Abstract
Lead (Pb) and stress co-occur as risk factors, share biological substrates and produce common adverse effects. We previously found that prenatal restraint stress (PS) or offspring stress (OS) could enhance maternal Pb-induced behavioral, brain neurotransmitter level and HPA axis changes. The current study examined how lifetime Pb exposure, consistent with human environmental exposure, interacts with stress. Dams were exposed to Pb beginning 2 mos prior to breeding (0, 50 or 150 ppm in drinking water), PS on gestational days 16 and 17, or the combination. Offspring continued on the same Pb exposure as the dam. A subset of Pb + PS offspring also received 3 additional stress challenges (OS), yielding 9 exposure groups/gender: 0-NS, 0-PS, 0-OS, 50-NS, 50-PS, 50-OS, 150-NS, 150-PS and 150-OS. As with maternal Pb [48], lifetime Pb and stress influenced Fixed Interval (FI) behavior primarily in females. Relative to 0-NS control, reductions in postreinforcement pause (PRP) times were seen only with combined Pb + PS (50-PS, 50-OS, 150-PS). Stress increased FI response rates when Pb alone was without effect (150-PS, 150-OS), but gradually mitigated rate increases produced by Pb alone (50-PS, 50-OS), effects that appear to be due primarily to PS, as they were of comparable magnitude in PS and OS groups. Individual subject data suggest that enhanced Pb and PS effects reflect increasing numbers of subjects shifting to the high end of the normal range of FI performance values, consistent with a dose-response type of Pb + stress additivity. Consistent with reports of cortico-striatal mediation of both interval timing (PRP) and FI rates, principal component analyses suggested potential mediation via altered frontal cortex norepinephrine, reduced nucleus accumbens dopaminergic control and enhanced striatal monoamine control. Altered FI performance, whether occurring through changes in response rate, PRP, or both, represent behavioral inefficiency and potentially sub-optimal or even dysfunctional resource/energy use.
Keywords: lead, stress, gender, Fixed Interval, postreinforcement pause, frontal cortex, norepinephrine
1. INTRODUCTION
Significant successes in lowering blood lead (PbB) levels have been achieved in countries that removed Pb from paint and gasoline. Even in such places, however, many residual consequences of the prior uses of Pb still remain. In the U.S., for example, such consequences include the lifetime elevated exposures experienced by the current elderly segment of the population, an exposure that has been posited to contribute to several diseases and disorders in this age group [1–8]. Sustained contamination from the prolonged use of Pb in paint and gasoline also underlies the current elevations of blood Pb that now preferentially impact low socioeconomic, medically underserved inner city children residing in old housing stock, i.e., the same communities that also sustain low follow up rates after initial identification of elevated Pb exposures [9; 10]. The impact of this problem has been significantly broadened by the growing recognition of adverse cognitive effects in children at increasingly lower blood Pb levels [11; 12]. Of course, elevated Pb burden also remains a significant problem in countries where leaded gasoline remains in use and/or where environmental regulations remain secondary to industrial development [13].
Although our understanding of the neurotoxic effects of Pb and the mechanism(s) by which they are achieved has increased substantially over the years, both as a result of human and experimental studies, this understanding reflects the effects of Pb exposure examined in isolation [14]. Human environmental Pb exposure, like all environmental chemical exposures, actually occurs in the context of numerous other risk factors for various diseases and disorders, including genetic and host risk factors, as well as other environmental exposures, some of which could conceivably have the potential to modify Pb-associated neurotoxicity [14]. For example, dietary calcium/iron levels can substantially alter Pb kinetics and its distribution across organ systems [15–18]. Time of the year (season) can change blood Pb levels [19], and aging has been shown to modify kinetics of Pb as well as its behavioral consequences [14; 20–22].
Stress is considered a risk factor for a variety of human diseases and disorders [23–31]. Higher levels of stress with consequent sustained elevation of glucocorticoids have been postulated to account for the increased incidence of many diseases and disorders found in low socioeconomic status (SES) communities [27; 32–36]. Like Pb exposure, stress may be present at any time throughout the lifespan, and, depending upon what has been deemed an individual’s allostatic load, i.e., the abrasion produced by the body’s necessity to respond repeatedly to stress challenges [37], it can have pronounced and, particularly in the case of prenatal stress, permanent adverse consequences [38–40].
In addition to co-occurrence as risk factors, particularly for low SES communities [41; 42], Pb and stress also share common biological substrates and produce similar adverse consequences [43–49]. Both Pb and stress, for example, act on the hypothalamic-pituitary-adrenal (HPA) axis [43; 45; 50–52]. In fact, maternal Pb exposure, like prenatal stress, induces a permanent change in HPA axis function that includes hypercortisolism as a consequence [43–45; 47; 48]. Both Pb and stress, moreover, have been associated with deficits in cognition and attention [11; 53–61], a finding that may reflect their common targeting of brain dopamine and glutamatergic systems in regions including nucleus accumbens and hippocampus that are critical to mediation of such behaviors [62–70].
Based upon their co-occurrence and shared biological substrates and consequences, we predicted the potential for enhanced toxicity in response to combined exposures to Pb and stress. Consistent with this assertion, enhanced effects were observed in behavioral, neurochemical and glucocorticoid outcomes in response to combined maternal Pb and stress in rats [45; 48; 49; 71]. For example, female offspring exposed maternally to 50 ppm Pb showed increases in response rates on a Fixed Interval (FI) schedule of reinforcement, a behavior highly sensitive to Pb and exhibiting extensive cross-species generality [72], that were even further increased in groups subjected to Pb combined with prenatal (PS) followed by offspring stress (OS). The magnitude of the 50 ppm Pb + stress changes produced effect levels comparable to those associated with a higher Pb exposure level, 150 ppm, indicative of Pb and PS + OS stress additivity [73; 74]. Corresponding changes in catecholamines, particularly frontal cortex NE, 5HT and 5HIAA were also seen [48]. Male offspring have shown enhanced effects of maternal Pb combined with prenatal stress in further delaying glucocorticoid negative feedback [45].
These prior studies of Pb exposure combined with PS ± OS were focused on determining the component of risk conferred specifically by early developmental Pb exposures. Human environmental Pb exposure is, of course, continuous across the life span. Therefore, through both acute effects of ongoing exposure, as well as accumulation and potential redistribution over time [20; 75–78], continuous or lifetime Pb exposure may exhibit a different pattern of interactions with stress than occurs with maternal Pb exposure alone. The current study used the same behavioral (Fixed Interval schedule-controlled performance), neurochemical and glucocorticoid measures employed in those studies of maternal Pb exposure to determine the interactions of lifetime Pb exposure, including levels just at those deemed to be of concern for children by the Centers for Disease Control, with PS ± OS.
Our prior studies have confirmed the importance of nucleus accumbens, striatal and frontal cortex monoamines in the mediation of Fixed Interval performance per se, as well as suggesting mediation of maternal Pb + stress effects [48; 63; 79–81]. The corticolimbic system, impacted by both Pb and stress, operates as a coordinated interacting network, rather than as individual regions with specific functions [82], with dysfunction attributable to disruption of corresponding excitatory-inhibitory balance in these systems. Therefore, in addition to characterizing the nature of lifetime Pb ± stress interactions, evaluation of the relationships between associated neurotransmitter and corticosterone changes with FI performance were explored to provide initial information on potential mechanisms of combined Pb and stress-induced behavioral consequences.
2. MATERIALS AND METHODS
2.1 Dams and Pb Exposure
Three-week-old female Long Evans rats (Charles River, Germantown, NY) were randomly assigned to receive distilled deionized water drinking solutions with Pb acetate at concentrations of 0, 50 or 150 ppm, the same concentrations used with maternal Pb exposure ± stress which yielded blood Pb levels (PbBs) in dams at weaning averaging < 3, 11–12, and 31 µg/dl, respectively [48]. These PbBs are associated with similar behavioral deficits in rodents and children [11; 56; 57].
Pb exposure of dams was initiated two months prior to breeding to ensure elevated bone Pb levels and Pb body burden at the time of conception, and was continued throughout lactation, consistent with human exposure. When females reached 3 mos of age, they were paired with 3 mos old male Long-Evans rats for breeding. Animals were housed in a vivarium room maintained at 22 ± 5 °C with a 12h light-dark cycle (lights on at 0700h). Standard rat chow diet was provided ad libitum. All experiments were carried out according to NIH Guidelines and were approved by the University of Medicine and Dentistry of New Jersey University Committee on Animal Resources where the study was carried out.
2.2 Breeding and Prenatal Stress
At pro-estrus, as determined by vaginal smears, female rats were mated with males (2:1) across two estrous cycles. The presence of vaginal plugs or sperm in vaginal smears collected in the early morning was considered indicative of pregnancy and deemed gestational day 1 (GD1). Pregnant females in each Pb-treated group were weighed and further randomly subdivided to a non-stress (NS) or prenatal stress (PS) condition. All pregnant females were individually housed for the remainder of pregnancy and lactation. This resulted in six Pb-stress conditions with the following numbers of dams: 0-NS (n=10), 0-PS (n=13), 50-NS (n=9), 50-PS (n=13), 150-NS (n=11), and 150-PS (n=10).
On GD 16 and 17, dams assigned to PS groups were weighed and subjected to a widely employed restraint stress procedure [83] consisting of three 45 min restraint sessions (0900, 1200 and 1500h) in plastic cylindrical devices carried out in a laboratory room; dams were returned to the home cage between the stress sessions. NS dams were weighed and subsequently left undisturbed in their home cages. Used in our previous study of Pb and stress, this protocol elevated corticosterone levels and altered catecholamine levels in frontal cortex and nucleus accumbens of dams [43]. The choice of days 16 and 17 was timed to correspond to the development of brain dopamine and glutamate systems/regions shown to be critical to the mediation of FI performance, and of glucocorticoid receptors critical to mediation of stress responses [84; 85].
2.3 Offspring Procedures
2.3.1 Pre-Behavioral Testing
At delivery (postnatal day 1: PND1), litter size was recorded and number of pups culled to 8 per litter, maintaining equal numbers of males and females wherever possible. Cross-fostering was not performed, as the intent of the study was to model the human environment. Pups were weighed and weaned at PND21, separated by gender, and littermates of the same gender were housed as pairs.
From weaning, pups were provided with unrestricted access to the same food (Laboratory Rodent Diet 5001, POMI Foods Inc.) and to the same Pb drinking solutions that their dam had received. At approximately 2 mos of age, brains and blood from a subset of male and female offspring from each of the 6 Pb-stress treatment groups defined above were harvested to provide basal determinations of corticosterone as well as levels of monoamines in frontal cortex, nucleus accumbens and striatum. Another subset was taken at 4 mos of age for the same corticosterone and neurochemical determinations.
Another subset was used for behavioral testing, which was initiated at 2 mos of age. At this time, a subset of offspring from each PS group (0-PS, 50-PS and 150-PS) was allocated to receive offspring stress (OS) treatment as adults that consisted of a series of three additional stress challenges (defined below). Thus, final Pb ± stress conditions included 9 groups/gender: 0-NS, 0-PS, 0-OS, 50-NS, 50-PS, 50-OS, 150-NS, 150-PS and 150-OS. Each gender group undergoing behavioral testing was comprised of a single offspring from a dam so as to preclude litter specific effects (n=9–11 per Pb ± stress group).
With the initiation of behavioral testing at 2 mos of age, offspring were provided with sufficient food to allow a 2–3 gm weight gain per day until male pups reached approximately 300 g and females 220 g. At this point, caloric intake was regulated for the duration of the experiment to maintain the above-stated body weights as required for behavioral evaluation. Because animals were pair-housed, individual feeding was accomplished by separating the residents through the introduction of a cage divider at the time of feeding which remained in place for approximately 90–120 min.
2.3.2 Behavioral Testing and Stress Challenges
Behavioral testing was carried out on an FI 1 min schedule of food reinforcement in 20 min sessions carried out 5 days a week (M-F) between 1000 and 1600h in operant chambers (30.5 cm × 24.5 cm × 21 cm; Med Associates Inc., St. Albans, Vermont) housed in sound-attenuated enclosures ventilated by a fan. Chambers were equipped with a grid floor, speaker, house light and three response levers configured horizontally on the front panel; only the right lever was active in these experiments. Behavioral contingencies and data were controlled by SoftCtrl™ Cumulative Record interface and Med-PC Version IV Research Control and Data Acquisition software.
Lever press response training was first carried out in automated overnight sessions using a concurrent variable time 60 sec fixed ratio (FR) 1 schedule. The schedule converted to FR 1 after 20 min or 10 FR 1 responses had occurred, and ended with 100 reinforcer deliveries [74]. The FI 1 min schedule was implemented in the next behavioral test session. The FI 1 min schedule provided reinforcement for the first occurrence of a designated response after a 1 min interval had elapsed. Reinforcement delivery (45 mg food pellet; Bioserv, Frenchtown, NJ) also initiated the next 1 min fixed interval. Sessions were initiated by the first response or after 5-min if no response had occurred, and ended following the completion of the 1- min interval that was in progress 20 min after the session began, or after a total of 21 min whichever occurred first. Responses during the 1 min interval itself had no programmed consequences. Performance on the FI schedule has been demonstrated to show high levels of cross-species generality [72] characterized by low rates early in the interval and increasing rates as the time to reinforcement availability approaches. The FI schedule has also proven to be sensitive to Pb alone across a range of different developmental periods of exposure and Pb exposure concentrations [86].
Standard FI performance measures were analyzed [72]. Overall response rate (total number of responses divided by total session time) evaluates integrated performance on the schedule that includes both responding and pausing (absence of responding). Time to the first response in the interval (post-reinforcement pause; PRP) is considered an index of timing behavior, since PRP time has been shown to be positively related to fixed interval length. A more precise measure of response rate is provided by run rate, which measures number of responses per minutes during the fixed interval after PRP time is subtracted out. Collectively, these measures assess efficiency of the acquisition of prototypical FI performance as they provide information related to behavioral mechanisms in the case of treatment effects, e.g., rate of response vs. timing deficiencies.
Tail blood was collected at the end of a behavioral session after approximately 3–5 FI sessions had been conducted for determinations of pre-stress challenge corticosterone levels. Offspring stress challenges were then begun in all OS groups and were imposed immediately prior to an FI session to measure its impact on subsequent FI performance. Stress challenges were carried out during the acquisition phase of FI training so that the ability to elicit group differences was maximized. Multiple rather than a single stress challenge was used to more closely mimic the human experience across the life span. The first such stress challenge, carried out prior to sessions 5–7, consisted of a single 45 min session of restraint stress in a plastic cylindrical device in a laboratory room. The use of the most intense stress before other stress challenges was based on its potential to increase sensitivity of the animals to subsequent stressors [87], thereby potentially maximizing the ability to detect treatment-related differences. Prior to session 13, cold stress, considered a mild physical stress, was imposed. Animals were placed in cages similar to home cages (without the bedding) at 4°C in a temperature-controlled room for 30 min prior to being placed in the operant chambers. Prior to session 21, OS groups underwent a 15 min test of locomotor activity in locomotor activity cages as a novel environment stressor. FI rats not assigned to offspring stress (e.g., NS and PS groups) were left undisturbed in their home cages during these periods. Blood samples were collected in OS groups immediately after the FI session that followed each stress challenge for corticosterone determinations. Behavioral testing on the FI schedule continued for an additional 120 sessions in all groups to determine time course and stability of any observed group differences. Results of the stress challenges per se will be reported elsewhere.
2.3.3 Post Behavioral Testing
Within one month after the completion of FI behavioral testing, blood was collected for a final corticosterone determination and brain regions were harvested for the measurement of brain monoamine levels as well as hippocampal cytosolic and nuclear glucocorticoid receptors.
2.4 PbB Analysis
PbB levels were measured in dams after 3 mos of Pb exposure (n=9–10/group), in dams at weaning (n=5–6/Pb ± stress group), in pups at two months of age (n=2/Pb ± stress group), and in a subset of Pb only offspring at the termination of behavioral testing (n=3–5/group) using the Lead Care III system according to the manufacturer’s instructions which has a limit of sensitivity of 3.3 µg/dl.
2.5 Neurochemical Determinations of Monoamines
Levels of (DA (dopamine), DOPAC (dihydroxyphenylacetic acid), HVA (homovanillic acid), NE (norepinephrine), 5-HT (serotonin) and 5-HIAA (5 hydroxyindoleacetic acid) from frontal cortex, nucleus accumbens and striatum were analyzed using HPLC with electrochemical detection as described in detail previously [48]. Concentrations of neurotransmitters were expressed in terms of ng/mg protein. DA turnover was calculated as the DOPAC/DA ratio.
2.6 Determination of Corticosterone Levels
Approximately 200 µl of blood from a tail nick was collected into pre-chilled tubes that were spun at 3500 rpm for 10 min. Serum was separated and stored at −20°C until the time of the assay. Serum corticosterone was measured using the commercially available ImmuChem™ Double Antibody Corticosterone 125I kit according to the manufacturer’s instructions (MP Biomedicals, Orangeburg, NY). All standards and samples were run in duplicate, counted using a Cobra II Auto Gamma counter and expressed in ng/ml. The minimum detectable level in this assay was 7.7ng/ml.
2.7 Western Blot Determination of Hippocampal Cytosolic and Nuclear Glucocorticoid Receptors
Hippocampal tissue was homogenized in 0.5 ml buffer/100 mg tissue (50 mM Tris buffer pH 7.2, at 4°C containing 1 mM EDTA, 10% w/v sucrose, and one tablet/10 ml of the protease inhibitor cocktail mix “Complete Mini”;Roche, Basel), then centrifuged for 5 min at 2000×g, followed by 105,000×g for 30 min and the final supernatant used as the cytosolic fraction. For the nuclear extraction, the pellet from low speed centrifugation from the homogenate was washed twice in homogenization buffer and then centrifuged (5 min, 2000×g). The washed pellet was re-suspended in 0.25 ml homogenization buffer containing 0.5 M NaCl, incubated for 1 h in ice in a shaker with vortexing, then centrifuged (8000×g) for 10 min at 4 °C. Samples were adjusted for protein levels using the BCA assay. Supernatants were mixed with Laemmli's sample buffer and boiled for 5 min.
Samples (40 µg protein) were loaded onto 8% bis-acrylamide gels and separated by SDS polyacrylamide gel electrophoresis. Separated proteins were electrophoretically transferred from gels to pure nitrocellulose membranes (Bio-Rad). Blots were blocked for 30 min in a Tris-buffered saline solution with 0.1% Tween-20 and 5% instant dried milk. Membranes were incubated overnight at 4°C with 5 µg/ml of the monoclonal mouse antibody glucocorticoid receptor (Affinity BioReagents, USA) that detects a band of approximately 97 kDa. Anti-B-actin (Sigma) measured at a molecular weight of 42kDa was used to confirm equal protein loading. Blots were subsequently incubated for 1 h at room temperature with secondary antibody and immuno-positive bands visualized using a Kodak BioMax Film. Optical density of GR reactive bands was quantified using NIH Image software [88].
2.8 Statistical Analysis
2.8.1 PbB Levels
Blood Pb levels were analyzed by ANOVA with Pb and treatment group (dams at 3 mos of Pb exposure, dams at weaning, offspring at 2 mos of age, offspring at the termination of behavioral testing) as between group factors. For statistically significant main effects or interactions, post-hoc Fishers protected least significant differences (PLSD) tests were used to compare treatment groups.
2.8.2 Biochemical Measures
Corticosterone
Overall ANOVAs including Pb, stress and gender as between group factors were first carried out to examine changes in basal (2 mos of age) and in final (post termination of behavioral testing) corticosterone levels. Because of the significant main effects and interactions involving gender for both basal (gender: F(1,89)= 75.4, p<0.0001; Pb x stress x gender: F(2,89)= 3.12, p=.049) and final corticosterone levels (gender: F(1,150)= 109.2, p<0.0001; Pb x stress x gender: F(4,150)= 4.9, p=.001), subsequent ANOVAs examining Pb and stress as between group factors were carried out separately for males and females for basal and for final determinations.
Glucocorticoid Receptors
Overall ANOVAs including Pb, stress and gender as between group factors were first carried out. Based on the confirmation of stress x gender differences for cytosolic glucocorticoid receptors (F(2,81)=3.29, p=0.042), subsequent ANOVAs based on Pb and stress were carried out separately for each gender for cytosolic and nuclear receptors.
Neurotransmitters
Neurotransmitter levels from behaviorally tested animals were calculated as a percent of the same gender 0-NS group mean values from the two month time point data (not shown) to incorporate a measure of longitudinal change over time. Overall ANOVAs for each neurotransmitter in each region that included Pb, stress and gender revealed pervasive (all comparisons except striatal NE) gender effects (frontal cortex for DA, DOPAC, DA TO, NE, 5-HT and HIAA, respectively: F= (1,167) 109.3, (1,163)41.04, (1,162)190.82, (1,166)65.29, (1,167)168.82, and (1,166)17.04, all p<0.0001; nucleus accumbens for DA, DOPAC, HVA, DA TO, NE, 5-HT and HIAA, respectively: F(1,168)57.68, (1,167)28.12, (1,163)587.82, (1,167)178.74, (1,165)13.96, (1,167)152.74, and (1,167)4.94, all p<0.0001 except NE (0.0003) and HIAA (0.0276); striatum for DA, DOPAC, HVA, DA TO, 5-HT and HIAA, respectively: F(1,166)=46.33, (1,166)199, (1,168)12.74, (1,164)166.7, (1,166)97.08, and (1,167)4.66, all p<0.0001 except HVA (0.0005) and HIAA (0.032)). Consequently, subsequent ANOVAs were then carried out for each neurotransmitter in each region separately for each gender that included Pb and stress as between group factors.
For all biochemical measures, main effects of Pb or stress were further examined using Fishers protected least significant differences (PLSD) tests. In the event of significant interactions, Fishers PLSD tests were used to compare Pb ± stress groups.
2.8.3 FI Performance
An overall repeated measures ANOVA (RMANOVA) was first carried out using Pb, stress and gender as between group factors and FI test sessions (except sessions preceded by stress challenges) as a within group factor for each FI performance measure (overall rate, PRP and run rate). Significant interactions with gender were found for all FI performance measures (overall rate: Pb x stress x gender x session: F(92,3887)=1.652, p=0.0001; run rate: Pb x stress x gender x session: F(92, 3887)= 1.367, p=0.012, postreinforcement pause time: Pb x session x gender: F(46, 3887)=1.935, p=.0002, stress x gender x session: F(46, 3887)=1.395, p=0.04).
Subsequently RMANOVAs were carried out for each gender for each FI performance measure using Pb and stress as between group factors and sessions as a within group factor to further characterize the effects. These RMANOVAs confirmed significant Pb x stress x session blocks in both genders for FI overall response rates (females: F(96,2016)=1.27, p=0.0406; males: (F(92,1886)=2.36, p<0.0001) and for female run rate (F(9,2016) = 1.3, p=0.029).
To further determine the basis of these overall response rate changes, subsequent analyses compared 0-NS control separately to each Pb ± stress condition (i.e., 0-NS vs. 0-PS and 0-OS; 0-NS vs. 50-NS, 50-PS and 50-OS, and 0-NS vs. 150-NS, 150-PS and 150-OS), using treatment group as a between group factor and sessions as a within group factor. This approach was utilized to: 1) examine PS and OS effects relative to control independently of Pb exposure; 2) minimize the number of comparisons, and 3) concurrently, to facilitate interpretability of outcomes, particularly of interaction effects given the number of factors examined. In essence it also provided a more conservative approach than an overall analysis across all conditions given the smaller degrees of freedom for each of the 3 major comparisons. In the event of significant outcomes in theses analyses, Fisher’s Protected Least Significant Difference tests were used to compare treatment groups (main effects) or treatment groups at each session block (interactions). However, given the notable trends toward increased response rates in the 0-PS and 0-OS groups, albeit non-significant, and the goal of further defining interaction effects of combined Pb and stress, post-hoc tests also included both the 0-PS and 0-OS groups and comparisons of groups within each condition.
In all above analyses, a p value of ≤0.05 was considered statistically significant.
2.8.4 Relationship of Biochemical Measures to FI Performance
To explore potential relationships between biochemical measures and the FI performance changes observed in female offspring, two analyses were carried out. First, simple linear regression analyses were used to explore whether final corticosterone levels correlated with the FI performance measures overall response rate and postreinforcement pause times at session blocks 10, 15 and 18.
Principal component analyses (PCA) are usefully applied to a set of variables to discover which form related subsets (components). As our first study characterizing lifetime Pb + stress effects, neurotransmitters in multiple brain regions were measured to assess neurotransmitter-specific vulnerabilities. PCA analyses were then used to explore relationships of the profile of neurotransmitter changes (ng/ml protein) to FI performance, i.e., to determine which neurotransmitter change(s) might cluster with FI performance. PCA were carried out separately for each of the 9 Pb ± stress groups to relate FI overall response rate from session block 10, a period in which maximal Pb ± stress differences were evident, with levels (ng/mg protein) of frontal cortex, nucleus accumbens and striatal monoamines. Similar analyses carried out on PRP values showed low relative strength of PRP itself in these models and are thus not presented.
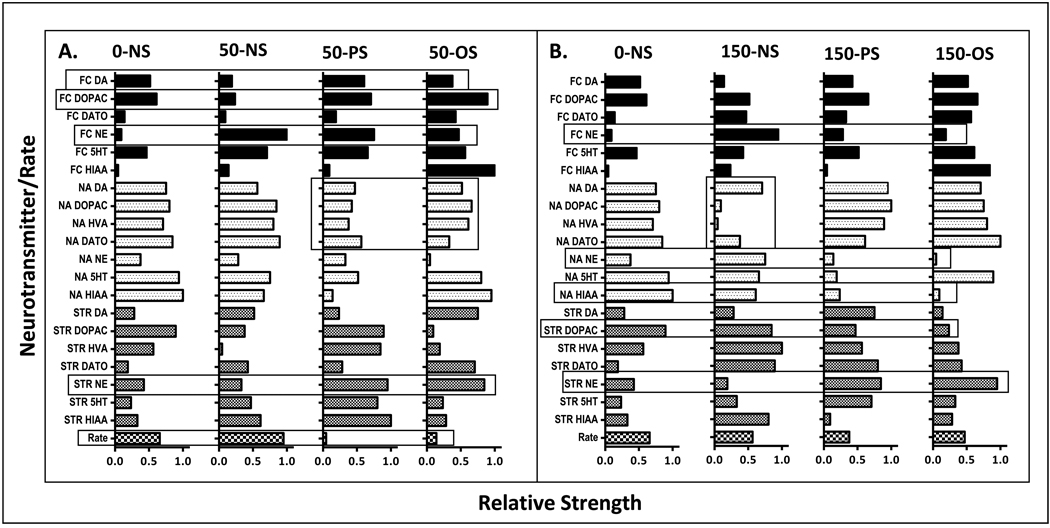
PCA were done using varimax rotation with Eigenvalues over 1 extracted and a maximum iteration for convergence of up to 100 rotations. Cumulative fits for rotated sums of squared loadings ranged from 85.21–93.01%. A total of 5–7 components with Eigenvalues over 1 emerged from these analyses. From each such PCA, the rotated matrix scores for the 20 neurotransmitters and the FI performance measures (21 total scores) were ranked from highest score in the first component (relative strength = 1.0) to lowest score in the final component (relative strength = 0.047) in a descending order based on relative units defined by 21/1.0=0.047 (Figure 10, x axis). These analyses were carried out to allow comparisons across Pb ± stress groups of the relative strength (position) of each neurotransmitter factor and its potential explanatory relation to FI overall response rate.
Figure 10.
Relative strengths of female offspring on a scale from 0 to 1.0 for each neurotransmitter and FI overall response rate (y axis) from session block 10 derived from principal component analyses carried out for each Pb-stress group comparing 0-NS control values to all 50 ppm group values (panel A) and 0-NS control values to all 150 ppm group values (panel B). Open bars encompassing a specific neurotransmitter show systematic changes across the groupings.
3. RESULTS
3.1 PbB Concentrations
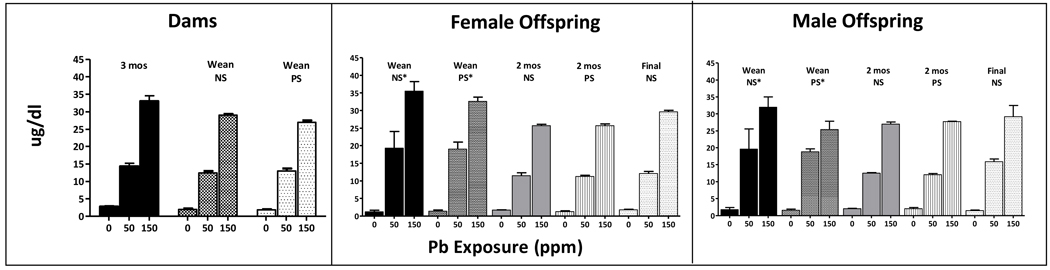
PbBs from dams measured after 3 mos of Pb exposure and at weaning, and from male and female offspring at 2 mos of age, and at the termination of behavioral testing are shown in Fig. 1. PbBs increased in a concentration-related manner (F(2,84)=1004, p<0.0001), with values averaging <3 µg/dl, 11–16 ug/dl and 25–33 µg/dl at 0, 50 and 150 ppm, respectively, across groups (p<0.0001, all comparisons). A main effect of treatment group (F(8,84)=4.62, p=0.0001) reflected the slightly higher levels of dams exposed to Pb for 3 mos (i.e., prior to breeding) than in all other groups (post hoc p values =0.0005–0.029). Values from offspring obtained at weaning following maternal Pb exposure reported in our previous studies [45; 48] are also depicted in Figure 1 for comparison, as these revealed higher PbBs in 50 ppm offspring than in dams at weaning at the 50 ppm exposure (12 vs. 19 µg/dl), but no differences in 150 ppm offspring (25–32 vs. 31 µg/dl).
Figure 1.
Mean ± S.E. blood Pb levels (µg/dl) of dams (left) measured after 3 mos of Pb exposure (n=9–10) and at weaning (n=5/6 per stress group), and of female offspring (middle) and of male offspring (right) measured at 2 mos of age (n=2/stress condition) and following the termination of behavioral testing (n=5/group for females and n=3/group for males). * values from offspring at weaning (n=3–8/stress group for females and n=2–9/stress group for males) as reported in our previous studies [45; 48].
3.2 Corticosterone Determinations
3.2.1 Basal Corticosterone
Females
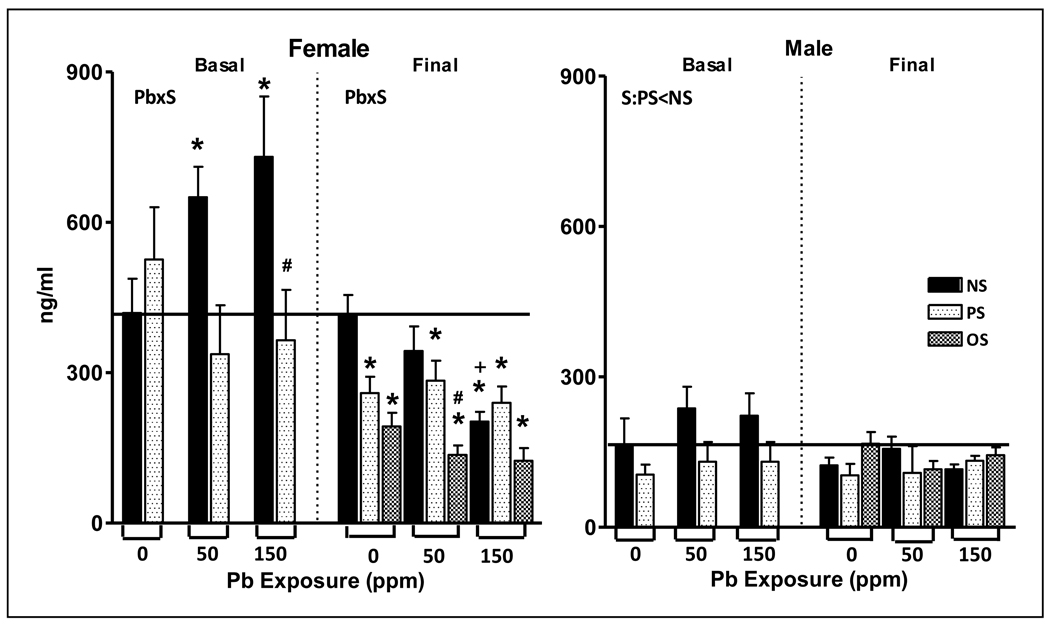
As shown in Figure 2, basal corticosterone levels exhibited Pb x stress interactions (F(2,39)=3.68, p=0.034) with subsequent post-hoc tests confirming Pb exposure-dependent increases of 55% (50-NS) and 74% (150-NS) of 0-NS control. PS (150-PS) reduced the increases in corticosterone produced by 150 ppm alone (150-NS) by approximately 50% (p=0.01).
Figure 2.
Mean ± S.E. corticosterone levels (ng/ml) of female (left) and male (right) offspring exposed to Pb (0, 50 or 150 ppm), and/or stress (none (NS), prenatal stress (PS) or prenatal followed by offspring stress (OS)) measured at approximately 2 mos of age (basal) and at the termination of behavioral testing (final; approximately 10 mos of age) in littermates of those offspring used in the 2 mos determinations. Solid horizontal line defines 0-NS control values. * signifies different from corresponding 0-NS control; # differs from corresponding Pb-NS value; + differs from 50-NS. Sample sizes: female basal corticosterone, n=5–10; male basal corticosterone, n=8–10; female final corticosterone, n=9–11; male final corticosterone, n=7–10.
Males
As in our prior studies, males maintained lower corticosterone levels than females [45]. Although males exhibited similar trends in Pb and stress effects as seen in females, only the effects of stress were significant (F(1,48)=5.97, p=0.0182), with PS values lower than NS values.
3.2.2 Final Corticosterone
Females
Final corticosterone levels exhibited Pb by stress interactions (F(4,79)=2.82, p=0.03) shown by post-hoc tests to include Pb-related reductions in corticosterone that were marginal at 50-NS (18%) but significant in the 150-NS group (52%, p<0.0001) relative to 0-NS control. PS also significantly reduced final corticosterone levels across Pb exposure concentrations (p=0.0024, 0.0178 and 0.0004 for the 0-PS, 50-PS and 150-PS groups, respectively), but even further reductions were produced by Pb + OS, with differences between 0-OS and 0-NS (p=<0.0001), and in 50-OS vs. 0-NS, 0-PS, 50-NS, and 50-PS groups (p= <0.0001, 0.009, <0.0001, and 0.001, respectively). While a similar trend was evident in the 150-OS group, overall levels of corticosterone at 150 ppm may have been too low to statistically detect further reductions.
Males
Final corticosterone values of males were quite low and did not demonstrate any influences of Pb or stress.
3.3 Hippocampal Glucocorticoid Receptors (GR)
3.3.1 Females
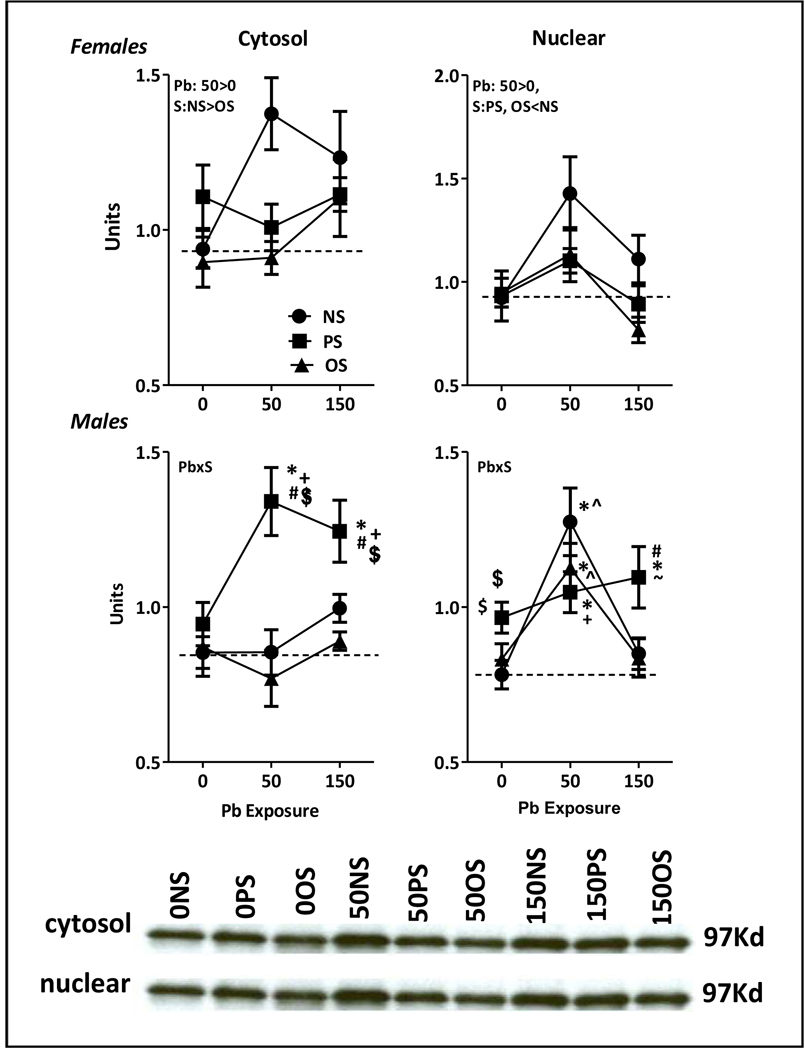
Relative to 0-NS controls, Pb increased cytosolic GR levels (Fig. 3, top left; F(2,42)=3.42, p=0.043) specifically at 150 ppm (p=0.014), while levels were reduced by stress (F(2,40)=3.8, p=0.031), specifically OS (p=0.0095). Pb also influenced nuclear GR receptor levels (F(2,44)=7.81, p=0.0013), with increases at 50 ppm (p=0.0025) but not at 150 ppm. Additionally, both PS and OS reduced nuclear receptor levels (stress: F(2,42)=3.306, p=0.046; NS vs. PS, p=0.045, NS vs. OS, p=0.032).
Figure 3.
Panel A. Group mean ± S.E. cytosolic (left column) and nuclear (right column) hippocampal glucocorticoid receptor density (units) in male (bottom row) and female (top row) behaviorally-tested offspring exposed to Pb, PS or OS in relation to Pb exposure level (ppm) as indicated. Dashed horizontal line depicts 0-NS control values. Pb=main effect of Pb, S=main effect of stress; PbxS= Pb by stress interaction in RMANOVA. Sample sizes: females: n=5–6; male: n=4–5. * differs from corresponding 0-NS control; # differs from 0-PS; +differs from corresponding Pb-NS group; $ differs from corresponding Pb-OS group; ^differs from 150-NS; ~ differs from 150-OS group. Panel B. Representative Western blots for female offspring for cytosolic (top row) and nuclear hippocampal (bottom row) glucocorticoid receptors at the designated 97Kd band.
3.3.2 Males
Pb x stress interactions were found for both cytosolic (F(4,32)=2.76, p=0.045) and nuclear hippocampal GR receptor levels (F(4,32)=3.46, p=0.018). Relative to 0-NS control, cytosolic receptor levels were increased in the 50-PS (p=0.0001) and 150-PS groups (p=0.0015) by levels of 58% and 46%, respectively. Nuclear receptor levels were also increased 34–63% by 50 ppm Pb across stress conditions (0-NS vs. 50-NS, 50-PS and 50-OS, p<0.0001, p=0.015, and 0.002, respectively). Increases were also still evident in the 150-PS group relative to 0-NS control (p=0.0008).
3.4 Fixed Interval Performance
3.4.1 Females
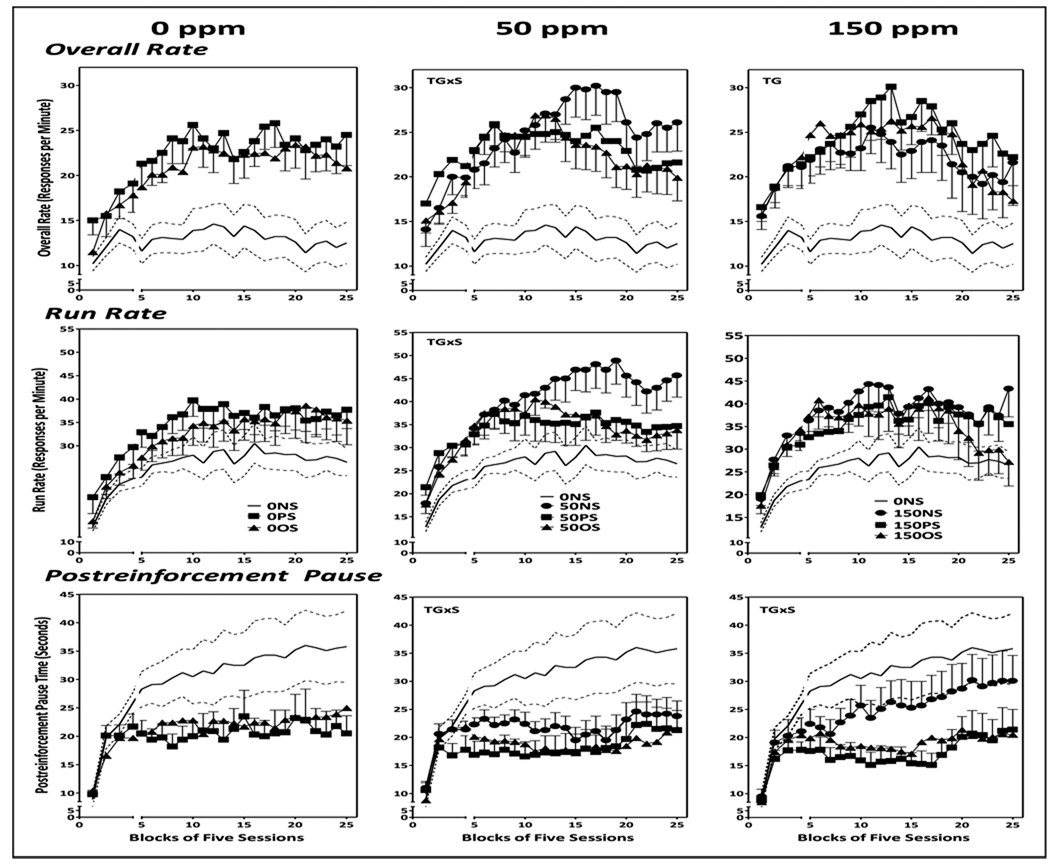
FI overall response rates of control (0-NS) females increased over the first 10–15 session blocks, after which a modest gradual decline was evident (Fig. 4; solid and dashed lines = mean ± SE for 0-NS values and is shown across all plots). Run rates increased until approximately session block 15 and remained stable thereafter. PRP times rose markedly over the first 4–5 session blocks, after which a continual gradual increase occurred across the course of behavioral test sessions.
Figure 4.
Group mean ± S.E. overall response rates (top row), run rates (middle row) and postreinforcement pause time (bottom row) of female offspring exposed to 0 ppm Pb (left column), 50 ppm Pb (middle column) or 150 ppm (right column) alone or in conjunction with PS or OS across 25 blocks of 5 sessions each. Solid line flanked by dashed lines represents 0NS group mean ± S.E. values and are depicted across all Pb exposure groups. The break in the X axis shows sessions prior to and following the imposition of stress challenges in offspring (see Methods). Sample sizes, n=9–11 per group.
0-NS vs. 0-PS and 0-OS (left column)
Despite the very suggestive increases in overall responses rates of the 0-PS and 0-OS groups as compared to 0-NS, (top left), only a marginal effect of treatment group on FI overall rates was found (F(2,48)=2.79, p=0.078), and there were no significant changes in run rate (middle row; treatment group: F(2,48)=.41, p=0.67; treatment group by session: F(48, 864)=0.99, p=0.49) or PRP times (bottom row; treatment group: F(2,48)=1.71, p=0.20; treatment group by session: F(48,864)=1.13, p=0.26).
0-NS vs. 50-NS, 50-PS and 50-OS (middle column)
A treatment group x session interaction for overall rate (F=(72,888)=1.7, p=0.004) reflected increased rates of the 50-NS, 50-PS and 50-OS groups relative to 0-NS control from session blocks 4–8 (all p values <0.05), but consistent significant increases only in the 50-NS group subsequent to session block 8 (all p values <0.05), even while rates of the 50-NS, 50-PS and 50-OS groups did not differ significantly from each other or from corresponding 0-PS or 0-OS values. Analysis of run rate demonstrated a significant main effect of treatment group (F(3,24)=2.94, p=0.046) based on increased rates in the 50-NS group (p=0.0056), as well as a significant treatment group x session interaction (F(72,888)=1.383, p=0.022), with a post-hoc profile across all blocks dominated by increased rates of the 50-NS group. PRP values were selectively decreased in the 50-PS and 50-OS groups (treatment group x sessions: F(72, 888)=1.93, p<0.0001) relative to 0-NS control that extended from session blocks 5–25 (all p values <0.05), even while values of the 50-PS and 50-OS groups did not differ significantly from each other, from 50-NS, or from corresponding 0-PS or 0-OS values.
0-NS vs. 150-NS, 150-PS and 150-OS (right column)
Analysis of overall rate showed a near significant effect of treatment group (top right; F(3,840)=2.76, p=0.056), but no interaction with sessions, despite clear trends towards initial increases approximating 100% followed by declines post session block 15. Given the near significance of the treatment group effect, post-hoc assessments were carried out that revealed increased rates in the 150-PS and 150-OS groups (p=0.009 and 0.044, respectively), but not the 150-NS group, even while none of these groups differed significantly from each other or from the corresponding 0-PS or 0-OS groups. No significant changes in run rates were observed (middle right). Increases in 150-PS and 150-OS group overall rates were largely paralleled by reductions in PRP times of approximately 50% (bottom right; treatment group x session: F(72,864)=1.55, p=0.003), but these were statistically significant only in the 150-PS group from session block 4–25 (all p values <0.05); 150-PS PRP times were significantly lower than 150-NS values from sessions blocks 9–17, but not from corresponding 0-PS or 150-OS values.
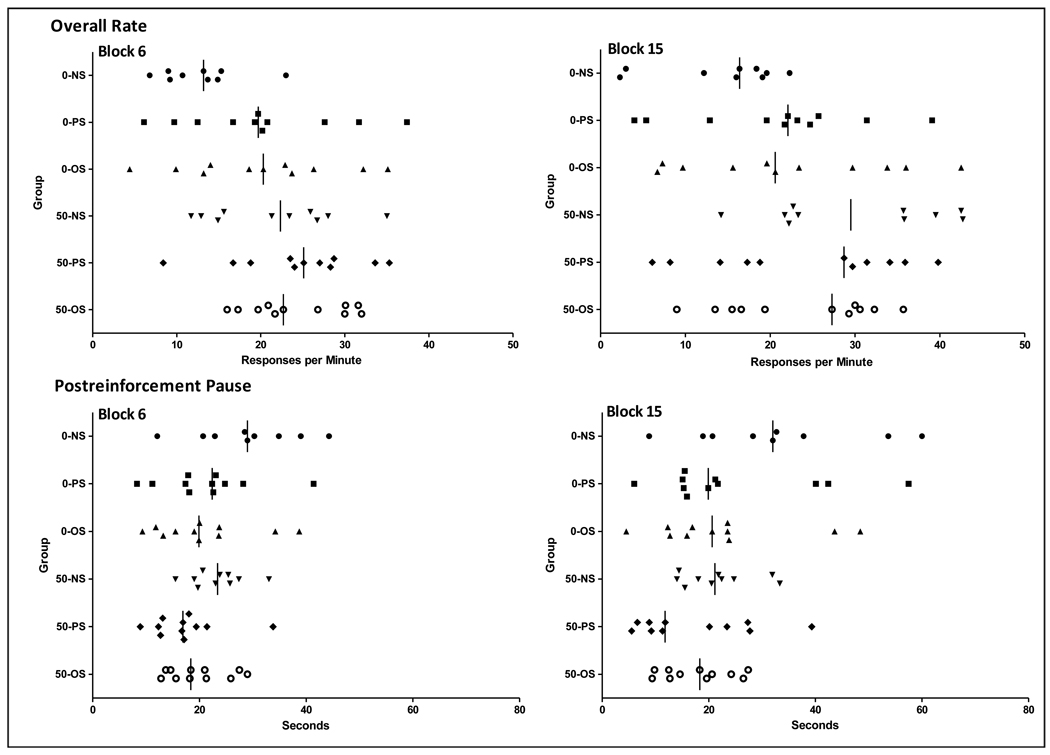
Figure 5 demonstrates that this pattern of Pb and stress additivity and statistical outcomes in females reflects shifts in the total number of subjects to the extreme ends of the normal (0-NS) distribution of FI performance measure values, as depicted for 0 and 50 ppm groups for session block 6 (sessions 25–30) and 15 (sessions 75–80). Consistent with our previous observations [89–91], overall rates (left) of most 0-NS subjects were below 20, while typically one or 2 of 10–12 subjects exhibits rates between 20–50 responses per minute at session block 6. The shift of additional subjects to the higher end of this normal response rate distribution begins with the 0-PS and 0-OS groups. This shift is further accompanied in the 50-NS group by an upward movement of the lower end of the distribution. With even more subjects shifted, 91% of 50-PS subjects and 100% of 50-OS subject values lie above the lower 40% of 50-NS values, and 82% and 91%, respectively, above the typical 20 ppm or less cluster of 0-NS controls. By session block 15, control subject values have tended to compress at approximately 20 ppm or below, while 0-PS and 0-OS subjects remain relatively stable. 50-NS subjects, however, continue to move to the high end of the distribution, while values of approximately half of the 50-PS and 50-OS subjects have now fallen to levels within the control range, accounting for the mitigated effects on rate seen in group analyses. The gradual shift upward of 0-NS PRP values between session block 6 and 15 is evident in 0-NS controls, and in a few of the 0-PS and 0-OS subjects. While no 50-NS subject had PRP values at the higher end of the distribution, individual 50-PS and 50-OS subjects are even more highly clustered at the low end of PRP values, both at session block 6, and slightly less so at session block 15. Indicative of Pb + stress additivity in terms of extreme subject values, 55% of 50-PS subjects and 45% of 50-OS subject values fall below 89% of the 0-NS, 91% of the 0-PS, 73% of the 0-OS and 100% of the 50-NS distributions at session block 15.
Figure 5.
FI overall response rates (top row; responses per minute) and PRP times (bottom row; seconds) of individual 0 and 50 ppm Pb ± stress group subjects at session block 6 (left) and session block 15 (right).
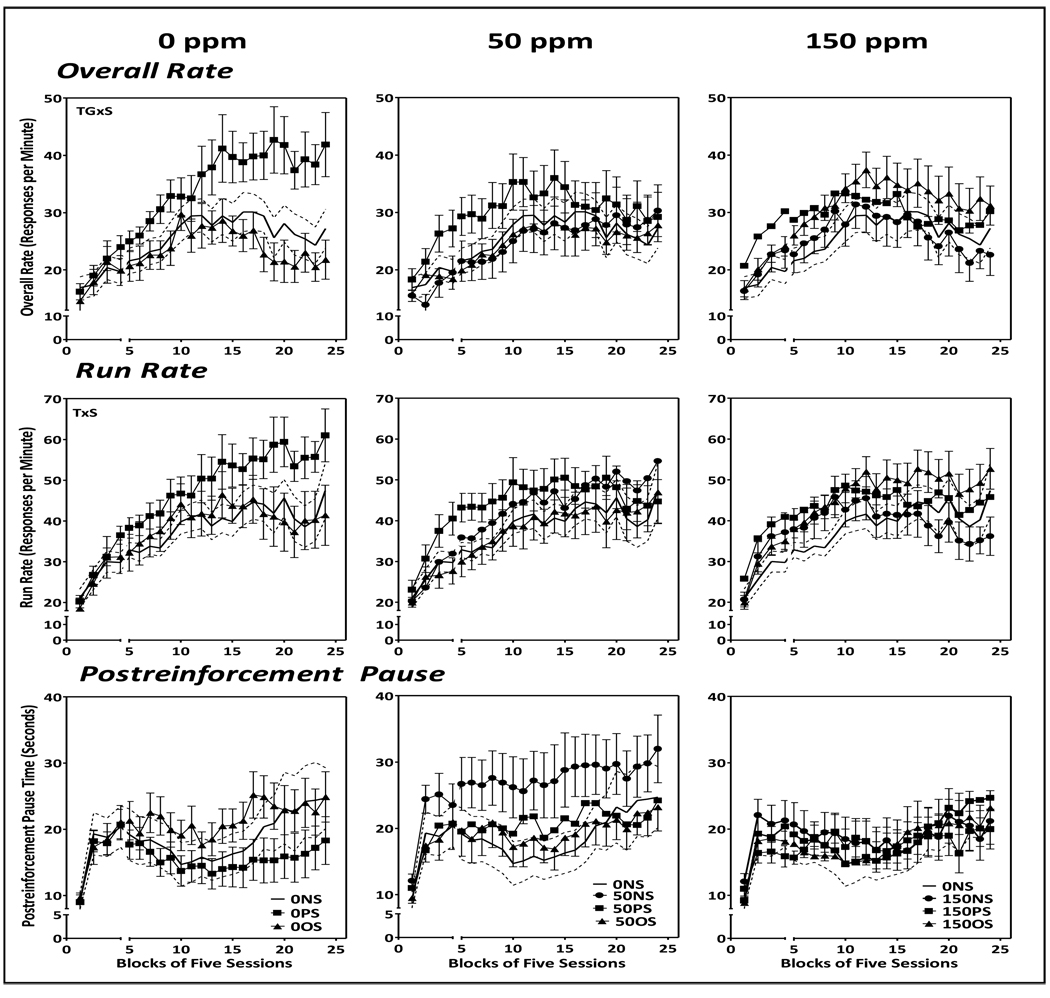
3.4.2 Males
Control (0-NS) males sustained overall response rate levels on the FI schedule (Fig. 6) that were approximately 2-fold higher than those of females. Overall rates increased over the first 10 session blocks and were followed thereafter by modest gradual reductions. Run rates increased out to approximately session block 15 before stabilizing. PRP times increased dramatically over the first 5 blocks of sessions, declined out to session blocks 10–12, and subsequently increased gradually over the duration of testing.
Figure 6.
Group mean ± S.E. overall response rates (top row), run rates (middle row) and postreinforcement pause time (bottom row) of male offspring exposed to 0 ppm Pb (left column), 50 ppm Pb (middle column) or 150 ppm (right column) alone or in conjunction with PS or OS across 25 blocks of 5 sessions each. Solid line flanked by dashed lines represents 0-NS group mean ± S.E. values and are depicted across all Pb exposure groups. The break in the X axis shows sessions prior to and following the imposition of stress challenges in offspring (see Methods). Sample sizes, n=9–11 per group.
0-NS vs. 0-PS and 0-OS (left column)
A treatment group x session interaction (F(46,621)=2.74, p<0.0001) for overall rate reflected the marked increases of the 0-PS group to levels almost 2-fold higher than those of the 0-NS (p=0.008) and 0-OS groups (p=0.028). These emerged at session block 13 and were sustained for the duration of testing (all p values <0.05) with differences from 0-NS and 0-OS values consistently from blocks 13–24 (all p values <0.05). While these effects were generally mirrored by run rate increases in the 0-PS group (treatment group x sessions: F(46,621)=1,48, p=0.025), the increases were less pronounced and not consistently significantly different from the 0-NS or 0-OS groups when analyzed by session block. While PRP times of the 0-PS group hovered at the short end of the distribution, they were not confirmed statistically.
0-NS vs. 50-NS, 50-PS and 50-OS and 0-NS vs. 150-NS, 150-PS and 150-OS (middle and right columns, respectively)
No changes in FI performance were observed in either the 50 (middle column) or 150 ppm groups (right column) in any measures of performance, indicating that the PS effects observed in control males (i.e., 0-PS group) were modified by Pb exposure (50-PS and 150-PS groups).
3.5 Brain Monoamine Levels
Neurotransmitter levels measured at the completion of behavioral testing were calculated as a percent of the 2 mos corresponding gender-specific group mean 0-NS value to incorporate longitudinal changes and their modification by Pb ± stress. In these analyses, no evidence was found for Pb ± stress related neurotransmitter changes in any region that paralleled the observed profile of FI performance changes for either males or females.
3.5.1 Frontal Cortex
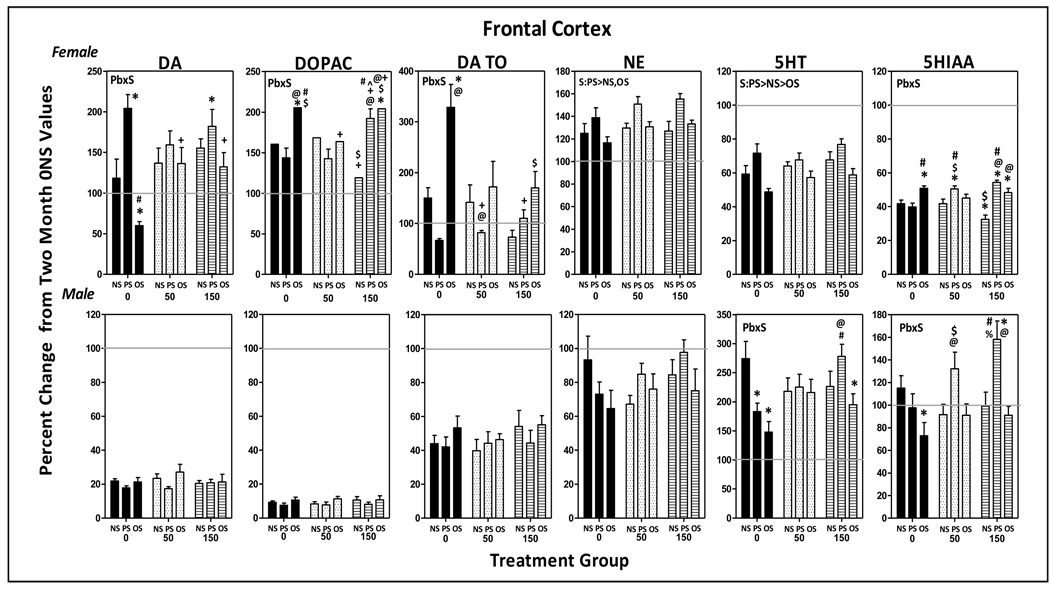
Females
As shown in Figure 7, levels of DA, DOPAC and DA TO had increased 25–75% in controls (0-NS) between 2 mos and the termination of behavioral testing. Although significant Pb x stress interactions were identified (DA: F(4,85)=3.51, p=0.0106; DOPAC: F(4,81)=2.91, p=0.026; DA TO: F(4,83)=4.107, p=0.004), the outcomes did not reflect simple Pb + stress-additive effects. Specifically, PS alone further increased DA levels, but only in the 0-PS and 150-PS groups (p=0.0007 and 0.0128, respectively). Only in the 0-OS group were DA levels further reduced (p=0.0187), while corresponding levels of DOPAC and DA TO were markedly increased (p=0.034 and <0.0001, respectively), presumably reflecting increased metabolism. DOPAC levels were also increased by 150-OS (p=0.039).
Figure 7.
Group mean ± S.E. values of frontal cortex levels of DA, DOPAC, DA TO, NE, 5HT and HIAA of female (top row) and male (bottom row) offspring in relation to Pb exposure and stress condition. Values are plotted as percent changes from corresponding group mean 0-NS values from littermates obtained at 2 mos of age. Solid gray horizontal lines depict the 100% of 2 mos values (no change from 2 mos of age). Sample sizes: females=6–11; males=8–11. Pb=main effect of Pb, S=main effect of stress; Pb x S = Pb by stress interaction in RMANOVAs. * differs from 0-NS control; # differs from 0-PS; + differs from 0-OS; $ differs from 50-NS; @ differs from corresponding Pb-NS group; ^ differs from corresponding 50 ppm group; & differs from 50-OS; % differs from 150-OS.
NE levels in 0-NS females increased approximately 25% between 2 mos of age and the termination of behavioral testing. NE levels further increased in response to PS across all Pb groups (stress: F(2,85)=11.2, p<0.0001, PS vs. NS, p=0.0002; PS vs. OS, p=0.0002). Levels of 5HT and 5HIAA had declined 40–60% in 0-NS controls by the end of behavioral testing. Reductions in 5-HT were attenuated by PS (F(2,85)=14.13. p<0.0001; PS vs. NS, p=0.015; PS vs. OS, p<0.0001) but further reduced by OS (OS vs. NS, p=0.012). The Pb x stress interaction (F(4,84)=9.41, p<0.0001) observed for 5HIAA confirmed the blunted reductions in 50-PS and 150-PS groups relative to 0-NS control (p=0.007 and 0.0002, respectively); such reductions were not observed in the 0-PS, 50-NS or 150-NS groups, indicating enhanced effects of 50-PS and 150-PS. Blunted reductions in 5HIAA were also observed in the 0-OS and 150-OS groups (p=0.0048 and 0.0387, respectively).
Males
Changes in neurotransmitter levels in 0-NS males between 2 mos of age and termination of behavioral testing were almost directly opposite to those seen in 0-NS females. DA and DOPAC levels of 0-NS males declined by 80–90%, DA TO by 50%, and NE by 10–20%. None of these trajectories were altered by Pb, stress or the combination. In contrast, 5HT levels increased in 0-NS males by over 250%, effects also showing Pb by stress interactions (F(4,78)=3.59, p=0.0098). Specifically, the increases seen in 0-NS males were blunted in the 0-PS, 0-OS and 150-OS groups (p=0.006, 0.0003 and 0.014, respectively). 5HIAA levels showed little change over time in 0-NS controls, but were notably elevated in the 150-PS group (Pb x stress interaction: F(4,77)=2.9, p=0.027, post hoc p=0.0109), but not the 0-PS group or 150-NS group, consistent with enhanced effects, with a similar trend in the 50-PS group. HIAA levels were reduced in the 0-OS group (p=0.021).
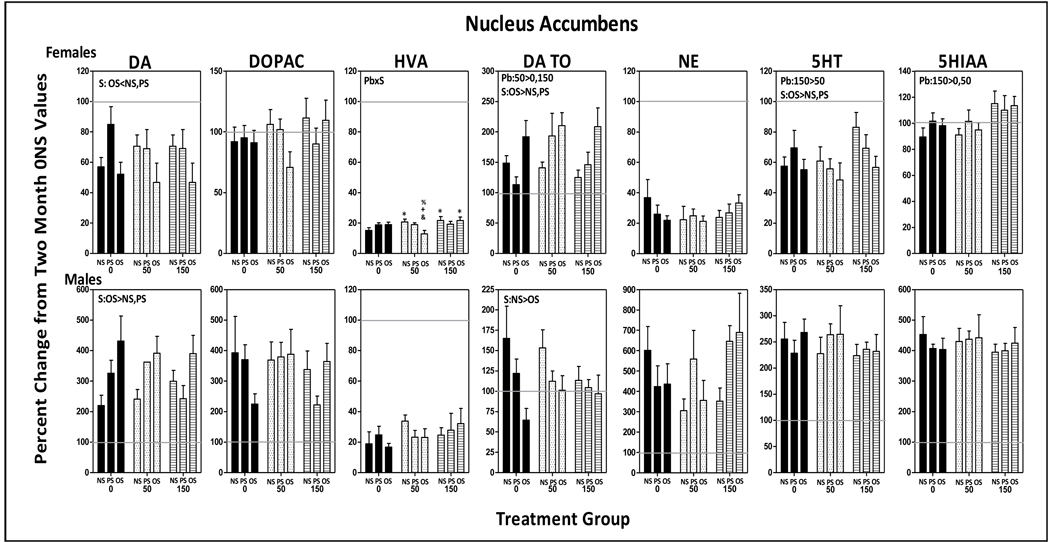
3.5.2.2 Nucleus Accumbens
Females
DA levels of 0-NS controls declined approximately 40% and HVA levels over 80% relative to 2 mos values, while DOPAC levels were unchanged, and DA TO values increased by 50% (Fig. 8). OS further reduced DA levels (stress: F(2,77)=7.49, p=0.0011; OS vs. NS, p=0.035; OS vs. PS, p=0.027). Pb x stress interactions for HVA (F(4,84)=3.00, p=0.023) were attributable to mitigated reductions in the 50-NS and 150-NS groups (p=0.048 and 0.0183, respectively), but with even further reductions in the 50-OS group (p=0.017). 5-HT levels of 0-NS controls declined approximately 40% from 2 mos values; OS further decreased 5-HT levels (stress: F(2,85)=3.82, p=0.0260; OS v s. NS, p=0.015; PS vs. OS, p=0.035). While 5HIAA values of 0-NS controls evidenced little change from 2 mos values, levels were increased by 150 ppm Pb across stress conditions (F2,83)=4.73, p=0.011; 0 vs. 150, p=0.006, 50 vs. 150, p=0.014).
Figure 8.
Group mean ± S.E. values of nucleus accumbens levels of DA, DOPAC, HVA, DA TO, NE, 5HT and HIAA of female (top row) and male (bottom row) offspring in relation to Pb exposure and stress condition. Values are plotted as percent changes from corresponding group mean 0-NS values from littermates obtained at 2 mos of age. Solid gray horizontal lines depict the 100% of 2 mos values (no change from 2 mos of age). Sample sizes: females=6–11; males=8–11. Pb=main effect of Pb, S=main effect of stress; Pb x S = Pb by stress interaction in RMANOVAs. * differs from 0-NS control; + differs from 0-OS; @ differs from corresponding Pb-NS group; & differs from 50-OS; % differs from 150-OS.
Males
Only the time-related changes in HVA (reductions of 80%) and DA TO (increase >150%) of 0-NS males corresponded to changes in 0-NS females. In contrast, levels of DA (>200%), NE (600%) and 5HT (250%) changed in directions opposite to those of 0-NS females. While DOPAC and 5HIAA levels showed little change over time in 0-NS females, they exhibited marked increases in 0-NS males. Pb, stress or the combination modified only DA and DA TO changes in males, with the increases in DA further elevated by OS across Pb exposures (F(2,81)=7.03, p=0.0015; OS vs. NS, p=0.0004; OS vs. PS, p=0.02), while OS significantly blunted the increases in DA TO (stress: F(2,81)=5.31, p=0.007; OS vs. NS, p=0.002).
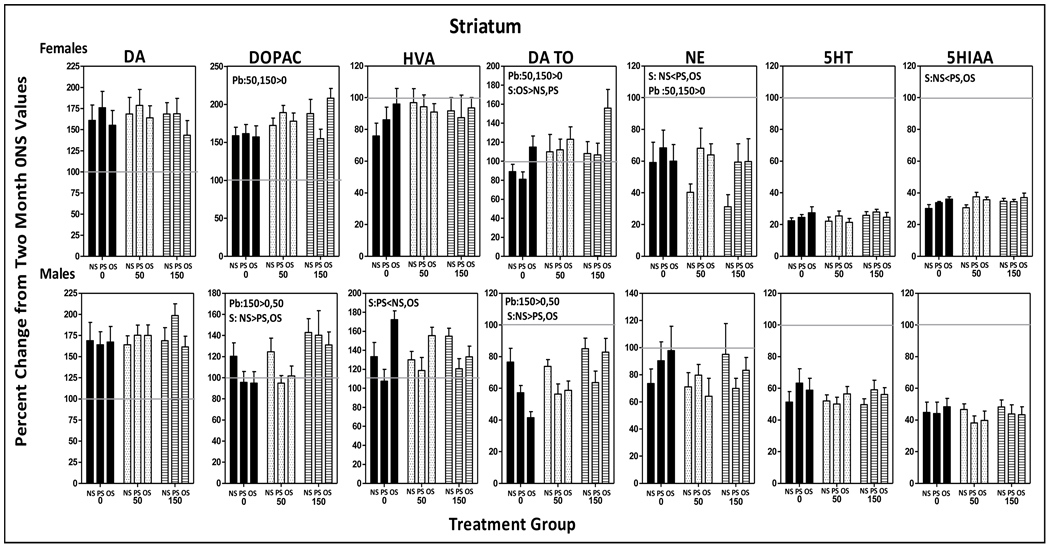
3.5.2.3 Striatum
Females
Increases in DA and DOPAC of greater than 150%, slight reductions in HVA, no changes in DA TO, decreases of approximately 40% in NE, and marked reductions in 5HT (80%) and 5HIAA (70%) levels were seen in 0-NS females compared to corresponding 2 mos values (Fig. 9). Pb further increased DOPAC (F(2,85)=3.23, p=0.045) at both 50 and 150 ppm (0 vs. 50, p=0.04; 0 vs. 150, p=0.017). OS further increased DA TO across Pb exposures (stress: F(2,81)=3.3, p=0.042; OS vs. NS, p=0.036, OS vs. PS, p=0.027), while both PS and OS blunted the reductions in NE (stress: F(2,73)=3.9, p=0.025; PS vs. NS, p=0.009, OS vs. NS, p=0.032).
Figure 9.
Group mean ± S.E. values of striatal levels of DA, DOPAC, HVA DA TO, NE, 5HT and HIAA of female (top row) and male (bottom row) offspring in relation to Pb exposure and stress condition. Values are plotted as percent changes from corresponding group mean 0-NS values from littermates obtained at 2 mos of age. Solid gray horizontal lines depict the 100% of 2 mos values (no change from 2 mos of age). Sample sizes: females=7–11; males=5–11. Pb=main effect of Pb, S=main effect of stress; Pb x S = Pb by stress interaction in RMANOVAs.
Males
Longitudinal changes over time in striatal neurotransmitters of 0-NS males were highly similar to those seen in females. 150 ppm Pb further increased DOPAC (F(2,81)=5.98, p=0.0038; 150 v s. 0, p=0.006; 150 vs. 50, p=0.009) and DA TO levels (F(2,63)=6.25, p=0.0034; 150 vs. 0, p=0.004, 150 vs. 50, p=0.01). PS reduced HVA levels (F(2,78)=8.45, p=0.0005; PS vs. NS, p=0.015; PS vs. OS, p=0.0002), and PS and OS reduced DA TO levels (F(2,79)=7.74, p=0.0008, PS vs. NS, p=0.0006; OS vs. NS, p=0.007).
3.6 Relationship of Biochemical Changes to FI Performance
Linear regression analyses relating final corticosterone levels to measures of FI performance (not shown) revealed no correlations when examined across all Pb ± stress conditions for any of the measures. When data were split by Pb exposure level or by stress or at the treatment group level, occasionally significant correlations were seen, but these were not consistent or systematic.
Outcomes from the PCA analyses (Fig. 10) that incorporated all neurotransmitters and block 10 mean FI overall response rates of females were used to explore potential neurochemical mechanisms of the mitigation of FI overall response rate increases in the 50-PS and 50-OS, but not the 50-NS group, post session block 8, as well as the increased rates of the 150-PS and 150-OS, but not the 150-NS group. In both cases, consistency with these changes would be best indicated by a pattern of relative strength changes in the Pb-NS group that differed from 0-NS, coupled with changes in the Pb-PS and Pb-OS groups that differed from both 0-NS and Pb-NS.
Comparisons of 0-NS and 50 ppm group plots (panel A) revealed decrements relative to 0-NS in the relative strength of frontal cortical DA and DOPAC at 50-NS that were not evident for 50-PS or 50-OS groups. Additionally, marked increases in the relative strength of frontal cortex NE were found for 50-NS relative to 0-NS, with similar but less pronounced increases in the 50-PS and 50-OS groups. Compared to either 0-NS or 50-NS, nucleus accumbens DA function was generally reduced, while striatal NE strength increased markedly in the 50-PS and 50-OS groups. Consistent with the post session block 8 mitigation of FI overall response rate increases in the 50-PS and 50-OS groups while 50-NS group rates remained elevated, the relative strengths values of overall rate in the models was considerably lower for the 50-PS and 50-OS groups.
For the 150 ppm-associated relative strength plots (panel B), frontal cortex NE levels were notably increased in the 150-NS group, but less so in the 150-PS and 150-OS groups relative to 0-NS control. Moreover, while nucleus accumbens NE levels increased in the 150-NS group, they were even further reduced relative to 0-NS control in the 150-PS and 150-OS groups; nucleus accumbens dopaminergic function was generally reduced in the 150-NS but not the 150-PS and 150-OS groups. In contrast, striatal NE levels were somewhat reduced in the 150-NS group, but markedly increased in the 150-PS and 150-OS groups, while striatal DOPAC levels in these two groups were reduced. Nucleus accumbens HIAA levels were reduced in the 150-NS group, but notably even further reduced in the 150-PS and 150-OS groups.
4. DISCUSSION
In addition to multiple effects of Pb, PS and OS alone, the current study also confirms that, when combined, lifetime Pb exposure and stress can produce enhanced effects in rats. In females, increased FI overall response rates as compared to 0-NS control were observed in the 150-PS and 150-OS but not the 150-NS, 0-PS or 0-OS groups. PRP times were reduced only with combined Pb and stress, i.e., in the 50-PS, 50-OS, and 150-PS, but not in the 0-PS, 0-OS, 50-NS or 150-NS groups (Fig. 4, Table 1). With respect to neurochemical changes, levels of frontal cortex 5-HIAA were elevated by PS in both genders at 50 and 150 ppm, but not at 0 ppm nor by Pb alone (Fig. 7), as were hippocampal cytosolic receptor levels in males (Fig. 3).These enhancements appeared to arise primarily from PS, as either they were seen only in the Pb-PS group, or were of comparable magnitude in the Pb-PS and Pb-OS groups. However, instances in which OS further modified the effects of combined Pb and PS, or influenced Pb effects directly were also observed, e.g., reductions in nucleus accumbens HVA levels occurred in 50-OS females but not in 50-NS or 0-OS females. Further, in males, frontal cortex 5-HIAA elevations were seen in 50-PS and 150-PS, but not 50-OS or 150-OS groups (Fig. 4). Thus, while the PS influence may predominate, OS can also directly influence outcomes. It remains to be determined whether OS effects reflect the cumulative impact of PS followed by OS, or direct OS effects, and to what extent the patterns of Pb and stress interactions are reflective of the specific behavioral experience.
Table 11.
Summary of Maternal vs. Lifetime Pb and Stress Effects on FI Performance Measures
| Maternal Pb2 | Lifetime Pb3 | |||
|---|---|---|---|---|
| Pb (ppm) | Overall Rate | PRP | Overall Rate | PRP |
| 0 ppm | ||||
| 0-PS | NE4 | NE | NE | NE |
| 0-OS | NE | *↓−23% | NE | NE |
| 50 ppm | ||||
| 50-NS | NE | NE | *↑95% | NE |
| 50-PS | NE | NE | *↑79.2% | *↓−42% |
| 50-OS | *↑64.9% | NE | *↑74.7% | *↓−39.3% |
| 150 ppm | ||||
| 150-NS | *↑42.4% | *↓−30.3% | NE | NE |
| 150-PS | NE | *↓−25.7% | *↑90.7% | *↓−44.7% |
| 150-OS | *↑59.2% | NE | *↑78.5% | NE |
Based on calculation of group mean values across session block post-stress challenge for both maternal and lifetime Pb exposure studies. All calculations represent percent of 0-NS control values; ↑represents increase; ↓ represents decrease
Data from [46].
Data from current study
NE = no significant effect
Individual subject data for FI performance (Fig. 6) shows that Pb + stress enhancements arise primarily from a Pb and stress additive movement of increasing numbers of subjects’ FI performance values to the extreme ends of the ‘normal distribution’ of performance values, likely accounting for the fact that statistically significant effects of Pb and stress are seen relative to 0-NS control, but generally not to 0-PS, 0-OS or Pb only groups. As such, enhanced Pb and stress effect represent a more subtle interaction consistent with a dose-response (increasing number of affected individuals), rather than a dose-effect phenomenon. That this pattern of individual responses does not reflect a ceiling effect for response rate is clear from the fact that female rats working on fixed ratio schedules in our laboratories achieve response rates ranging from 80 to 100 responses per minute (our unpublished data). Changes in prototypical FI performance, regardless of whether they arise from alterations in response rate, PRP times, or both, represent highly inefficient performance. Rate decreases may lengthen the time to reinforcement or decrease reinforcement frequency, while inappropriately timed rate increases decrease reinforcement density and can represent sub-optimal or even dysfunctional resource/energy use, as outlined in foraging theory [92].
4.1 Lifetime Pb ± Stress-Induced Reductions in PRP in Female Offspring as a Potential Timing Disruption
Combined lifetime Pb and PS, but not lifetime Pb alone, reduced PRP times (current study), while no such interactions occurred with maternal Pb exposure [48] and stress (Table 1). This specific effect of lifetime Pb and PS may reflect the more protracted exposure relative to maternal Pb only, consistent with cumulative toxicity. Reductions in PRP, consistent with initiation of responding earlier in the fixed interval, represent a highly inefficient strategy that can neither accelerate reinforcement delivery nor increase number of reinforcer deliveries.
One interpretation of reductions in PRP values on an FI schedule is a deficit in timing abilities (temporal discrimination), consistent with the perception of time passing more quickly [93]. Interestingly, FI performance of children and infants has been demonstrated to be a perfect surrogate for impulsivity (measured using self-control paradigms) [94; 95], a diagnostic component of attention deficit disorder that has also been hypothesized to include both a timing disturbance [96] and aversion to delays [97]. In that context, both Pb and stress have been posited as risk factors for attention deficit disorder [54; 98]. Reductions in PRP could suggest a higher risk for temporal discrimination dysfunction, particularly for girls that have been subjected to elevated Pb exposure and prenatal stress. Future research examining the impact of Pb and stress specifically on timing behavior and on self-control will assist in interpreting these interactions.
4.2 Potential Neurochemical Mediators of Pb + Stress-Associated FI Performance Changes
As noted above, combined lifetime Pb and PS, but not Pb alone, reduced PRP values. In the case of overall response rates, stress combined with either maternal or lifetime Pb increased overall response rates when Pb alone was without effect (i.e., 50-OS with maternal Pb, and 150-PS and 150-OS with lifetime Pb; Table 1), but mitigated increases in FI rates produced by Pb alone (e.g., 150-PS (although not OS) maternal Pb exposure, and 50-PS and 50-OS lifetime Pb).
As shown in both human and animal studies [99], cortico-striatal/dopamine circuitry is critical to interval timing/temporal discrimination, to which PRP has been related. Current understanding of neurochemical mediation of FI response rates is limited, but our own studies show roles for frontal cortex, nucleus accumbens and striatum in the mediation of FI response rates [63; 79–81]. Thus, it is not surprising that no single neurotransmitter change in any of the regions examined in the current study paralleled Pb ± stress-induced FI performance changes in either gender, particularly given that they were based upon a single measurement in time. In addition, the differential patterns of changes between 50 ppm and 150 ppm Pb exposure conditions in behavior, neurotransmitters and glucocorticoids suggest that different mechanisms may be operative at the different Pb exposure concentrations.
For such reasons, and even though carried out somewhat differently following maternal [48] vs. lifetime Pb exposure, PCA were used to explore potential clustering of neurotransmitter changes with FI response rates. These analyses suggested disruptions in corticolimbic catecholaminergic balance, especially NE, as potential mediators of both maternal [48] and lifetime Pb ± stress-associated FI response rate changes. Interestingly, frontal cortex NE assumed a higher relative strength in virtually all groups exposed to lifetime Pb ± stress compared to 0-NS (Fig. 10 and data not shown). Differences in frontal cortex NE relative strengths also fit a pattern consistent with expected group differences (changes in the Pb-NS group that differed from 0-NS, coupled with changes in the Pb-PS and Pb-OS groups that differed from both 0-NS and Pb-NS) as well as with expected 50 vs. 150 ppm outcome differences, consistent with their potential to be served by different underlying mechanisms. Frontal cortical NE has been suggested to provide an excitatory influence on HPA axis function and to modulate signals broadly across the brain, at least in response to acute stress [100; 101]. Additionally, systemic NE alters temporal information processing in humans [102] and systemic destruction of central noradrenergic neurons using the selective neurotoxin N-(2-chloroethyl0-n-ethyl-2-bromobenzylamine impaired acquisition of a temporal discrimination although not the ability to remember temporal durations in rats [103]. Both Pb exposure and prenatal stress have been associated with changes in NE, but the nature of these changes have not been systematic, an outcome not surprising given that these results are derived from studies that differ significantly in methodology [104–107]. There were other notable changes in PCA profiles as well that included a generalized reduction in nucleus accumbens DA function with increased FI rates (50-PS, 50-OS, 150-NS) and the observation that striatal NE was largely uninfluenced by Pb alone, but notably increased in Pb + stress groups.
Collectively, these observations certainly suggest cortico-striatal NE imbalance as one priority for evaluation as a potential mediator of Pb ± stress-associated FI rate and PRP changes. Strategies to pursue neurochemical mechanisms must consider, however, our recent demonstration that brain monoamine levels change over time per se in the absence of any experimental manipulations, and that such trajectories can be dramatically altered by behavioral experience and further by Pb ± stress [71]. Such findings raise questions as to whether neurochemical profiles collected following extensive behavioral experience have been modified by that behavioral experience, and whether, instead, profiles collected prior to the initiation of behavioral experience better serve as mechanistic guidance. Future studies could derive neurotransmitter profiles from littermates of animals designated for behavioral evaluation immediately prior to implementing testing.
4.3 Complexities of Interpretation of Pb + Stress Interactions and Non-Linearity of Effects
Several outcomes appear to suggest that Pb can reverse effects of stress, or that stress reverses Pb effects. For example, while 0-PS males ultimately exhibited an approximate 2-fold increase in FI response rates (Fig. 6), corresponding effects were not seen in either the 50-PS or 150-PS groups. Similarly, in females, the mitigation of 50 ppm-associated FI rate increases by PS and OS, the lower rates of the 150-NS group relative to 150-PS and 150-OS, and the enhancement of Pb-associated PRP reductions by stress may all provoke such interpretations. However, significant underlying complexities, including potentially different underlying dose-effect functions for each of the independent variables and for each of the Pb exposure concentrations, as well as the magnitude of change possible for each such variable and for outcome measures must all be considered for any interpretation. Instances where one variable (e.g., stress) alters the outcome relative to control, while combined variables (stress and Pb) apparently reverse effects to control, can reflect e.g., the concurrent influences of multiple underlying mechanisms, dose-effect curves with different peak effect or saturation levels, and/or nonlinear dose-effect curves as opposed to a return to normal or control function. We have shown, for example, that nucleus accumbens dopamine levels relate to FI response rates via an inverse U-shaped function [63].
In fact, non-linear dose effect functions are common to both Pb and stress [11; 45; 48; 63; 108–112]. Although the basis for such curves remains unclear, the ability of Pb2+ to substitute for Ca2+, more potently than Ca2+ itself in some cases, may underlie Pb non-linearity. Indeed, with some substitutions of Pb2+ for Ca2+, enhanced effects of Pb2+ are observed at low concentrations and reductions at higher concentrations, actions which are jointly determined by the concentrations of Ca2+ present [113]. The broad array of outcomes which are affected by Pb in U-shaped dose effect curves [114]could reflect therefore both the competing concentrations of Pb2+ and Ca2+, coupled with the extensive physiological and homeostatic functions of Ca2+ that span all organs and systems. Collectively, the non-linear relationships between corticosterone and its outcomes and Pb exposure and its outcomes argue against any simple interpretations that Pb reverses the effects of PS or PS+OS, or that stress reverses effects of Pb. It also underscores the complexities that will be inherent in determining mechanisms of interactions and the need for creative strategies to elaborate such mechanisms.
4.4 Public Health Implications
The current findings have significant implications for public health and risk assessment. Our findings of enhanced effects of combined lifetime Pb and stress, as well as their associated gender differences, clearly underscore the critical need to consider the health consequences of environmental chemical exposures in relevant human contexts, i.e., in association with other pertinent extant risk factors; studies of Pb alone may underestimate its true risks. In addition, the enhancement of Pb effects by prenatal stress indicates the need for screening of pregnant women at high risk for both Pb exposure and stress, since the current postnatal only screening programs will already have missed these windows of vulnerability. Recent reports of the protracted biological consequences of decreased glucocorticoid and increased pro-inflammatory signaling engendered by early life low SES conditions makes this even more important [29].
ACKNOWLEDGEMENTS
Supported in part by grants ES01212 (D. Cory-Slechta) and ES01247 (T. Gasiewciz).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.van Wijngaarden E, Campbell JR, Cory-Slechta DA. Bone lead levels are associated with measures of memory impairment in older adults. Neurotoxicol. 2009;30:572–580. doi: 10.1016/j.neuro.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Y, Schwartz J, Sparrow D, Aro A, Weiss ST, Hu H. Bone lead and blood lead levels in relation to baseline blood pressure and the prospective development of hypertension: the Normative Aging Study. Am. J. Epidemiol. 2001;153:164–171. doi: 10.1093/aje/153.2.164. [DOI] [PubMed] [Google Scholar]
- 3.Gerr F, Letz R, Stokes L, Chettle D, McNeill F, Kaye W. Association between bone lead concentration and blood pressure among young adults. Am. J. Ind. Med. 2002;42:98–106. doi: 10.1002/ajim.10096. [DOI] [PubMed] [Google Scholar]
- 4.Kim R, Hu H, Rotnitzky A, Bellinger D, Needleman H. A longitudinal study of chronic lead exposure and physical growth in Boston children. Environ. Health Perspect. 1995;103:952–957. doi: 10.1289/ehp.95103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opler MG, Brown AS, Graziano JH, Desai M, Zheng W, Schaefer C, Factor-Litvak P, Susser ES. Prenatal lead exposure, delta-aminolevulinic acid, and schizophrenia. Environ. Health Perspect. 2004;112:548–552. doi: 10.1289/ehp.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajan P, Kelsey KT, Schwartz JD, Bellinger DC, Weuve J, Sparrow D, Spiro A, 3rd, Smith TJ, Nie H, Hu H, Wright RO. Lead burden and psychiatric symptoms and the modifying influence of the delta-aminolevulinic acid dehydratase (ALAD) polymorphism: the VA Normative Aging Study. Am. J. Epidemiol. 2007;166:1400–1408. doi: 10.1093/aje/kwm220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhodes D, Spiro A, 3rd, Aro A, Hu H. Relationship of bone and blood lead levels to psychiatric symptoms: the normative aging study. J. Occup. Environ. Med. 2003;45:1144–1151. doi: 10.1097/01.jom.0000094995.23808.7b. [DOI] [PubMed] [Google Scholar]
- 8.Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114:1388–1394. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- 9.Haley VB, Talbot TO. Geographic analysis of blood lead levels in New York State children born 1994–1997. Environ. Health Perspect. 2004;112:1577–1582. doi: 10.1289/ehp.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kemper AR, Cohn LM, Fant KE, Dombkowski KJ, Hudson SR. Follow-up testing among children with elevated screening blood lead levels. JAMA. 2005;293:2232–2237. doi: 10.1001/jama.293.18.2232. [DOI] [PubMed] [Google Scholar]
- 11.Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N. Engl. J. Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ. Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He K, Wang S, Zhang J. Blood lead levels of children and its trend in China. Sci. Total Environ. 2009;407:3986–3993. doi: 10.1016/j.scitotenv.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Cory-Slechta DA. Studying toxicants as single chemicals: does this strategy adequately identify neurotoxic risk? Neurotoxicol. 2005 doi: 10.1016/j.neuro.2004.12.007. in press. [DOI] [PubMed] [Google Scholar]
- 15.Hashmi NS, Kachru DN, Khandelwal S, Tandon SK. Interrelationship between iron deficiency and lead intoxication (Part 2) Biol. Trace Elem. Res. 1989;22:299–307. doi: 10.1007/BF02916618. [DOI] [PubMed] [Google Scholar]
- 16.Hashmi NS, Kachru DN, Tandon SK. Interrelationship between iron deficiency and lead intoxication (Part 1) Biol. Trace Elem. Res. 1989;22:287–297. doi: 10.1007/BF02916617. [DOI] [PubMed] [Google Scholar]
- 17.Mahaffey KR, Goyer R, Haseman JK. Dose-response to lead ingestion in rats fed low dietary calcium. J. Lab. Clin. Med. 1973;82:92–100. [PubMed] [Google Scholar]
- 18.Mahaffey KR. Nutritional factors and susceptibility to lead toxicity. Environ. Health Perspect. 1974;7:107–112. doi: 10.1289/ehp.747107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemp FW, Neti PV, Howell RW, Wenger P, Louria DB, Bogden JD. Elevated blood lead concentrations and vitamin D deficiency in winter and summer in young urban children. Environ. Health Perspect. 2007;115:630–635. doi: 10.1289/ehp.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cory-Slechta DA. Alterations in tissue Pb distribution and hematopoietic indices during advanced age. Arch. Toxicol. 1990;64:31–37. doi: 10.1007/BF01973373. [DOI] [PubMed] [Google Scholar]
- 21.Cory-Slechta DA, Pokora MJ. Pokora, Behavioral manifestations of prolonged lead exposure initiated at different stages of the life cycle: I. Schedule-controlled responding. Neurotoxicol. 1991;12:745–760. [PubMed] [Google Scholar]
- 22.Cory-Slechta DA, Pokora MJ, Widzowski DV. Behavioral manifestations of prolonged lead exposure initiated at different stages of the life cycle: II. Delayed spatial alternation. Neurotoxicol. 1991;12:761–776. [PubMed] [Google Scholar]
- 23.Bruce MA, Beech BM, Sims M, Brown TN, Wyatt SB, Taylor HA, Williams DR, Crook E. Social environmental stressors, psychological factors, and kidney disease. J. Investig. Med. 2009;57:583–589. doi: 10.231/JIM.0b013e31819dbb91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Vriendt T, Moreno LA, De Henauw S. Chronic stress and obesity in adolescents: scientific evidence and methodological issues for epidemiological research. Nutr Metab Cardiovasc Dis. 2009;19:511–519. doi: 10.1016/j.numecd.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figueredo VM. The time has come for physicians to take notice: the impact of psychosocial stressors on the heart. Am. J. Med. 2009;122:704–712. doi: 10.1016/j.amjmed.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Flaherty EG, Thompson R, Litrownik AJ, Zolotor AJ, Dubowitz H, Runyan DK, English DJ, Everson MD. Adverse childhood exposures and reported child health at age 12. Acad Pediatr. 2009;9:150–156. doi: 10.1016/j.acap.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Kitaoka-Higashiguchi K, Morikawa Y, Miura K, Sakurai M, Ishizaki M, Kido T, Naruse Y, Nakagawa H. Burnout and risk factors for arteriosclerotic disease: follow-up study. J Occup Health. 2009;51:123–131. doi: 10.1539/joh.l8104. [DOI] [PubMed] [Google Scholar]
- 29.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vere CC, Streba CT, Streba LM, Ionescu AG, Sima F. Psychosocial stress and liver disease status. World J Gastroenterol. 2009;15:2980–2986. doi: 10.3748/wjg.15.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Von Korff M, Alonso J, Ormel J, Angermeyer M, Bruffaerts R, Fleiz C, de Girolamo G, Kessler RC, Kovess-Masfety V, Posada-Villa J, Scott KM, Uda H. Childhood psychosocial stressors and adult onset arthritis: broad spectrum risk factors and allostatic load. Pain. 2009;143:76–83. doi: 10.1016/j.pain.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev. Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- 33.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 34.Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosom. Med. 2006;68:414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- 35.Lupien SJ, King S, Meaney MJ, McEwen BS. Child's stress hormone levels correlate with mother's socioeconomic status and depressive state. Biol. Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- 36.Pallares ME, Scacchi Bernasconi PA, Feleder C, Cutrera RA. Effects of prenatal stress on motor performance and anxiety behavior in Swiss mice. Physiol. Behav. 2007;92:951–956. doi: 10.1016/j.physbeh.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 37.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 38.Beydoun H, Saftlas AF. Physical and mental health outcomes of prenatal maternal stress in human and animal studies: a review of recent evidence. Paediatr. Perinat. Epidemiol. 2008;22:438–466. doi: 10.1111/j.1365-3016.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 39.McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57 Suppl 2:S11–S15. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rinaudo PF, Lamb J. Fetal origins of perinatal morbidity and/or adult disease. Semin Reprod Med. 2008;26:436–445. doi: 10.1055/s-0028-1087109. [DOI] [PubMed] [Google Scholar]
- 41.Jones RL, Homa DM, Meyer PA, Brody DJ, Caldwell KL, Pirkle JL, Brown MJ. Trends in blood lead levels and blood lead testing among US children aged 1 to 5 years, 1988–2004. Pediatrics. 2009;123:e376–e385. doi: 10.1542/peds.2007-3608. [DOI] [PubMed] [Google Scholar]
- 42.Gaitens JM, Dixon SL, Jacobs DE, Nagaraja J, Strauss W, Wilson JW, Ashley PJ. Exposure of U.S. children to residential dust lead, 1999–2004: I. Housing and demographic factors. Environ. Health Perspect. 2009;117:461–467. doi: 10.1289/ehp.11917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environ. Health Perspect. 2004;112:717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cory-Slechta DA, Virgolini MB, Rossi-George A, Thiruchelvam M, Lisek R, Weston D. Lifetime consequences of combined maternal lead and stress. Basic Clin Pharmacol Toxicol. 2008;102:218–227. doi: 10.1111/j.1742-7843.2007.00189.x. [DOI] [PubMed] [Google Scholar]
- 45.Rossi-George A, Virgolini MB, Weston D, Cory-Slechta DA. Alterations in glucocorticoid negative feedback following maternal Pb, prenatal stress and the combination: a potential biological unifying mechanism for their corresponding disease profiles. Toxicol. Appl. Pharmacol. 2009;234:117–127. doi: 10.1016/j.taap.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Virgolini MB, Chen K, Weston DD, Bauter MR, Cory-Slechta DA. Interactions of chronic lead exposure and intermittent stress: consequences for brain catecholamine systems and associated behaviors and HPA axis function. Toxicol. Sci. 2005;87:469–482. doi: 10.1093/toxsci/kfi269. [DOI] [PubMed] [Google Scholar]
- 47.Virgolini MB, Bauter MR, Weston DD, Cory-Slechta DA. Permanent alterations in stress responsivity in female offspring subjected to combined maternal lead exposure and/or stress. Neurotoxicol. 2006;27:11–21. doi: 10.1016/j.neuro.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Virgolini MB, Rossi-George A, Lisek R, Weston DD, Thiruchelvam M, Cory-Slechta DA. CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicol. 2008;29:812–827. doi: 10.1016/j.neuro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Virgolini MB, Rossi-George A, Weston D, Cory-Slechta DA. Influence of low level maternal Pb exposure and prenatal stress on offspring stress challenge responsivity. Neurotoxicol. 2008;29:928–939. doi: 10.1016/j.neuro.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vyskocil A, Smejkalova J, Lacinova V. Dose-related stress reaction in male rats chronically exposed to lead acetate. Sb. Ved. Pr. Lek. Fak. Karlovy Univerzity Hradci Kralove. 1991;34:393–401. [PubMed] [Google Scholar]
- 51.de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 52.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 53.Anderson NB, Armstead CA. Toward understanding the asociation of socioeconomic status and health: A new challenge for the biopsychosocial approach. Psychosom. Med. 1995;57:213–225. doi: 10.1097/00006842-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Bellinger DC, Hu H, Titlebaum L, Needleman HL. Attentional correlates of dentin and bone lead levels in adolescents. Arch. Environ. Health. 1994;49:98–105. doi: 10.1080/00039896.1994.9937461. [DOI] [PubMed] [Google Scholar]
- 55.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu. Rev. Psychol. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 56.Canfield RL, Gendle MH, Cory-Slechta DA. Impaired neuropsychological functioning in lead-exposed children. Dev Neuropsychol. 2004;26:513–540. doi: 10.1207/s15326942dn2601_8. [DOI] [PubMed] [Google Scholar]
- 57.Cory-Slechta DA. Relationships between lead-induced learning impairments and changes in dopaminergic, cholinergic and glutamatergic neurotransmitter system functions. Annu. Rev. Pharmacol. Toxicol. 1995;35:391–415. doi: 10.1146/annurev.pa.35.040195.002135. [DOI] [PubMed] [Google Scholar]
- 58.Dietrich KN, Ris MD, Succop PA, Berger OG, Bornschein R. Early exposure to lead and juvenile delinquency. Neurotoxicol. Teratol. 2001;23:511–518. doi: 10.1016/s0892-0362(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 59.Dohrenwend BP. Social status and stressful life events. J. Pers. Soc. Psychol. 1973;28:225–235. doi: 10.1037/h0035718. [DOI] [PubMed] [Google Scholar]
- 60.Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. Journal of the American Medical Association. 1996;275:363–369. [PubMed] [Google Scholar]
- 61.Schwartz J. Low-level lead exposure and children's IQ: a meta-analysis and search for a threshold. Environ. Res. 1994;65:42–55. doi: 10.1006/enrs.1994.1020. [DOI] [PubMed] [Google Scholar]
- 62.Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur. J. Neurosci. 2000;12:973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- 63.Cory-Slechta DA, O’Mara DJ, Brockel BJ. Nucleus accumbens dopaminergic mediation of fixed interval schedule-controlled behavior and its modulation by low-level lead exposure. J.Pharmacol. Exp. Ther. 1998;286:794–805. [PubMed] [Google Scholar]
- 64.Cory-Slechta DA, O’Mara DJ, Brockel BJ. Learning versus performance impairments following regional administration of MK-801 into nucleus accumbens and dorsomedial striatum. Behav. Brain Res. 1999;102:181–194. doi: 10.1016/s0166-4328(99)00015-7. [DOI] [PubMed] [Google Scholar]
- 65.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lowy M, Gault L, Yammamato B. Adrenalectomy attenuates stress induced elevation in extracellular glutamate concentration in hippocampus. J. Neurosci. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- 67.Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: Implications for dopamine-associated psychiatric disorders. Biol. Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- 68.Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8716–8720. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pokora MJ, Richfield EK, Cory-Slechta DA. Preferential vulnerability of nucleus accumbens dopamine binding sites to low-level lead exposure: Time course of effects and interactions with chronic dopamine agonist treatments. J. Neurochem. 1996;67:1540–1550. doi: 10.1046/j.1471-4159.1996.67041540.x. [DOI] [PubMed] [Google Scholar]
- 70.Rouge-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur. J. Neurosci. 1998;10:3903–3907. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 71.Cory-Slechta DA, Virgolini MB, Rossi-George A, Weston D, Thiruchelvam M. Experimental manipulations blunt time-induced changes in brain monoamine levels and completely reverse stress, but not Pb+/−stress-related modifications to these trajectories. Behav. Brain Res. 2009;205:76–87. doi: 10.1016/j.bbr.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelleher RT, Morse WH. Determinants of the behavioral effects of drugs. In: Tedeschi R, editor. Importance of Fundamental Principles in Drug Evaluation. New York: Raven Press; 1969. pp. 383–405. [Google Scholar]
- 73.Cory-Slechta DA, Weiss B. Alterations in schedule-controlled behavior of rodents correlated with prolonged lead exposure. In: Balster RL, editor. Behavioral Pharmacology: The Current Status. New York: Alan R. Liss; 1985. pp. 487–501. [Google Scholar]
- 74.Cory-Slechta DA, Weiss B, Cox C. Performance and exposure indices of rats exposed to low concentrations of lead. Toxicol. Appl. Pharmacol. 1985;78:291–299. doi: 10.1016/0041-008x(85)90292-3. [DOI] [PubMed] [Google Scholar]
- 75.Cory-Slechta DA, Weiss B, Cox C. Tissue distribution of Pb in adult vs. old rats: a pilot study. Toxicology. 1989;59:139–150. doi: 10.1016/0300-483x(89)90052-8. [DOI] [PubMed] [Google Scholar]
- 76.Cory-Slechta DA. Lead exposure during advanced age: Alterations in kinetics and biochemical effects. Toxicol. Appl. Pharmacol. 1990;104:67–78. doi: 10.1016/0041-008x(90)90283-z. [DOI] [PubMed] [Google Scholar]
- 77.Drasch GA, Bohm J, Baur C. Lead in human bones. Investigations of an occupationally nonexposed population in southern Bavaria (FRG). I Adults. Sci. Total Environ. 1987;64:303–315. doi: 10.1016/0048-9697(87)90252-x. [DOI] [PubMed] [Google Scholar]
- 78.Manea-Krichten M, Patterson C, Miller G, Settle D, Erel Y. Comparative increases of lead and barium with age in human tooth enamel, rib and ulna. Sci. Total Environ. 1991;107:179–203. doi: 10.1016/0048-9697(91)90259-h. [DOI] [PubMed] [Google Scholar]
- 79.Cory-Slechta DA, Pazmino R, Bare C. The critical role of the nucleus accumbens dopamine systems in the mediation of fixed interval schedule-controlled operant behavior. Brain Res. 1997;764:253–256. doi: 10.1016/s0006-8993(97)00591-x. [DOI] [PubMed] [Google Scholar]
- 80.Cory-Slechta DA, Brockel BJ, O’Mara DJ. Lead exposure and dorsomedial striatum mediation of fixed interval schedule-controlled behavior. Neurotoxicol. 2002;23:313–327. doi: 10.1016/s0161-813x(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 81.Evans SB, Cory-Slechta DA. Prefrontal cortical manipulations alter the effects of intra-ventral striatal dopamine antagonists on fixed-interval performance in the rat. Behavioral Brain Research. 2000;17:45–58. doi: 10.1016/s0166-4328(99)00108-4. [DOI] [PubMed] [Google Scholar]
- 82.Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog. Neurobiol. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 83.Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114:1635–1644. doi: 10.1210/endo-114-5-1635. [DOI] [PubMed] [Google Scholar]
- 84.Diaz R, Sokoloff P, Fuxe K. Codistribution of the dopamine D3 receptor and glucocorticoid receptor mRNAs during striatal prenatal development in the rat. Neurosci. Lett. 1997;227:119–122. doi: 10.1016/s0304-3940(97)00316-9. [DOI] [PubMed] [Google Scholar]
- 85.Yi SJ, Masters JN, Baram TZ. Glucocorticoid receptor mRNA ontogeny in the fetal and postnatal rat forebrain. Mol. Cell. Neurosci. 1994;5:385–393. doi: 10.1006/mcne.1994.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cory-Slechta DA. Comparative neurobehavioral toxicology of heavy metals. In: Suzuki T, editor. Toxicology of Metals. New York: CRC Press; 1996. pp. 537–560. [Google Scholar]
- 87.Koolhaas JM, Meerlo P, De Boer SF, Strubbe JH, Bohus B. The temporal dynamics of the stress response. Neurosci. Biobehav. Rev. 1997;21:775–782. doi: 10.1016/s0149-7634(96)00057-7. [DOI] [PubMed] [Google Scholar]
- 88.Little HJ, Croft AP, O’Callaghan MJ, Brooks SP, Wang G, Shaw SG. Selective increases in regional brain glucocorticoid: a novel effect of chronic alcohol. Neuroscience. 2008;156:1017–1027. doi: 10.1016/j.neuroscience.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 89.Cory-Slechta DA, Thompson T. Behavioral toxicity of chronic postweaning lead exposure in the rat. Toxicol. Appl. Pharmacol. 1979;47:151–159. doi: 10.1016/0041-008x(79)90082-6. [DOI] [PubMed] [Google Scholar]
- 90.Cory-Slechta DA, Weiss B, Cox C. Delayed behavioral toxicity of lead with increasing exposure concentration. Toxicol. Appl. Pharmacol. 1983;71:342–352. doi: 10.1016/0041-008x(83)90021-2. [DOI] [PubMed] [Google Scholar]
- 91.Cory-Slechta DA, Weiss B, Cox C. Performance and exposure indices of rats exposed to low concentrations of lead. Toxicol. Appl. Pharmacol. 1985;78:291–299. doi: 10.1016/0041-008x(85)90292-3. [DOI] [PubMed] [Google Scholar]
- 92.Stephens DW, Krebs JR. Foraging Theory. Princeton, NJ: Princeton University Press; 1986. [Google Scholar]
- 93.Rubia K, Halari R, Christakou A, Taylor E. Impulsiveness as a timing disturbance: neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009;364:1919–1931. doi: 10.1098/rstb.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Darcheville JC, Riviere V, Wearden JH. Fixed-interval performance and self-control in children. J. Exp. Anal. Behav. 1992;57:187–199. doi: 10.1901/jeab.1992.57-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Darcheville JC, Riviere V, Wearden JH. Fixed-interval performance and self-control in infants. J. Exp. Anal. Behav. 1993;60:239–254. doi: 10.1901/jeab.1993.60-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wittmann M, Paulus MP. Decision making, impulsivity and time perception. Trends Cogn Sci. 2008;12:7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 97.Sonuga-Barke EJS, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion-I. The effect of delay on choice. Journal of Child Psychology and Psychiatry. 1992;33:387–398. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- 98.Grizenko N, Shayan YR, Polotskaia A, Ter-Stepanian M, Joober R. Relation of maternal stress during pregnancy to symptom severity and response to treatment in children with ADHD. J. Psychiatry Neurosci. 2008;33:10–16. [PMC free article] [PubMed] [Google Scholar]
- 99.Meck WH, Penney TB, Pouthas V. Cortico-striatal representation of time in animals and humans. Curr. Opin. Neurobiol. 2008;18:145–152. doi: 10.1016/j.conb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 100.Arnsten AF. Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog. Brain Res. 2000;126:183–192. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- 101.Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 102.Rammsayer TH, Hennig J, Haag A, Lange N. Effects of noradrenergic activity on temporal information processing in humans. Q. J. Exp. Psychol. B. 2001;54:247–258. doi: 10.1080/02724990143000036. [DOI] [PubMed] [Google Scholar]
- 103.al-Zahrani SS, al-Ruwaitea AS, Ho MY, Bradshaw CM, Szabadi E. Destruction of central noradrenergic neurones with DSP4 impairs the acquisition of temporal discrimination but does not affect memory for duration in a delayed conditional discrimination task. Psychopharmacology (Berl) 1997;130:166–173. doi: 10.1007/s002130050225. [DOI] [PubMed] [Google Scholar]
- 104.Flora GJS, Seth PK, Flora SJS. Recoveries in lead induced alterations in rat brain biogenic amine levels following combined chelation therapy with meso 2,3-dimercaptosuccinic acid and calcium disodium versenate. Biogenic Amines. 1997;13:79–90. [Google Scholar]
- 105.Hrdina PD, Hanin I, Dubas TC. Neurochemical correlates of lead toxicity. In: Thomas JA, editor. Lead Toxicity. Baltimore: Urban & Schwarzenberg; 1980. pp. 273–300. [Google Scholar]
- 106.Schneider ML, Clarke SA, Kraemer GW, Roughton EC, Lubach GR, Rimm-Kaufman S, Schmidt D, Ebert M. Prenatal stress alters brain biogenic amine levels in primates. Dev. Psychopathol. 1998;10:427–440. doi: 10.1017/s0954579498001679. [DOI] [PubMed] [Google Scholar]
- 107.Takahashi LK, Turner JG, Kalin NH. Prenatal stress alters brain catecholaminergic activity and potentiates stress-induced behavior in adult rats. Brain Res. 1992;574:131–137. doi: 10.1016/0006-8993(92)90809-n. [DOI] [PubMed] [Google Scholar]
- 108.Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol. Sci. 2006;27:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 109.Park CR, Campbell AM, Woodson JC, Smith TP, Fleshner M, Diamond DM. Permissive influence of stress in the expression of a u-shaped relationship between serum corticosterone levels and spatial memory errors in rats. Dose Response. 2006;4:55–74. doi: 10.2203/dose-response.004.01.005.Park. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, Khairova R, Zhou R, Yuan P, Machado-Vieira R, McEwen BS, Manji HK. Dynamic regulation of mitochondrial function by glucocorticoids. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3543–3548. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davis JM, Svendsgaard DJ. U-shaped dose-response curve-shaped dose-response curves: Their occurrence and implications for risk assessment. J. Toxicol. Environ. Health. 1990;30:71–83. doi: 10.1080/15287399009531412. [DOI] [PubMed] [Google Scholar]
- 112.Leasure JL, Giddabasappa A, Chaney S, Johnson JE, Jr, Pothakos K, Lau YS, Fox DA. Low-level human equivalent gestational lead exposure produces sex-specific motor and coordination abnormalities and late-onset obesity in year-old mice. Environ. Health Perspect. 2008;116:355–361. doi: 10.1289/ehp.10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kern M, Audesirk G. Stimulatory and inhibitory effects of inorganic lead on calcineurin. Toxicology. 2000;150:171–178. doi: 10.1016/s0300-483x(00)00258-4. [DOI] [PubMed] [Google Scholar]
- 114.Pounds JG. Effect of lead intoxication on calcium homeostasis and calcium-mediated cell function: a review. Neurotoxicol. 1984;5:295–331. [PubMed] [Google Scholar]