Abstract
Chronic treatment with benzodiazepines, which positively modulate GABAA receptors, can lead to the development of tolerance. Similar effects might also occur during chronic treatment with positive modulators acting at other sites on GABAA receptors (e.g., neuroactive steroids). In the current study, tolerance and cross tolerance were examined in 7 rats treated daily with the neuroactive steroid pregnanolone (25.6 mg/kg/day) and responding under a fixed ratio 10 schedule of food presentation. Dose-effect curves were determined for positive GABAA modulators (pregnanolone, flunitrazepam, midazolam, and pentobarbital) as well as other drugs (ketamine and morphine) before, during and after chronic treatment. Initially, daily pregnanolone administration increased responding, although tolerance developed to the rate-increasing effects after 14 weeks; tolerance did not develop to the rate-decreasing effects. The potencies of pregnanolone, midazolam and morphine to decrease responding did not change during treatment, while flunitrazepam was more potent and pentobarbital and ketamine were less potent during treatment as compared to before treatment. After treatment, pregnanolone and midazolam were more potent than before treatment. The development of tolerance to the rate-increasing effects of pregnanolone indicates that neuroadaptations occur during chronic treatment; that tolerance develops to only some effects suggests that the behavioral consequences of these neuroadaptations are limited.
Keywords: neuroactive steroids, scheduled-controlled behavior, benzodiazepines, tolerance, rats
Introduction
Gamma-aminobutyric acid (GABA) is the primary inhibitory neurotransmitter in the mammalian nervous system and is involved in numerous processes including development and organization of the central nervous system (Andang and Lendahl, 2008), endocrine and neuroendocrine responses (Cullinan et al., 2008; Morrow et al., 1995), and regulation of mood and anxiety states (Paul and Purdy, 1992; Smith et al., 2007). Many effects of GABA are mediated by GABAA receptors, which are ligand-gated chloride channels that have several modulatory sites; benzodiazepines, barbiturates, and neuroactive steroids act at these distinct sites. Positive modulation at these sites enhances the effects of GABA, thereby facilitating chloride conductance (Allan and Harris, 1986; Majewska et al., 1986; Tallman and Gallager, 1985).
Positive GABAA modulators acting at benzodiazepine or barbiturate sites have long been used therapeutically in the treatment of anxiety, sleep disorders, and seizure disorders. Although benzodiazepines have a larger margin of safety than barbiturates, the use of these drugs can be limited by the development of tolerance and dependence, with benzodiazepine withdrawal symptoms ranging from uncomfortable (increased anxiety and insomnia) to potentially dangerous (seizures). Like benzodiazepines, drugs acting at the neuroactive steroid site, including pregnanolone, positively modulate GABAA receptors resulting in anxiolytic (i.e. Akwa et al., 1999), sedative (i.e. Lancel et al., 1997) and anticonvulsant (i.e. Kokate et al., 1998; Reddy and Rogawski, 2000a) effects; however, neuroactive steroids appear to differ from benzodiazepines in several important ways. One difference involves effects that occur when positive modulators are administered repeatedly with tolerance developing to benzodiazepines under many different conditions (e.g., McMillan, 1992; Löscher et al., 1996; Gerak et al., 2008; Gerak, 2009) and developing to neuroactive steroids under a much more limited range of conditions. For example, tolerance to the anticonvulsant effects of benzodiazepines is readily apparent (Gonsalves and Gallagher, 1987; Löscher et al., 1996), although tolerance does not develop to the anticonvulsant effects of pregnanolone (Kokate et al., 1998) or another neuroactive steroid, ganaxolone (Reddy and Rogawski, 2000b). Such differences might provide a clinical advantage for neuroactive steroids as compared to benzodiazepines.
There are conditions under which tolerance can develop to neuroactive steroids, although it appears to be selective with tolerance developing differentially depending on the particular neuroactive steroid studied or the dependent variable measured. For example, tolerance develops to the anesthetic (Zhu et al., 2004) and memory-impairing (Türkmen et al., 2006) effects of allopregnanolone and to the effects of minaxolone on locomotor activity (Marshall et al., 1997), but not to the rate-decreasing effects of pregnanolone on schedule-controlled responding (McMahon and France, 2002a). Direct comparisons between different neuroactive steroids are few, and the effects of chronic treatment must be compared across studies and dependent variables; however, one study examined the anesthetic effects of pregnanolone and allopregnanolone and found no evidence for acute tolerance to either neuroactive steroid (Zhu et al., 2001). Taken together, these studies suggest that treatment with neuroactive steroids produces differential tolerance depending upon the treatment dose, species, dependent variable, or route, frequency or duration of administration.
This study systematically increased the dose and frequency of pregnanolone administered chronically in order to examine the development of tolerance to and dependence on a neuroactive steroid. To test whether tolerance developed, response rates and the potency of pregnanolone were compared before, during and after chronic pregnanolone treatment. Cross tolerance to positive GABAA modulators acting at other sites was examined by comparing potencies of the benzodiazepines midazolam and flunitrazepam and the barbiturate pentobarbital obtained during and after chronic treatment to their potencies obtained before chronic pregnanolone treatment. In addition, the NMDA receptor antagonist ketamine and the mu opioid receptor agonist morphine were also studied before, during and after chronic pregnanolone treatment. Another consequence of chronic treatment is the development of dependence, which is evidenced by the emergence of withdrawal when treatment is discontinued. In studies that did not find evidence of tolerance to neuroactive steroids, discontinuation of treatment resulted in an increased susceptibility to pentylenetetrazol-induced seizures (Reddy and Rogawski, 2000a) and disruption in rates of food-maintained responding (McMahon and France, 2002a), suggesting that dependence can develop during chronic neuroactive steroid treatment in the absence of tolerance. One reliable indicator of withdrawal is suppression of responding maintained by food presentation (Ator et al., 2000; Ator and Griffiths, 2003; Gellert and Sparber 1977). To the extent that the current treatment conditions result in dependence, it would be indicated by disruptions in responding following temporary suspension of chronic pregnanolone treatment.
Methods
Animals
Seven male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) weighing 250-300 g upon arrival were individually housed in a temperature- and humidity-controlled vivarium on a 14 hour light/10 hour dark cycle. Weights were initially increased and then maintained at 320-330 g for the duration of the study by providing measured amounts of rodent chow daily (Harlan Teklad, Madison, WI, USA). Rats had ad libitum access to water in the home cage and grain-based pellets during experimental sessions (BioServ, Inc., Frenchtown, NJ, USA). All experimental procedures were carried out under guidelines provided by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio and in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, 1996).
Apparatus and procedure
Experimental sessions were conducted daily at the same time. White noise was used in the testing room to reduce extraneous noise. Standard operant boxes were contained in ventilated, sound-attenuating chambers (Med Associates, Inc., St. Albans, VT, USA). The operant boxes (30 × 24 × 21 cm) were constructed of clear polycarbonate and aluminum. One side of the box was a response panel with two levers, two stimulus lights, a pellet dispenser and food cup. The floor of the box was constructed of stainless steel rods. During sessions, rats responded on one of the two levers under a fixed ratio 10 schedule for food. Daily sessions consisted of two to eight 15-minute cycles, each divided into a 10-minute timeout, when responses had no programmed consequence, followed by a 5-minute response period during which time the stimulus light above the active lever was illuminated. Ten consecutive responses on the active lever resulted in the delivery of a single food pellet with a maximum of 10 pellets delivered during each cycle. Responses on the inactive lever reset the count on the active lever to 0. Assignment of the left or right lever as the active lever was counterbalanced among rats. Stimulus lights were extinguished after 5 minutes or the delivery of 10 food pellets, whichever occurred first. Any time remaining between delivery of 10 food pellets and the next cycle was a timeout. Data were collected using MED-PC IV software (Med Associates, Inc., St. Albans, VT, USA).
During training sessions, vehicle or sham injections were given during the first minute of each cycle. Training continued until animals satisfied the following testing criteria: response rates for each cycle within ±20% of the mean for individual sessions and mean response rates for a single session within ±20% of the mean of 10 consecutive sessions, including the current session. Tests were conducted every third session as long as the testing criteria were satisfied during the intervening training sessions. If criteria were not met between tests, additional training sessions were conducted until testing criteria were satisfied for 2 consecutive sessions. To determine the treatment dose of pregnanolone and the interval between pregnanolone administration and sessions, time course studies were conducted prior to chronic treatment by giving pregnanolone on the first cycle, followed by seven sham cycles. Dose-effect curves were determined by administering vehicle on the first cycle followed by increasing doses of the test drug; injections were given during the first minute of each cycle. Cumulative doses increased in 0.25 or 0.50 log unit increments until response rates decreased to less than 10% of control rates. Dose-effect curves were determined for pregnanolone, flunitrazepam, midazolam, pentobarbital, ketamine, and morphine before chronic pregnanolone treatment.
Once dose-effect curves for all six drugs were determined, rats were treated daily with increasing doses of pregnanolone; an outline of the experimental design can be found in Table 1. Chronic treatment continued for a total of 20 weeks. At the start of chronic treatment, rats received one injection of 5.6 mg/kg of pregnanolone in the home cage one hour before sessions. Because tolerance was not evident after 2 weeks of treatment with a single dose of pregnanolone, the frequency of dosing was increased to twice daily injections of 5.6 mg/kg of pregnanolone (total dose: 11.2 mg/kg/day), given five hours and one hour prior to sessions, for 5 weeks. Thereafter, a third daily injection of 5.6 mg/kg of pregnanolone was added four hours after the second injection (approximately two hours after sessions; total dose: 16.8 mg/kg/day of pregnanolone); this treatment continued for 2 more weeks. Response rates and the potency of pregnanolone determined at this time indicated no development of tolerance; as a result, the treatment dose was further increased. For the remaining 11 weeks of treatment, one dose of 10.0 mg/kg of pregnanolone was given five hours prior to sessions, a second dose of 5.6 mg/kg was given one hour prior to sessions, and a third dose of 10.0 mg/kg was given four hours after the second injection (total dose: 25.6 mg/kg/day of pregnanolone). Throughout the study, dose-effect curves were determined twice weekly, beginning with a pregnanolone dose-effect curve on the fourth day of treatment and a flunitrazepam dose-effect curve on the seventh day of treatment. Thereafter, pregnanolone and flunitrazepam dose-effect curves were determined each week while treatment conditions were changing with the exception of week 7 during which ketamine dose-effect curves were determined twice. The final treatment conditions were established by week 12; for the next 11 weeks, dose-effect curves for other drugs were determined when rats received pregnanolone before the session so that the interval between the last pregnanolone injection and the session was one hour. The third injection of pregnanolone was not given on testing days to avoid adverse effects. To test for the development of dependence as measured by disruptions in rates of food-maintained responding, pregnanolone treatment was temporarily suspended for 25 hours and rats received vehicle instead of pregnanolone for the three consecutive injections that preceded the session; vehicle was administered during the first cycle of sessions. On separate occasions, the vehicle cycle was followed by redetermination of dose-effect curves for each of the 6 drugs. For 2 weeks following termination of chronic pregnanolone treatment, rats received three vehicle injections daily and dose-effect curves for pregnanolone and flunitrazepam were determined. Four weeks after termination of treatment dose-effect curves for all drugs were redetermined.
Table 1.
Outline of experimental design detailing tests conducted before, during and after chronic pregnanolone treatment.
| Treatment dose (mg/kg/day) |
Week of treatment | Test | |
|---|---|---|---|
| Before | 0 | -- | Pregnanolone time course |
| 0 | -- | Dose-effect curves for all drugs | |
| During | 5.6 | 1, 2 | Pregnanolone and flunitrazepam dose-effect curvesa |
| 11.2 | 3-6 | Pregnanolone and flunitrazepam dose-effect curvesa | |
| 11.2 | 7 | Ketamine dose-effect curves | |
| 16.8 | 8, 9 | Pregnanolone and flunitrazepam dose-effect curvesa | |
| 25.6 | 10, 11 | Pregnanolone and flunitrazepam dose-effect curvesa | |
| 25.6 | 12-20 | Dose-effect curves for all drugs | |
| After | 0 | 2 weeks after | Pregnanolone and flunitrazepam dose-effect curvesa |
| 0 | 4 weeks after | Dose-effect curves for all drugs |
Pregnanolone dose-effect curves were determined on the fourth day of each week and flunitrazepam dose-effect curves were determined on the seventh day of each week.
Drugs
Pregnanolone (5ß-pregnan-3α-ol-20-one; Steraloids, Inc., Newport, RI, USA) was dissolved in 45% (w/v) 2-hydroxypropyl-γ-cyclodextrin (Sigma-Aldrich, St. Louis, MO, USA). Sodium pentobarbital (Sigma-Aldrich, St. Louis, MO, USA) and morphine sulfate (Research Technology Branch, National Institute on Drug Abuse, Rockville MD, USA) were dissolved in 0.9% saline. Ketamine hydrochloride (Fort Dodge Laboratories, Fort Dodge, IA, USA) and midazolam hydrochloride (Bedford Laboratories, Bedford, OH, USA) were purchased in solution and diluted in 0.9% saline. Flunitrazepam (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 20% emulphor, 10% ethanol and 70% saline. All drugs were administered i.p.
Data analyses
Control response rates were determined for each rat prior to chronic treatment by first averaging response rates across cycles within a training session, then across the 10 training sessions that immediately preceded the start of daily pregnanolone treatment. A paired t-test was used to determine changes in rates before and after chronic pregnanolone treatment. Time course data were analyzed by two-way repeated measures analysis of variance (ANOVA) using GraphPad 5.01 Software (San Diego, CA, USA, www.graphpad.com) with pregnanolone dose and time since injection as the between-subjects factors. Significant ANOVAs were followed with Bonferroni's post hoc test. A one-way repeated measures ANOVA was used to compare response rates on the first cycle (vehicle injection) of weekly test sessions before, during and after treatment; post hoc comparisons were made using Dunnett's Multiple Comparison Test to compare response rates on the first cycle of the session during and after treatment to response rates before treatment. Statistical tests were deemed significant when p < 0.05.
The dose of drug needed to decrease response rates to 50% of control rates (ED50) was estimated by fitting straight lines to individual dose-response curves for each rat; each line was estimated using one dose that produced response rates greater than or equal to 75% of control rates and one dose that produced response rates less than or equal to 25% of control rates and all doses in between. Potency ratios were calculated for each animal by dividing the ED50 values during and after treatsment by the ED50 value before treatment to determine changes in potency that might indicate the development of tolerance or cross tolerance during chronic treatment. Significant changes in potencies during and after treatment relative to before treatment were detected when the 95% confidence intervals of the potency ratios averaged across rats did not include 1.
Results
Control rates of responding
Prior to chronic treatment, mean control response rates varied across individual rats from 0.45 to 0.89 responses per second, with a group mean (±SEM) of 0.67±0.06 responses per second. Rates of responding obtained four weeks after treatment (group mean: 0.64±0.05 responses per second) were not significantly different from those obtained before treatment (t(6) = 0.79, p > 0.05).
Time course studies
The rate-decreasing effects of a single, bolus dose of 5.6 mg/kg of pregnanolone were evident 15 minutes after administration (Fig. 1). A significant time × dose interaction (F(21,120) = 4.11, p < 0.001) and a significant main effect of dose (F(3,120) = 26.97, p < 0.001) were detected. Bonferroni post hoc tests revealed decreased response rates for 60 minutes after 5.6 mg/kg of pregnanolone; response rates were not different from control rates 75 minutes after administration. There was no significant change in rates of responding following administration of 1.0 (data not shown) or 3.2 mg/kg of pregnanolone compared to vehicle.
Figure 1.
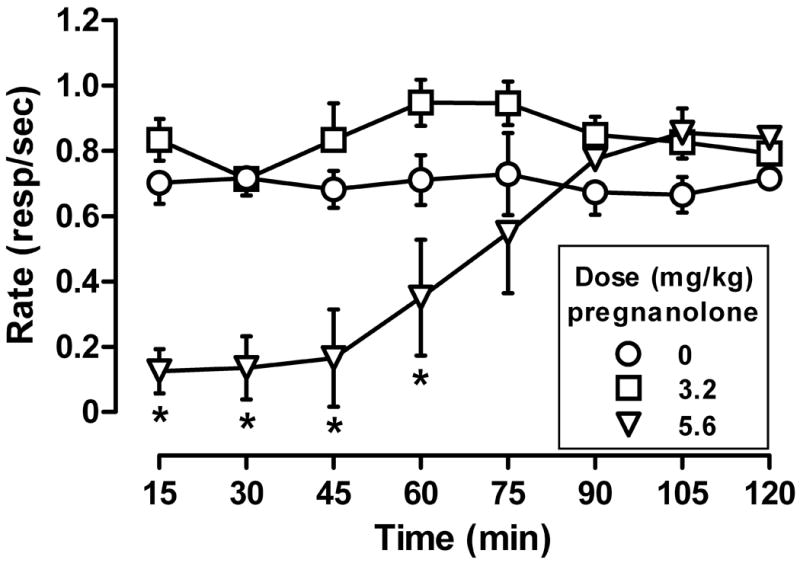
Time-course for the rate-decreasing effects of pregnanolone. A bolus dose of 5.6 mg/kg of pregnanolone significantly decreases responding for 60 minutes. Abscissa: time since pregnanolone injection in minutes. Ordinate: mean response rates (±SEM) expressed as number of responses per second. * Response rates significantly different from vehicle (open circles; p < 0.05).
Daily treatment with pregnanolone
During chronic pregnanolone treatment, response rates on the first cycle of sessions were increased relative to response rates obtained on the first cycle of sessions before chronic treatment (F(16,119) = 6.85, p < 0.001). This increase was evident on the first day of treatment, resulting in average response rates of 0.92±0.07 responses per second. For 13 weeks, response rates on the first cycle of weekly test sessions were increased relative to rates obtained during the first cycle of the test session immediately before treatment began, regardless of daily pregnanolone dose (compare triangles and circle, Fig. 2); when pregnanolone treatment was suspended for 25 hours before the session (squares, weeks 6 and 12, Fig. 2), rates on the first cycle decreased and were no different from before treatment. Tolerance developed to the rate-increasing effects of pregnanolone by the 14th week of treatment. Thereafter, suspension of treatment for 25 hours before sessions had no effect on response rates (squares, week 18, Fig. 2), and there was no change in rates four weeks after chronic pregnanolone treatment was terminated (diamond, Fig. 2).
Figure 2.
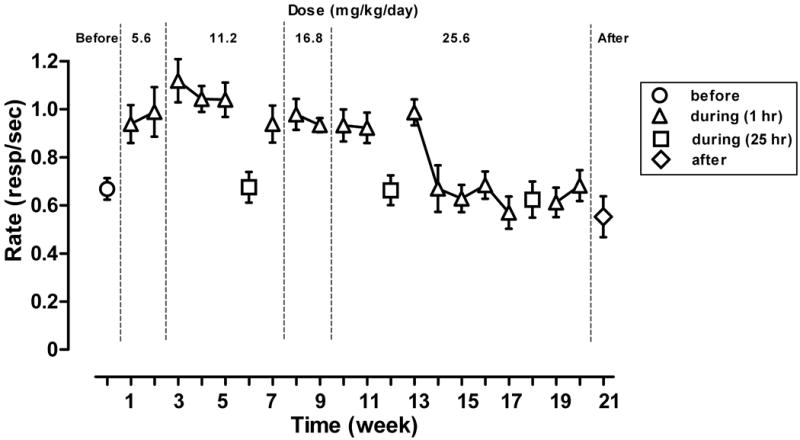
Response rates on the first cycle of weekly test sessions before (circles), during (triangles: pregnanolone last given 1 hr prior to session; squares: pregnanolone last given 25 hr prior to session), and after (diamonds) chronic pregnanolone treatment. Data points represent rates obtained on the first cycle of a single test session. Abscissa: week of chronic treatment. Ordinate: response rates (±SEM) expressed as number of responses per second.
Dose-effect curves determined before treatment
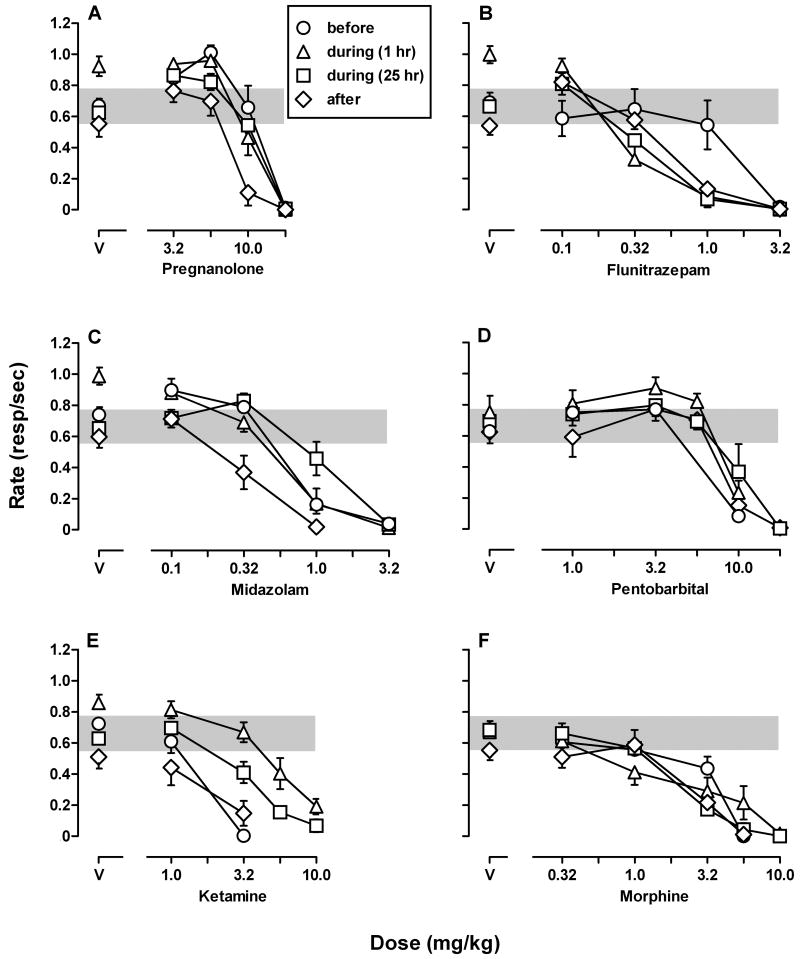
Prior to chronic pregnanolone treatment, small doses of pregnanolone and midazolam increased rates of responding and larger doses had no effect or decreased rates (open circles, Fig. 3A and 3C). The highest response rates occurred at a cumulative dose of 5.6 mg/kg of pregnanolone, which increased rates from 0.67±0.06 under control conditions to 1.01±0.05 responses per second. A dose of 0.1 mg/kg of midazolam increased responding to 0.90±0.07 responses per second. Flunitrazepam, pentobarbital, ketamine and morphine only decreased rates (open circles, Fig. 3B, 3D, 3E and 3F).
Figure 3.
Cumulative dose-effect curves for pregnanolone (A), flunitrazepam (B), midazolam (C), pentobarbital (D), ketamine (E), and morphine (F) before (circles), during (triangles: pregnanolone last administered 1 hour prior to session; squares: pregnanolone last administered 25 hours prior to session), and after (diamonds) chronic pregnanolone treatment. During chronic treatment, dose-effect curves obtained when pregnanolone was last given 1 hr prior to the session (triangles) were determined during week 11 for pregnanolone (A) and flunitrazepam (B) and during week 12 for midazolam (C) and ketamine (E), before tolerance developed to the rate-increasing effects of pregnanolone (points above V); dose-effect curves for pentobarbital (D) and morphine (F) were determined during week 16 of treatment after tolerance developed to the rate-increasing effects of pregnanolone. Abscissae: cumulative dose in mg/kg body weight. Ordinates: response rates expressed as number of responses per second. Gray shading indicates the 95% confidence intervals of control response rates prior to chronic treatment. See Table 2 for significant potency ratios.
Dose-effect curves determined during treatment
During chronic treatment, small doses of pregnanolone, midazolam and flunitrazepam increased rates of responding and larger doses had no effect or decreased rates (open triangles, Fig. 3A, 3B and 3C). When pregnanolone treatment was suspended for 25 hours, small doses of pregnanolone and flunitrazepam increased rates of responding and larger doses decreased rates (open squares, Fig. 3A and 3B). Potency ratios with 95% confidence intervals for each drug and treatment condition are listed in Table 2. During pregnanolone treatment, there was no change in the potencies of pregnanolone (Fig. 3A), midazolam (Fig. 3C), or morphine (Fig. 3F) to decrease response rates as compared to their potencies before chronic treatment. The potency of flunitrazepam (Fig. 3B) was increased during chronic pregnanolone treatment; there was a 3-fold shift leftward in the flunitrazepam dose-effect curve during treatment. Pentobarbital (Fig. 3D) and ketamine (Fig. 3E) were significantly less potent in decreasing response rates during chronic treatment; there was a significant 1.5-fold shift rightward in the pentobarbital dose-effect curve and a significant 5-fold shift rightward in the ketamine dose-effect curve. When pregnanolone treatment was suspended for 25 hours, there was a significant 2-fold shift rightward in the midazolam dose-effect curve and a significant 3-fold shift rightward in the ketamine dose-effect curve. The potencies of pregnanolone and morphine to decrease rates were unchanged when treatment was suspended for 25 hours compared to before treatment. The potency of flunitrazepam was increased when pregnanolone treatment was suspended for 25 hours resulting in a 3-fold shift leftward in the flunitrazepam dose-effect curve.
Table 2.
Mean ED50 values and potency ratios with 95% confidence intervals (CI) for the rate-decreasing effects of pregnanolone, flunitrazepam, midazolam, morphine, ketamine, and pentobarbital before, during, and after daily pregnanolone treatment. Dose-effect curves were determined 1 and 25 hours after the previous pregnanolone dose.
| ED50 (mg/kg) | Potency Ratioa | |||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Pregnanolone | ||||
| Before | 12.95 | 10.68-15.21 | ||
| During | ||||
| 1 hour | 12.01 | 7.68-16.35 | 0.97 | 0.56-1.38 |
| 25 hours | 11.85 | 8.73-14.98 | 0.94 | 0.67-1.20 |
| After | 7.94 | 5.49-10.39 | 0.63* | 0.43-0.82 |
| Flunitrazepam | ||||
| Before | 1.72 | 1.03-2.40 | ||
| During | ||||
| 1 hour | 0.58 | 0.08-1.08 | 0.49* | 0.17-0.82 |
| 25 hours | 0.55 | 0.26-0.84 | 0.33* | 0.19-0.47 |
| After | 1.63 | 0.27-3.00 | 0.92 | 0.43-1.40 |
| Midazolam | ||||
| Before | 0.85 | 0.44-1.26 | ||
| During | ||||
| 1 hour | 0.85 | 0.48-1.22 | 1.23 | 0.58-1.88 |
| 25 hours | 1.45 | 0.86-2.04 | 1.96* | 1.09-2.82 |
| After | 0.37 | 0.20-0.56 | 0.55* | 0.22-0.88 |
| Pentobarbital | ||||
| Before | 6.73 | 6.05-7.41 | ||
| During | ||||
| 1 hour | 9.92 | 7.53-12.31 | 1.48* | 1.14-1.83 |
| 25 hours | 9.48 | 5.84-13.11 | 1.42 | 0.84-2.00 |
| After | 8.79 | 6.7-10.89 | 1.32 | 1.00-1.64 |
| Ketamine | ||||
| Before | 1.62 | 1.27-1.97 | ||
| During | ||||
| 1 hour | 7.43 | 4.65-10.22 | 4.90* | 2.43-7.37 |
| 25 hours | 4.34 | 2.69-5.99 | 2.88* | 1.48-4.28 |
| After | 1.67 | 1.24-2.11 | 1.10 | 0.70-1.50 |
| Morphine | ||||
| Before | 3.05 | 2.04-4.06 | ||
| During | ||||
| 1 hour | 3.11 | 0.50-5.72 | 1.24 | -0.05-2.53 |
| 25 hours | 2.16 | 1.50-2.83 | 0.85 | 0.39-1.30 |
| After | 2.04 | 1.36-2.72 | 0.79 | 0.31-1.28 |
Potency ratio relative to ED50 values before treatment.
Indicate significant differences from before treatment.
Dose-effect curves determined after treatment
After chronic treatment, pregnanolone and midazolam were significantly more potent in decreasing response rates relative to before treatment as indicated by 2-fold leftward shifts. The potencies of flunitrazepam, pentobarbital, ketamine, and morphine following treatment were not different from before treatment (Table 2).
Discussion
This study examined the development of tolerance and cross tolerance during chronic pregnanolone treatment. Daily treatment with pregnanolone increased responding for several weeks; however, by the 14th week of treatment, tolerance developed to the rate-increasing effects of pregnanolone and response rates were not different from rates before treatment. Larger doses of pregnanolone decreased responding; systematically increasing the daily treatment dose did not shift the rate-decreasing portion of the pregnanolone dose-effect curve rightward, indicating a lack of tolerance to the rate-decreasing effects of pregnanolone. When pregnanolone treatment was suspended for 25 hours, rates of food-maintained responding were not different from rates obtained before chronic treatment, regardless of whether tolerance had developed; that rates were not disrupted suggests that dependence did not develop.
Despite similarities in the acute behavioral effects (e.g. sedation and anticonvulsant effects) of positive GABAA modulators acting at different sites, the effects of chronic treatment with benzodiazepines or with neuroactive steroids appear to differ widely. Repeated treatment with benzodiazepines leads to the development of tolerance to the anxiolytic (e.g. Fernandes et al., 1999), sedative (e.g. McMillan, 1992), and anticonvulsant effects (e.g. Izzo et al., 2001) in rats and mice. Tolerance also develops to the rate-decreasing effects of benzodiazepines under a variety of conditions (e.g. Cohen and Sanger, 1994; Gerak, 2009; McMahon and France, 2002b). Although, under some conditions, sensitivity to neuroactive steroids decreases with repeated administration, the conditions under which tolerance develops to neuroactive steroids appear to be narrow. For example, tolerance develops to the memory-impairing (Türkmen et al., 2006), anticonvulsant (Członkowska et al., 2001) and anesthetic effects (Zhu et al., 2004; Türkmen et al., 2008) of allopregnanolone; however, tolerance does not develop either to allopregnanolone when its effects on the components of sleep are measured (Damianisch et al., 2001) or to the anticonvulsant effects of pregnanolone (Kokate et al., 1998). In addition, strain differences have been noted in the development of tolerance to the hypothermic effects of allopregnanolone in mice selectively bred for high or low sensitivity to ethanol-induced hypothermia (Palmer et al., 2002). In the current study, tolerance developed to the rate-increasing effects of pregnanolone, and not to its rate-decreasing effects in this or other (McMahon and France, 2002a) studies. Although some of the differences in the development of tolerance to neuroactive steroids noted across studies might be due to the species studied, or route and duration of administration, these explanations cannot account for other inconsistencies, such as the differential development of tolerance observed in the current study. Thus, decreased sensitivity to neuroactive steroids occurs under a limited set of conditions, possibly indicating an important difference between neuroactive steroids and benzodiazepines.
Following acute (Gerak et al., 2008) and chronic treatment (McMahon and France 2002a) with benzodiazepines, cross tolerance to other benzodiazepines develops; however, cross tolerance does not develop to neuroactive steroids (Gerak et al., 2008; Gerak 2009; McMahon and France 2002a, 2002b; McMahon et al., 2007). Although cross tolerance to benzodiazepines has been shown in subjects treated chronically with neuroactive steroids (McMahon and France 2002a; Reddy and Rogawski 2000b), in the current study, chronic pregnanolone treatment did not result in cross tolerance to benzodiazepines and produced a modest 1.5-fold shift to the right in the pentobarbital dose-effect curve. One possible explanation for differential development of cross tolerance is that the neuroadaptations that occur at GABAA receptors during chronic treatment differ depending on the site of action of the treatment drug. For example, in vitro studies have shown that subunit composition of GABAA receptors is an important determinant of the affinity of positive modulators (Baburin et al., 2008; Benke et al., 1996; Kohm et al., 2006). Chronic benzodiazepine treatment results in decreased benzodiazepine binding (Wu et al., 1994a) likely due to changes in the relative numbers of α1 and γ2 subunits (Biggio et al., 2003; Primus and Gallager, 1992; Wu et al., 1994b); in contrast, chronic neuroactive steroid treatment alters expression of α4 mRNA (Smith et al., 2007). Thus, the neuroadaptations of GABAA receptors during chronic benzodiazepine or neuroactive steroid treatment seem to differ. Changes in subunit composition during chronic pregnanolone treatment might also have differentially affected the potencies of the two benzodiazepines in the current study. Whatever the reason for the lack of cross tolerance and the differential change in potencies of flunitrazepam and midazolam, differences in the development of cross tolerance between benzodiazepines and neuroactive steroids suggests that the mechanism by which tolerance develops varies across positive GABAA modulators.
When chronic benzodiazepine treatment is abruptly terminated, behavioral signs of withdrawal such as increased anxiety, insomnia, muscle tremors and seizures are readily apparent (Ator and Griffiths, 2003; Woods et al., 1992). Discontinuation of chronic benzodiazepine treatment can also disrupt operant responding for food (Ator et al., 2000) and is considered a sensitive measure of withdrawal, often noted in the absence of other physiological withdrawal signs (Emmett-Oglesby et al., 1990). Given the similarities between benzodiazepines and neuroactive steroids administered acutely, including the ability of neuroactive steroids to reverse withdrawal in diazepam-dependent monkeys (McMahon et al., 2007), it was expected that large doses of pregnanolone given repeatedly would result in the development of dependence and that withdrawal signs following discontinuation of pregnanolone treatment would be similar to those that emerge following termination of chronic benzodiazepine treatment. Despite large doses given repeatedly, pregnanolone treatment failed to result in a disruption of food-maintained responding or any other overt behavioral signs of withdrawal, suggesting that, at least under these conditions, dependence does not develop to pregnanolone.
During pregnanolone treatment, sensitivity to ketamine decreased. There is evidence that NMDA receptors have distinct modulatory sites for neuroactive steroids (Park-Chung et al., 1997), and these sites could play an important role in the changes in sensitivity to ketamine that were observed during chronic pregnanolone treatment. In addition, the decreased potency of ketamine during treatment might be due to compensatory changes occurring at NMDA receptors in response to enhanced GABAergic neurotransmission as a result of daily pregnanolone treatment. Compensatory changes in the glutamatergic system are also evident during chronic benzodiazepine treatment (Izzo et al., 2001). Changes in sensitivity to ketamine were temporary; four weeks after termination of daily pregnanolone treatment the potency of ketamine was no different from before treatment. The apparent cross tolerance to ketamine in addition to tolerance to the rate-increasing effects of pregnanolone provide evidence that complex interactions occur between the GABAergic and glutamatergic neurotransmitter systems during chronic pregnanolone treatment.
After chronic treatment, sensitivity to pregnanolone and midazolam was modestly increased suggesting that GABAA receptors were still changed long after daily pregnanolone treatment was terminated. It is known that changes in subunit composition of GABAA receptors can affect the functional properties of the receptor (Baburin et al., 2008) and that drug treatment can alter subunit expression (Yu et al., 1996) and coupling efficiency of the various binding sites on GABAA receptors (Yu and Ticku, 1995), although it is not known which of the changes in GABAA receptors account for the development of tolerance to the rate-increasing effects of pregnanolone or which changes persist after termination of treatment. Importantly, the potency of morphine, a drug that does not have actions at GABAA receptors, was unchanged by chronic pregnanolone treatment further supporting the notion of selective changes at GABAA receptors.
In summary, daily treatment with pregnanolone resulted in selective tolerance to the rate-increasing but not rate-decreasing effects of pregnanolone. Chronic pregnanolone treatment did not result in cross tolerance to the benzodiazepines flunitrazepam or midazolam, although cross tolerance to ketamine developed, and temporary suspension of treatment failed to result in any behavioral signs of withdrawal. Despite having similar modulatory actions at GABAA receptors, benzodiazepines and neuroactive steroids appear to differ in the development of tolerance and dependence, suggesting that neuroactive steroids may be an effective therapeutic target for the treatment of insomnia and seizure disorders while minimizing the unwanted effects that occur with repeated benzodiazepine use.
Acknowledgments
The authors thank R. Lopez, O. Dominguez, and B. Harrington for expert technical assistance.
The project described was supported by Grant DA017240 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
References
- Allan AM, Harris RA. Anesthetic and convulsant barbiturates alter gamma-aminobutyric acid-stimulated chloride flux across brain membranes. J Pharmacol Exp Ther. 1986;238:763–768. [PubMed] [Google Scholar]
- Akwa Y, Purdy RH, Koob GF, Britton KT. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–125. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- Andang M, Lendahl U. Ion fluxes and neurotransmitters signaling in neural development. Curr Opin Neurobiol. 2008;18:232–236. doi: 10.1016/j.conb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;5:S55–S72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Ator NA, Weerts EM, Kaminski BJ, Kautz MA, Griffiths RR. Zaleplon and triazolam physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. Drug Alcohol Depend. 2000;61:69–84. doi: 10.1016/s0376-8716(00)00122-8. [DOI] [PubMed] [Google Scholar]
- Baburin I, Kohm S, Timin E, Hohaus A, Sieghart W, Hering S. Estimating the efficiency of benzodiazepines on GABAA receptors comprising γ1 or γ2 subunits. Brit J Pharmacol. 2008;155:424–433. doi: 10.1038/bjp.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke D, Honer M, Michel C, Mohler H. GABAA receptor subtypes differentiated by their γ-subunit variance: prevalence, pharmacology and subunit architecture. Neuropharmacol. 1996;35:1413–1423. doi: 10.1016/s0028-3908(96)00068-8. [DOI] [PubMed] [Google Scholar]
- Biggio G, Dazzi L, Biggio F, Mancuso L, Talani G, Busonero F, et al. Molecular mechanisms of tolerance to and withdrawal of GABAA receptor modulators. Eur Neuropsychopharmacol. 2003;13:411–423. doi: 10.1016/j.euroneuro.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Cohen C, Sanger DJ. Tolerance, cross tolerance and dependence measured by operant responding in rats treated with triazolam via osmotic minipumps. Psychopharmacol. 1994;115:86–94. doi: 10.1007/BF02244756. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct. 2008;213:63–72. doi: 10.1007/s00429-008-0192-2. [DOI] [PubMed] [Google Scholar]
- Członkowska AI, Krzaścik P, Sienkiewicz-Jarosz H, Siemiatkowski M, Szyndler J, Maciejak P, Bidziński A, Płaźnik A. Tolerance to the anticonvulsant activity of midazolam and allopregnanolone in a model of picrotoxin seizures. Eur J Pharmacol. 2001;425:121–127. doi: 10.1016/s0014-2999(01)01183-9. [DOI] [PubMed] [Google Scholar]
- Damianisch K, Rupprecht R, Lancel M. The influence of subchronic administration of the neurosteroid allopregnanolone on sleep in the rat. Neuropsychopharmacol. 2001;25:576–584. doi: 10.1016/S0893-133X(01)00242-1. [DOI] [PubMed] [Google Scholar]
- Emmett-Oglesby MW, Mathis DA, Moon RTY, Lal H. Animal models of drug withdrawal symptoms. Psychopharmacol. 1990;101:292–309. doi: 10.1007/BF02244046. [DOI] [PubMed] [Google Scholar]
- Fernandes C, Arnot MI, Irvine EE, Bateson AN, Martin IL, File SE. The effect of treatment regimen on the development of tolerance to the sedative and anxiolytic effects of diazepam. Psychopharmacol. 1999;145:251–259. doi: 10.1007/s002130051056. [DOI] [PubMed] [Google Scholar]
- Gerak LR. Selective changes in sensitivity to benzodiazepines, and not other positive GABAA modulators, in rats receiving flunitrazepam chronically. Psychopharmacol. 2009;204:667–677. doi: 10.1007/s00213-009-1497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, McMahon LR, France CP. Acute cross tolerance to midazolam, and not pentobarbital and pregnanolone, after a single dose of chlordiazepoxide in monkeys discriminating midazolam. Behav Pharmacol. 2008;19:796–804. doi: 10.1097/FBP.0b013e32831c3b40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert VF, Sparber SB. A comparison of the effects of naloxone upon body weight loss and suppression of fixed-ratio operant behavior in morphine-dependent rats. J Pharmacol Exp Ther. 1977;201:44–54. [PubMed] [Google Scholar]
- Gonsalves SF, Gallagher DW. Time course for development of anticonvulsant tolerance and GABAergic subsensitivity after chronic diazepam. Brain Res. 1987;405:94–99. doi: 10.1016/0006-8993(87)90993-0. [DOI] [PubMed] [Google Scholar]
- Izzo E, Auta J, Impaganatiello F, Pesold C, Guidotto A, Costa E. Glutamic acid decarboxylase and glutamate receptor changes during tolerance and dependence to benzodiazepines. PNAS. 2001;98:3483–3488. doi: 10.1073/pnas.051628698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohm S, Baburin I, Timin EN, Hohaus A, Sieghart W, Hering S. Pharmacological properties of GABAA receptors containing γ1 subunits. Mol Pharmacol. 2006;69:640–649. doi: 10.1124/mol.105.017236. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Yamaguchi S, Pannell LK, Rajamani U, Carroll DM, Grossman AB, Rogawski MA. Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. J Pharmacol Exp Ther. 1998;287:553–558. [PubMed] [Google Scholar]
- Lancel M, Faulhaber J, Schiffelholz T, Romeo E, Di MF, Holsboer F, Rupprecht R. Allopregnanolone affects sleep in a benzodiazepine-like fashion. J Pharmacol Exp Ther. 1997;282:1213–1218. [PubMed] [Google Scholar]
- Löscher W, Rundfeldt C, Hönack D, Ebert U. Long-term studies on anticonvulsant tolerance and withdrawal characteristics of benzodiazepine receptor ligands in different seizure models in mice. I. Comparison of diazepam, clonazepam, clobazam and abecarnil. J Pharmacol Exp Ther. 1996;279:561–572. [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Marshall FH, Stratton SC, Mullings J, Ford E, Worton SP, Oakley NR, Hagan RM. Development of tolerance in mice to the sedative effects of the neuroactive steroid minaxolone following chronic exposure. Pharmacol Biochem Behav. 1997;58:1–8. doi: 10.1016/s0091-3057(96)00132-3. [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CF. Acute and chronic effects of the neuroactive steroid pregnanolone on schedule-controlled responding in rhesus monkeys. Behav Pharmacol. 2002a;13:545–555. doi: 10.1097/00008877-200211000-00004. [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CF. Daily treatment with diazepam differentially modifies sensitivity to the effects of γ-aminobutyric acidA modulators on schedule-controlled responding in rhesus monkeys. J Pharmacol Exp Ther. 2002b;300:1017–1025. doi: 10.1124/jpet.300.3.1017. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Javors MA, France CF. Changes in relative potency among positive GABAA receptor modulators upon discontinuation of chronic benzodiazepine treatment in rhesus monkeys. Psychopharmacol. 2007;192:135–145. doi: 10.1007/s00213-006-0692-9. [DOI] [PubMed] [Google Scholar]
- McMillan DE. Effects of drugs on behavior before and during chronic diazepam administration. Eur J Pharmacol. 1992;215:145–152. doi: 10.1016/0014-2999(92)90022-v. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Devaud LL, Purdy RH, Paul SM. Neuroactive steroid modulators of the stress response. Ann NY Acad Sci. 1995;771:257–272. doi: 10.1111/j.1749-6632.1995.tb44687.x. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Moyer MR, Crabbe JC, Phillips TJ. Initial sensitivity, tolerance and cross tolerance to allopregnanolone- and ethanol-induced hypothermia in selected mouse lines. Psychopharmacol. 2002;162:313–322. doi: 10.1007/s00213-002-1106-2. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Wu FS, Purdy RH, Malayev AA, Gibbs TT, Farb DH. Distinct sites for inverse modulation of N-methyl-D-aspartate receptors by sulfated steroids. Mol Pharmacol. 1997;52:1113–1123. doi: 10.1124/mol.52.6.1113. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Primus RJ, Gallager DW. GABAA receptor subunit mRNA levels are differentially influenced by chronic FG 7142 and diazepam exposure. Eur J Pharmacol. 1992;226:21–28. doi: 10.1016/0922-4106(92)90078-a. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after nerosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000a;294:909–915. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000b;295:1241–1248. [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABAA receptors: focus on the α4 and δ subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallman JF, Gallager DW. The GABA-ergic system: A locus of benzodiazepine action. Ann Rev Neurosci. 1985;8:21–44. doi: 10.1146/annurev.ne.08.030185.000321. [DOI] [PubMed] [Google Scholar]
- Türkmen S, Löfgren M, Birzniece V, Bäckström T, Johansson IM. Tolerance development to Morris water maze test impairments induced by acute allopregnanolone. Neuroscience. 2006;139:651–659. doi: 10.1016/j.neuroscience.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Türkmen S, Wahlström G, Bäckström T, Johansson IM. Persistence of tolerance to the anesthetic effect of allopregnanolone in male rats. Eur J Pharmacol. 2008;592:73–80. doi: 10.1016/j.ejphar.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev. 1992;44:151–347. [PubMed] [Google Scholar]
- Wu Y, Rosenberg HC, Chiu TH, Ramsey-Williams V. Regional changes in [3H]zolpidem binding to brain benzodiazepine receptors in flurazepam tolerant rat: comparison with changes in [3H]flunitrazepam binding. J Pharmacol Exp Ther. 1994a;268:675–682. [PubMed] [Google Scholar]
- Wu Y, Rosenberg HC, Chiu TH, Zhao TJ. Subunit- and brain region-specific reduction of GABAA receptor subunit mRNAs during chronic treatment of rats with diazepam. J Mol Neurosci. 1994b;5:105–120. doi: 10.1007/BF02736752. [DOI] [PubMed] [Google Scholar]
- Yu R, Follesa P, Ticku MK. Down-regulation of the GABA receptor subunits mRNA levels in mammalian cultured cortical neurons following chronic neurosteroid treatment. Brain Res Mol Brain Res. 1996;41:163–168. doi: 10.1016/0169-328x(96)00087-3. [DOI] [PubMed] [Google Scholar]
- Yu R, Ticku MK. Chronic neurosteroid treatment produces functional heterologous uncoupling of the γ-aminobutyric acid type A/benzodiazepine receptor complex in mammalian cortical neurons. Mol Pharmacol. 1995;47:603–610. [PubMed] [Google Scholar]
- Zhu D, Birzniece V, Bäckström T, Whalström G. Dynamic aspects of acute tolerance to allopregnanolone using anesthesia threshold in male rats. Br J Anaesth. 2004;93:560–570. doi: 10.1093/bja/aeh233. [DOI] [PubMed] [Google Scholar]
- Zhu D, Wang T, Bäckström T, Whalström G. Evaluation and comparison of the pharmacokinetic and pharmacodynamic properties of allopregnanolone and pregnanolone at induction of anaesthesia in the male rat. Br J Anaesth. 2001;86:403–412. doi: 10.1093/bja/86.3.403. [DOI] [PubMed] [Google Scholar]