, Patrono C. Platelet cyclooxygenase inhibition by low-dose aspirin is not reflected consistently by platelet function assays: implications for aspirin "resistance". J Am Coll Cardiol. 2009;53:667–677. doi: 10.1016/j.jacc.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 11.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Meneveau N, Steg PG, Ferrieres J, Danchin N, Becquemont L. French Registry of Acute STE, Non STEMII. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 12.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, Sabatine MS. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 13.Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, Payot L, Brugier D, Cayla G, Beygui F, Bensimon G, Funck-Brentano C, Montalescot G. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 14.Braunwald E, Angiolillo D, Bates E, Berger PB, Bhatt D, Cannon CP, Furman MI, Gurbel P, Michelson AD, Peterson E, Wiviott S. Antiplatelet therapy and platelet function testing. Clin Cardiol. 2008;31:I36. doi: 10.1002/clc.20371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serebruany VL, Goto S. The challenge of monitoring platelet response after clopidogrel. Eur Heart J. 2008;29:2833–2834. doi: 10.1093/eurheartj/ehn494. [DOI] [PubMed] [Google Scholar]
- 16.Labarthe B, Theroux P, Angioi M, Ghitescu M. Matching the evaluation of the clinical efficacy of clopidogrel to platelet function tests relevant to the biological properties of the drug. J Am Coll Cardiol. 2005;46:638–645. doi: 10.1016/j.jacc.2005.02.092. [DOI] [PubMed] [Google Scholar]
- 17.Gurbel PA, Bliden KP, Samara W, Yoho JA, Hayes K, Fissha MZ, Tantry US. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol. 2005;46:1827–1832. doi: 10.1016/j.jacc.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 18.Lordkipanidze M, Pharand C, Nguyen TA, Schampaert E, Palisaitis DA, Diodati JG. Comparison of four tests to assess inhibition of platelet function by clopidogrel in stable coronary artery disease patients. Eur Heart J. 2008;29:2877–2885. doi: 10.1093/eurheartj/ehn419. [DOI] [PubMed] [Google Scholar]
- 19.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–666. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 20.Ruggeri ZM, Dent JA, Saldivar E. Contribution of distinct adhesive interactions to platelet aggregation in flowing blood. Blood. 1999;94:172–178. [PubMed] [Google Scholar]
- 21.Kasirer-Friede A, Cozzi MR, Mazzucato M, De Marco L, Ruggeri ZM, Shattil SJ. Signaling through GP Ib-IX-V activates αIIbβ3 independently of other receptors. Blood. 2004;103:3403–3411. doi: 10.1182/blood-2003-10-3664. [DOI] [PubMed] [Google Scholar]
- 22.Mazzucato M, Cozzi MR, Pradella P, Ruggeri ZM, De Marco L. Distinct roles of ADP receptors in von Willebrand factor-mediated platelet signaling and activation under high flow. Blood. 2004;104:3221–3227. doi: 10.1182/blood-2004-03-1145. [DOI] [PubMed] [Google Scholar]
- 23.Turner NA, Moake JL, McIntire LV. Blockade of adenosine diphosphate receptors P2Y 12 and P2Y 1 is required to inhibit platelet aggregation in whole blood under flow. Blood. 2001;98:3340–3345. doi: 10.1182/blood.v98.12.3340. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz UR, Geiger J, Walter U, Eigenthaler M. Flow cytometry analysis of intracellular VASP phosphorylation for the assessment of activating and inhibitory signal transduction pathways in human platelets--definition and detection of ticlopidine/clopidogrel effects. Thromb Haemost. 1999;82:1145–1152. [PubMed] [Google Scholar]
- 25.Fujimura Y, Titani K, Holland LZ, Roberts JR, Kostel P, Ruggeri ZM, Zimmerman TS. A heparin-binding domain of human von Willebrand factor. Characterization and localization to a tryptic fragment extending from amino acid residue Val-449 to Lys-728. J Biol Chem. 1987;262:1734–1739. [PubMed] [Google Scholar]
- 26.Andre P, LaRocca T, Delaney SM, Lin PH, Vincent D, Sinha U, Conley PB, Phillips DR. Anticoagulants (thrombin inhibitors) and aspirin synergize with P2Y12 receptor antagonism in thrombosis. Circulation. 2003;108:2697–2703. doi: 10.1161/01.CIR.0000093279.36628.12. [DOI] [PubMed] [Google Scholar]
- 27.Jantzen HM, Milstone DS, Gousset L, Conley PB, Mortensen RM. Impaired activation of murine platelets lacking Gαi2. J Clin Invest. 2001;108:477–483. doi: 10.1172/JCI12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frere C, Cuisset T, Quilici J, Camoin L, Carvajal J, Morange PE, Lambert M, Juhan-Vague I, Bonnet JL, Alessi MC. ADP-induced platelet aggregation and platelet reactivity index VASP are good predictive markers for clinical outcomes in non-ST elevation acute coronary syndrome. Thromb Haemost. 2007;98:838–843. [PubMed] [Google Scholar]
- 29.Blindt R, Stellbrink K, de Taeye A, Muller R, Kiefer P, Yagmur E, Weber C, Kelm M, Hoffmann R. The significance of vasodilator-stimulated phosphoprotein for risk stratification of stent thrombosis. Thromb Haemost. 2007;98:1329–1334. [PubMed] [Google Scholar]
- 30.Morel O, Faure A, Ohlmann P, Jesel L, Desprez D, Grunebaum L, Bareiss P. Impaired platelet responsiveness to clopidogrel identified by flow cytometric vasodilator-stimulated phosphoprotein (VASP) phosphorylation in patients with subacute stent thrombosis. Thromb Haemost. 2007;98:896–899. [PubMed] [Google Scholar]
- 31.Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, Collins R, Liu LS group Cc. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1607–1621. doi: 10.1016/S0140-6736(05)67660-X. S0140-6736(05)67660-X [pii] 10.1016/S0140-6736(05)67660-X [doi]. [DOI] [PubMed] [Google Scholar]
- 32.André P, Delaney SM, LaRocca T, Vincent D, DeGuzman F, Jurek M, Koller B, Phillips DR, Conley PB. P2Y 12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest. 2003;112:398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goto S, Tamura N, Ishida H, Ruggeri ZM. Dependence of platelet thrombus stability on sustained glycoprotein IIb/IIIa activation through adenosine 5'-diphosphate receptor stimulation and cyclic calcium signaling. J Am Coll Cardiol. 2005;47:155–162. doi: 10.1016/j.jacc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 34.Aleil B, Ravanat C, Cazenave JP, Rochoux G, Heitz A, Gachet C. Flow cytometric analysis of intraplatelet VASP phosphorylation for the detection of clopidogrel resistance in patients with ischemic cardiovascular diseases. J Thromb Haemost. 2005;3:85–92. doi: 10.1111/j.1538-7836.2004.01063.x. [DOI] [PubMed] [Google Scholar]
- 35.Srinivasan S, Mir F, Huang JS, Khasawneh FT, Lam SC, Le Breton GC. The P2Y12 antagonists, 2-methylthioadenosine 5'-monophosphate triethylammonium salt and cangrelor (ARC69931MX), can inhibit human platelet aggregation through a Gi-independent increase in cAMP levels. J Biol Chem. 2009;284:16108–16117. doi: 10.1074/jbc.M809780200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Jukema JW, Huisman MV. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J. 2007;154:221–231. doi: 10.1016/j.ahj.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Buonamici P, Marcucci R, Migliorini A, Gensini GF, Santini A, Paniccia R, Moschi G, Gori AM, Abbate R, Antoniucci D. Impact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosis. J Am Coll Cardiol. 2007;49:2312–2317. doi: 10.1016/j.jacc.2007.01.094. [DOI] [PubMed] [Google Scholar]
- 38.Gori AM, Marcucci R, Paniccia R, Giusti B, Fedi S, Antonucci E, Buonamici P, Antoniucci D, Gensini GF, Abbate R. Thrombotic events in high risk patients are predicted by evaluating different pathways of platelet function. Thromb Haemost. 2008;100:1136–1145. doi: 10.1160/th08-06-0374. [DOI] [PubMed] [Google Scholar]
- 39.Pinto Slottow TL, Bonello L, Gavini R, Beauzile P, Sushinsky SJ, Scheinowitz M, Kaneshige K, Xue Z, Torguson R, Tantry U, Pichard AD, Satler LF, Suddath WO, Kent K, Gurbel P, Waksman R. Prevalence of aspirin and clopidogrel resistance among patients with and without drug-eluting stent thrombosis. Am J Cardiol. 2009;104:525–530. doi: 10.1016/j.amjcard.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Cuisset T, Frere C, Poyet R, Quilici J, Gaborit B, Bali L, Brissy O, Lambert M, Morange PE, Alessi MC, Bonnet JL. Clopidogrel response: Head-to-head comparison of different platelet assays to identify clopidogrel non responder patients after coronary stenting. Arch Cardiovasc Dis. 2010;103:39–45. doi: 10.1016/j.acvd.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Ruggeri ZM. Platelet adhesion under flow. Microcirculation. 2009;16:58–83. doi: 10.1080/10739680802651477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goto S, Tamura N, Sakakibara M, Ikeda Y, Handa S. Effects of ticlopidine on von Willebrand factor-mediated shear-induced platelet activation and aggregation. Platelets. 2001;12:406–414. doi: 10.1080/09537100120078377. [DOI] [PubMed] [Google Scholar]
- 43.Hezard N, Metz D, Nazeyrollas P, Droulle C, Potron G, Nguyen P. PFA-100 and flow cytometry: can they challenge aggregometry to assess antiplatelet agents, other than GPIIbIIIa blockers, in coronary angioplasty? Thromb Res. 2002;108:43–47. doi: 10.1016/s0049-3848(02)00391-2. [DOI] [PubMed] [Google Scholar]
- 44.Raman S, Jilma B. Time lag in platelet function inhibition by clopidogrel in stroke patients as measured by PFA-100. J Thromb Haemost. 2004;2:2278–2279. doi: 10.1111/j.1538-7836.2004.01046.x. [DOI] [PubMed] [Google Scholar]
- 45.Golanski J, Pluta J, Baraniak J, Watala C. Limited usefulness of the PFA-100 for the monitoring of ADP receptor antagonists--in vitro experience. Clin Chem Lab Med. 2004;42:25–29. doi: 10.1515/CCLM.2004.006. 10.1515/CCLM.2004.006 [doi]. [DOI] [PubMed] [Google Scholar]
- 46.Geiger J, Teichmann L, Grossmann R, Aktas B, Steigerwald U, Walter U, Schinzel R. Monitoring of clopidogrel action: comparison of methods. Clin Chem. 2005;51:957–965. doi: 10.1373/clinchem.2004.047050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


