Abstract
Background
Mycobacterium avium (MAC) lives and replicates in macrophages and causes disseminated disease in immunocompromised individuals. As a host response to control disease, many macrophages become apoptotic a few days after MAC infection. In this study, we hypothesized that MAC can survive autophagic and apoptotic macrophages and spread.
Methods
Electron, time-lapse video, fluorescence microscopy. Apoptosis was determined by ELISA and TUNEL assays. Autophagy was seen by migration of LC3-1.
Results
Apoptotic macrophages harbor chiefly viable MAC. MAC escapes both the vacuole and the macrophage once apoptosis is triggered, leaving the bacteria free to infect nearby macrophages in the process of spreading. In addition, some MAC species will have apoptotic bodies and are released in healthy macrophages following apoptotic body ingestion. Because autophagy precedes apoptosis, it was established that heat killed MAC, and viable MAC induces autophagy in macrophages at similar rates, but MAC still survives.
Conclusion
MAC spreading from cell-to-cell is triggered by the macrophage’s attempt to kill the bacterium, undergoing apoptosis.
Keywords: Mycobacterium avium, macrophages, apoptosis, spread, escape
1. Introduction
Organisms of the Mycobacterium avium complex (MAC) are opportunistic pathogens causing disease in individuals with immunosuppression, chronic lung conditions, such as emphysema and bronchiectasis, as well as healthy populations [1]. The bacteria is thought to be primarily acquired via both the GI and respiratory tracts. After crossing the mucosal barrier, MAC is taken up by and replicate in macrophages. MAC survives within vacuoles that do not acidify or fuse with lysosomes [2–6]. MAC-infected macrophages undergo apoptosis three to seven days after infection [7], as an innate defense mechanism [8]. Macrophages employ several different strategies to eliminate intracellular pathogens, among those, the production of toxic products such as superoxide anion, nitric oxide, and antimicrobial peptides [9]. More recently, it has become evident that autophagy and apoptosis are components of the killing mechanism of the host. Pathogens, however, have evolved strategies to overcome the host killing; and Salmonella typhimurium [10], Shigella flexneri [11] and Yersinia pseudotuberculosis [12], for instance, have developed ways to survive apoptosis.
Elucidating the mechanism(s) by which MAC spreads is critical for understanding disease. Previous work has suggested that plasmin may be involved during early dissemination of MAC from the lung to other organs [13]; whereas, neutrophils [14] and CD4 + T cells reduce the degree of dissemination in mice [15, 16]. In Mycobacterium tuberculosis, genes in the region of difference-1 (RD1), such as esat-6, cfp-10, [17], as well as other genes such as hbha [18] appear to play a role in bacterial dissemination. MAC, in contrast, does not contain genes of close sequence similarity to genes in the RD1 region and MAC’s use of hbha has not been characterized in terms of dissemination [19]. This leaves much unknown as to how MAC moves from one macrophage to another. Previous studies have shown that MAC, passed through macrophages, became more efficient at entering fresh macrophages by using mechanisms independent of complement receptor 3 to be internalized [20]. Once inside of the “second” macrophage, MAC resides in vacuoles that differ from MAC vacuoles in the primary macrophage [21, 22]. We assume that this increased invasive phenotype is the predominant phenotype state of MAC within the host, and associated with the spreading of the bacteria. Another innate immunity process macrophages undergo to help control infection is autophagy. In fact, autophagy of macrophages infected by M. tuberculosis results in diminishing bacterial survival, and M. tuberculosis has developed mechanisms to suppress it [23]. Recent literature suggests a close relationship between autophagy and apoptosis, and in the case of intracellular pathogens, the attempt to kill the pathogen by autophagy precedes apoptosis [4, 24]. To date, the role of autophagy of macrophages on MAC infection has not been elucidated. In the current study, we demonstrate that MAC-infected macrophages undergo autophagy and that MAC is able to survive autophagic macrophages, as well as being capable of escaping macrophages upon induction of apoptosis.
2. Results
2.1 Course of M. avium infection of macrophages
Primary macrophages, as well as mononuclear phagocyte cell lines infected with MAC in culture, undergo apoptosis after 4 – 6 days [21, 22], and MAC escaping apoptotic macrophages infects surrounding phagocytes [21, 22].
To determine whether MAC of IG phenotype (obtained from monocyte-derived macrophages) was comparable to MAC of EG phenotype (obtained from broth) regarding the ability to grow inside fresh macrophage monolayers, we infected fresh monolayers with both EG and IG strains and determined viability over time. EG MAC increased in viability from 83% the first day after infection to 89% on the seventh day after infection. Similarly, the population of bacteria that had previously been exposed to monocyte-derived macrophages (IG) increased in viability from 76% the first day after infection to 96% the seventh day, demonstrating that both EG and IG were able to survive and have robust growth in monocyte-derived macrophages. The viability of the IG strain was decreased at time 0, when compared with the viability of EG strain, because some of the intracellular bacteria (IG) lose viability upon macrophage apoptosis.
2.2 MAC infection outcome
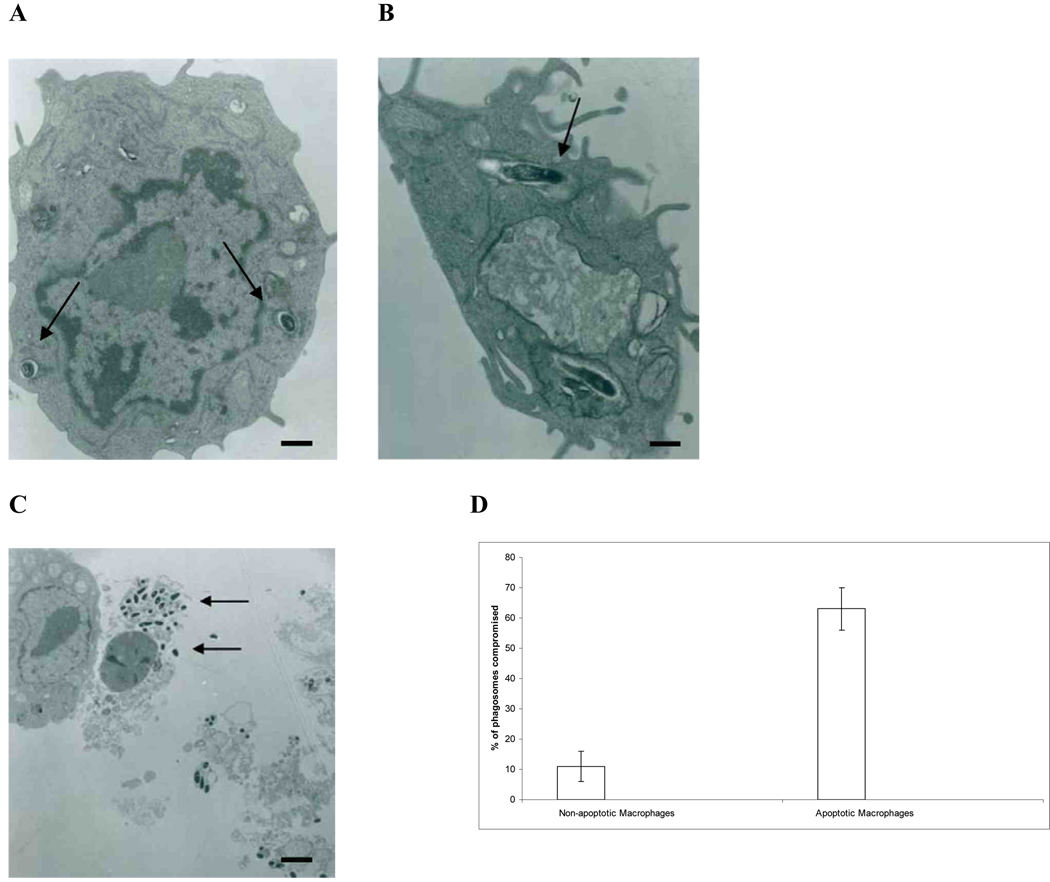
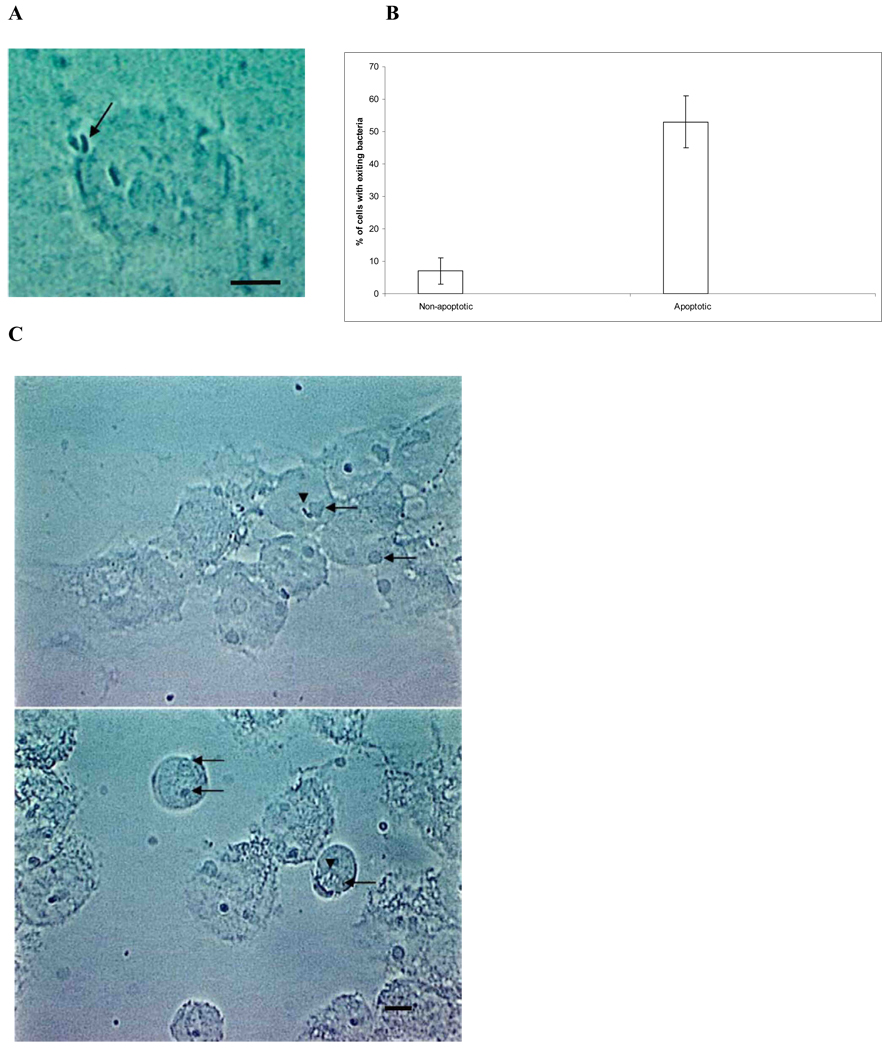
To identify the outcome of MAC once monocyte-derived macrophages undergo apoptosis, human monocyte-derived macrophages were infected with MAC and TEMs were prepared of both infected non-apoptotic and apoptotic macrophages. Fig. 1A is a representative picture that shows that the vacuolar membrane surrounding MAC in non-apoptotic monocyte-derived macrophages remained intact, while in apoptotic monocyte-derived macrophages, the vacuole membrane was compromised (Fig. 1B). In 300 cells examined, 11 ± 5% of adherent, infected cells had the MAC vacuole membrane compromised, while 63 ± 7% of the detached, apoptotic cells showed loss of MAC vacuole membrane. These micrographs also showed that MAC escapes the apoptotic human monocyte-derived macrophage to the extra-cellular space, likely following necrosis of the macrophages (Fig. 1C). The exit from apoptotic human monocyte-derived macrophages was further confirmed by video microscopy (Fig. 2A and 2B). The video also evidenced that apoptotic monocyte-derived macrophages are taken up by uninfected macrophages. The fresh “second macrophages” ingested MAC-infected apoptotic bodies, becoming infected with MAC (Fig. 2C). It was also observed that intracellular bacteria in apoptotic macrophages appear to encounter the cytoplasm membrane in a randomized fashion by moving in Brownian movement. The encounters become more frequent once macrophages turned into apoptotic bodies. These data indicated that MAC is capable of leaving both the vacuole and macrophage after induction of apoptosis, but the escape apparently depends on the randomized encounter with the macrophage cytoplasmic membrane. Since sometimes MAC escape does not occur before apoptotic macrophages are ingested by fresh macrophages, it was paramount to determine the outcome of the infection in those cases. It was seen that MAC inside apoptotic bodies and ingested by a secondary macrophage could still leave the apoptotic cell and reside inside of a vacuole compartment (Fig. 2C). In some cells, when the bacterium did not leave the macrophage, MAC was seen co-inhabiting the vacuolar space with an apoptotic body (Fig. 2C). Comparable data were obtained using THP-1 macrophages (data not shown).
Fig. 1.
Electron microscopy images of MAC in apoptotic macrophages. (A) TEM of MAC-infected macrophages that are not apoptotic. Arrows indicate membrane bound vacuoles containing bacteria. Notice that the vacuolar membrane appears to be intact. 10,500 × magnification (bar = 5 µm). (B) TEM of MAC in an apoptotic macrophage. Arrow indicates an area where the integrity of the vacuole membrane appears to have host integrity. 12,500 × magnification (bar = 2 µm). (C) TEM of MAC-infected apoptotic macrophages being ingested by healthy macrophages (bar = 100 µm). Arrows indicate bacteria escaping the apoptotic macrophage. 2,500 × magnification. (D) Quantification of the assay based on 200 cells.
Fig. 2.
Video microscopy images of MAC in apoptotic macrophages. (A) Representative image from video microscopy, showing MAC leaving the apoptotic macrophages (arrow), while one other organism remains intracellularly (bar = 5 µm). (B) Quantification if MAC leaving apoptotic macrophages based on 200 cells. (C) Four days after the apoptotic bodies (arrows) were added to a new macrophage monolayer, MAC (arrowheads) was observed inside fresh macrophages (bar = 100 µm). A large number of apoptotic bodies are also seen, including some occupying the same vacuole with free MAC.
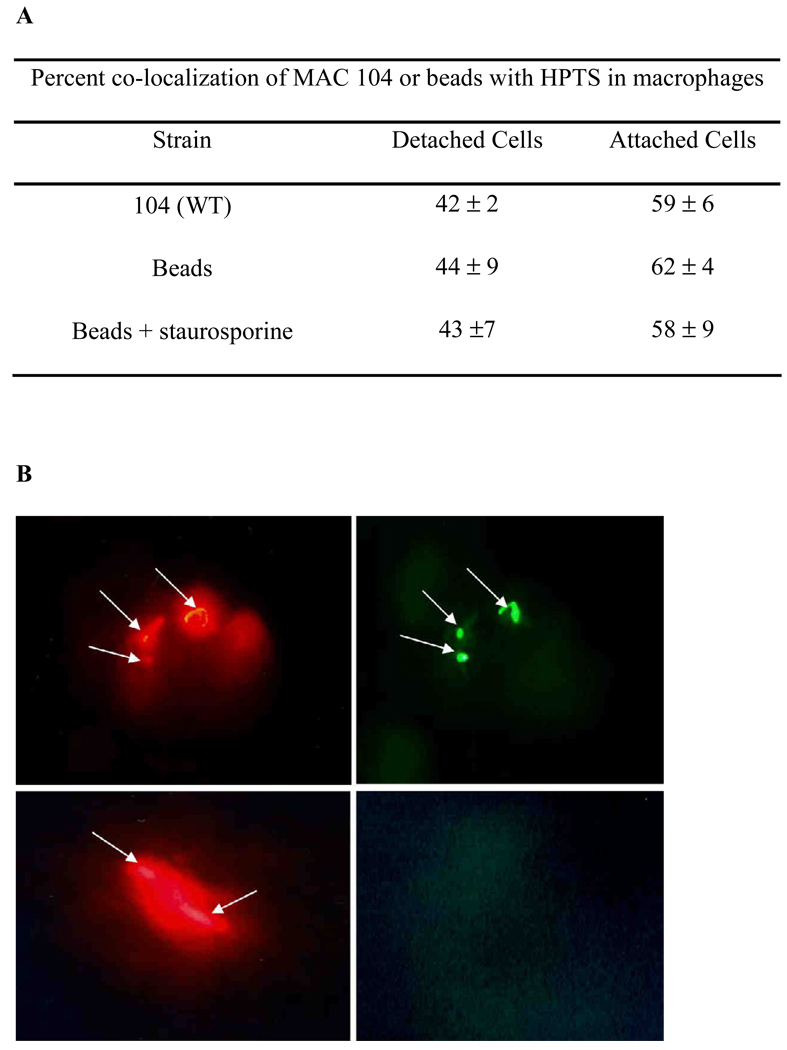
Since it would require that the vacuole membrane lose integrity upon macrophage apoptosis, we examined whether MAC actively lyses the vacuole membrane of apoptotic cells in response to apoptosis. As shown in Fig. 3, we observed that at four days post-infection 59% of the MAC co-localized with HPTS in attached cells (non-apoptotic), while 42% of MAC co-localized with HPTS in detached cells (apoptotic), supporting the observation of loss of vacuolar membrane integrity. While HPTS can lose the fluorescein in presence of acidic conditions, MAC phagosome is not acidic, and it was not seen in the reported assays (data not shown). It was also determined that the vacuolar membrane may lose integrity as an effect of apoptosis. The percent co-localization, 44% in attached cells and 62% in detached cells, was similar between latex-beads and MAC-infected macrophages, suggesting that the compromised vacuolar membrane is not specific to MAC.
Fig. 3.
Fluorescent microscopy of MAC and HPTS in macrophages. Four days after Raw 264.7 macrophages were infected by the wild-type Texas Red-stained MAC in the presence of HPTS, attached and detached cells were, separately, collected and analyzed for co-localization on a fluorescent microscope. Values are from 3 repetitions of the assay. Examples are shown in figures: A and B show bacteria (red) and green (HPTS in the vacuole), indicating intact vacuole membrane. C & D show bacteria (red) and no vacuole (green), indicating that the vacuole is leaking.
2.3 Apoptosis
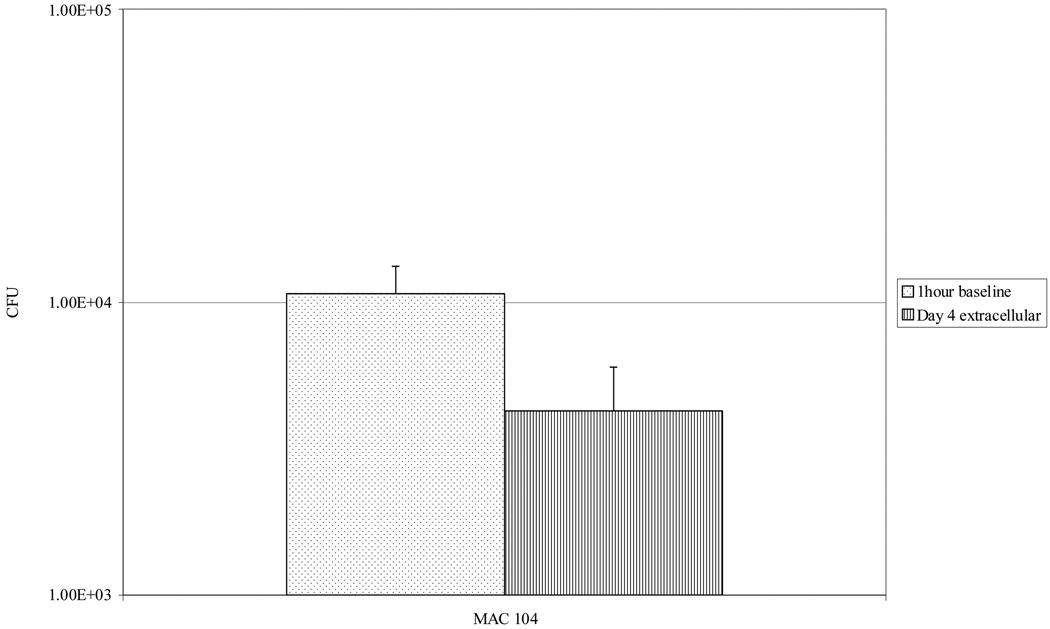
Seventeen percent of the attached macrophages and 98 percent of the detached monocyte-derived macrophages, THP-1 and Raw cells stained positive, indicating that apoptosis is a common effect of MAC infection. Similar results were obtained with Raw 264.7 cells (19 ± 6 of attached and 97 ± 1 of detached cells). We also quantified MAC escaping from THP-1 macrophages by collecting extracellular bacteria after 4 days of infection. Viable bacteria were consistently recovered from the extra-cellular environment (Fig. 4).
Fig. 4.
Amount of extra-cellular MAC 4 days post-infection of macrophages. Raw 264.7 cells were infected by MAC 104, and the number of intracellular bacteria was determined 1 h later by plating for CFU. Four days after infection, the number of extra-cellular bacteria was determined by collecting the supernatant, separating cellular debris by differential centrifugation, then plating for CFU. Bars represent mean and standard deviation values from three experiments.
2.4 Fate of bacteria within apoptotic macrophages
When adding apoptotic THP-1 macrophages within viable intracellular bacteria to fresh macrophages, it was noticed that a percentage of the bacteria population was killed following ingestion, but the survivors replicated after 2 days. In contrast, monolayers infected with free bacteria showed a continuous increase of intracellular bacteria (Table 1). To determine whether MAC viability changes when macrophage apoptosis is inhibited by using a pan-caspase inhibitor MAC-infected monolayers were treated with Z-VAD-FMK (R & D Systems, Inc, MN) and macrophage viability and MAC colony forming units (CFU) determined. Table 2 shows that, when apoptosis is inhibited, MAC survival increases.
Table 1.
Fate of MAC associated with apoptotic THP-1 macrophages following ingestion by fresh macrophages.
| Experimental groups | % macrophages with apoptotic cells a 103/104 | # viable bacteria 2 h b | # viable bacteria day 2 b | # viable bacteria day 5 b |
|---|---|---|---|---|
| Uninfected control | 0/0 | 0 | 0 | 0 |
| MAC 101-infected Mø | 43% / 61% | 2.8 ± 0.4 × 104 | 7.6 ± 0.4 × 103 | 5.1 ± 0.4 × 104 |
| MAC 104-infected Mø | 46% / 67% | 3.5 ± 0.6 × 104 | 9.8 ± 0.2 × 103 | 8.4 ± 0.6 × 104 |
| MAC 101 control (MOI 1) | 0 | 6.5 ± 0.5 × 104 | 8.9 ± 0.3 × 104 | 7.3 ± 0.3 × 105 |
| MAC 104 control (MOI 1) | 0 | 6.1 ± 0.6 × 104 | 8.2 ± 0.6 × 104 | 9.1 ± 0.4 × 105 |
Fresh macrophage monolayers were infected with either 103 or 104 apoptotic macrophages.
1 × 105 fresh macrophages were infected with 104 apoptotic macrophages.
Table 2.
Survival of intracellular MAC following apoptosis of Raw 264.7 macrophages.
| Number of bacteria in apoptotic cells (Day 0) a | Days after apoptosis (% viable organisms) b | ||
|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |
| 3.6 ± 0.7 × 104 (apoptotic) c | 71 ± 4 | 63 ± 7 | 61 ± 5 |
| 5.1 ± 0.4 × 105 (non-apoptotic) c | 92 ± 3 | 120 ± 11 | 132 ± 8 |
| 4.7 ± 0.5 × 105 (treated with Z-VAD-FMK) d | 90 ± 4 | 109 ± 8 | 125 ± 6 |
Day 0 is 5 days after infection of monolayer
Mean ± SD of 4 experiments. The percent is calculated based on the number of CFU at Day 0.
Apoptotic cells were detached cells. Non-apoptotic cells were attached cells.
Z-VAD-FMK was used at the concentration of 5 µM before and every two days following infection.
2.5 Autophagy
Since MAC has mechanisms to overcome host cell apoptosis and autophagy, we hypothesized that if MAC-infected Raw 264.7 macrophages induce autophagy, MAC should be able to survive. Raw 264.7 cells were chosen for the experiments because it was easier to work with those cells among all macrophages. One and 2 days after infection, the percent of MAC 104-infected and heat-killed MAC 104-infected cells macrophages undergoing autophagy was not significantly different from uninfected Raw 264.7 macrophages. Four days post-infection, however, 4.6% of uninfected macrophages, while 8.1% of the MAC 104-infected, and 11.9% of the heat-killed MAC 104-infected Raw 264.7 macrophages, were positive for autophagy (p < 0.05 comparing uninfected macrophages exposed to both live- and heat-killed MAC 104).
To determine whether macrophages undergoing autophagy were the macrophages were infected, Raw 264.7 macrophages were exposed to rhodamine-labeled MAC 104, and 4 days later, extra-cellular bacteria were killed with antibiotic treatment and then cells were stained for LC3. Of the cells that were infected by MAC, 21.4% showed a pattern compatible to autophagy, compared with 4.6 ± 0.4% of uninfected macrophages (p < 0.05). These data indicate that MAC-infected macrophages frequently undergo autophagy (Table 2).
To examine whether MAC survives to autophagy, macrophages were treated with rapamycin and the bacterial load determined after 48 h. Ninety-two percent of the inoculum survived inside macrophages treated with rapamycin, compared to 94% in untreated control macrophages.
3. Discussion and conclusion
Macrophages infected with MAC undergo apoptosis, in contrast to macrophages infected with M. tuberculosis [25, 26]. Apoptosis, therefore, is an effective host innate immunity mechanism to control the progression of the infection [8]. MAC, however, has evolved strategies to overcome host killing. We report that MAC can survive in apoptotic macrophages, and many of the organisms escape the dying cells and infect other, adjacent, macrophages.
Fratazzi and colleagues found that 90% of MAC from macrophages that are undergoing apoptosis lose viability [8]. In contrast, Pais and colleagues determined that MAC viability was unchanged upon treatment of macrophages with staurosporine, an inducer of apoptosis [27]. The data shown in the present study indicate that some MAC can survive the apoptotic process and then escape apoptotic bodies.
Escape from the phagocyte requires that the bacterium lyse the vacuole and the cytoplasm membrane. Listeria monocytogenes is the classical example of a bacterium that actively escapes the phagosome [28]. We had hypothesized that MAC would rupture the vacuole membrane by an active mechanism; however, our results suggest that, upon induction of apoptosis, the vacuole membrane loses integrity and MAC is delivered to the cytoplasm. Microscopic observation indicates that the encounter between the bacterium and the macrophage’s cytoplasmic membrane was randomized and became more frequent when apoptotic bodies were formed. As far as we can tell from video observation, MAC depends on Brownian movement to encounter the macrophage cytoplasmic membrane. How MAC lyses the macrophage membrane is unknown. The genomic RD1 region appears to have a role in cell-to-cell spread of M. tuberculosis [17, 29]. However, MAC lacks genes of close sequence similarity to genes in the M. tuberculosis region. Mycobacterium marinum can also escape the phagosome and likely uses actin tails to spread from host cells [30], suggesting that MAC uses a different mechanism of dissemination from both M. marinum and M. tuberculosis. A recent report by Hagedorn and colleagues confirmed our observations [31] that MAC does not exit phagocytic cells in a similar fashion to M. marinum and M. tuberculosis. In fact, MAC has been shown to disrupt actin fibers [32] and, therefore, tail formation in cytosol would be difficult to happen. Recent work in the laboratory has identified MAC mutants that cannot escape apoptotic macrophages and disseminate (data shown). Further study of these mutants may shed some light on the mechanism of escape.
It would not be unusual for MAC to contain genetic baggage that allows it to survive under diverse conditions for short periods of time. Alternatively, MAC within apoptotic bodies is also able to cause infection to secondary macrophages ingesting the apoptotic body without exposure to the extra-cellular space. The relative occurrence of these three modes of spreading is not currently known, and further research would shed light onto the most predominant mechanism of dissemination used by MAC. Our results showed that once MAC associated with apoptotic macrophages is taken up by fresh macrophages, a percentage of MAC organisms are killed, while the remaining viable bacteria replicate intracellularly.
The virulent M. tuberculosis strain H37Rv induces less macrophage apoptosis than the attenuated H37Ra strain, suggesting an apoptosis block by virulent M. tuberculosis [33], nonetheless, apoptosis still occurs upon infection by either strain. Hayashi and colleagues [34] found that MAC sonicate induced apoptosis in both human monocyte derived and THP-1 macrophages. Similarly, we found murine Raw 264.7 and human THP-1 macrophages to have similar amounts of apoptosis triggered by live MAC. However, without side by side comparison of intact and lysed MAC or virulent and avirulent MAC strains, we are unable to say if apoptosis is actively induced or inhibited by live and virulent MAC compared to heat-killed or avirulent MAC.
In macrophages infected by M. tuberculosis, autophagy results in double-membrane phagosomes that mature into phagolysosomes and inhibit bacterial survival [23]. Similarly, we found a reduction in the ability of MAC to survive once macrophages become autophagic, although a fraction of the intracellular bacteria is still capable of surviving. The observation by de Chastellier and Thilo [5] indicates that MAC in cholesterol-deprived macrophages is located in double-membrane bound vacuoles, but upon the addition of cholesterol, MAC was again located in typical vacuoles, suggesting that some MAC strains survive autophagy in macrophages. Since MAC is known to live within environmental amoeba [35], and amoeba has been shown to undergo autophagy [36], it would be of value to determine if MAC survives amoebic autophagy, and if the MAC survival mechanism in macrophages evolved due to the bacterial environmental lifestyle.
Based on the evidence gathered in this study, we propose a model for how MAC spreads from macrophage to macrophage. In this model, bacteria are phagocytosed into the macrophage, and apoptosis is induced a few days later. Upon apoptosis, some bacteria are killed, while other bacteria escape the apoptotic bodies to the extra-cellular space, where they can be efficiently phagocytosed by another macrophage and the cycle likely repeats. Other bacteria, however, stay in the apoptotic bodies, and once those are taken up by new macrophages, some bacteria effectively infect the new macrophage, while others are killed. One of the main questions in the interaction between MAC and macrophages regards if MAC tries to kill the host macrophages, inducing apoptosis, or is it the host cell that tries to eliminate the pathogen. So far, no definitions exist to this question.
Future studies will dissect the molecular mechanisms of apoptosis and autophagy in macrophages and how MAC deals with them.
4. Materials and methods
4.1 Bacteria
MAC strains 101 and 104 were isolated from the blood of AIDS patients and are virulent in mice [37, 38]. For extra-cellular grown bacteria (EG), MAC from frozen stocks was cultured on 7H10 Middlebrook agar supplemented with oleic acid, albumin, dextrose and catalase (OADC) (Hardy Diagnostics, Santa Maria CA, USA) for 10 days. Intracellular grown bacteria (IG) were obtained by infecting human monocyte-derived macrophage monolayers for 4 days as previously reported [21].
4.2 Macrophages and Viability
Three kinds of macrophages were used in the studies. Human monocyte-derived macrophages were obtained as previously reported [39]. Approximately 99% of the cells matured to macrophages (data not shown [39]). Collected mononuclear fraction was washed and counted to establish a suspension of 5 × 105 cells/mL. Viability was determined as previously described [39]. Serum (FBS) used has been previously screened to support cell viability. THP-1 (ATCC, Manassas VA, USA) phagocytes were cultured in RPMI-1640 medium (Gibco, Carlsbad CA, USA) supplemented with 10% heat inactivated fetal bovine serum (FBS, Gemini, Woodland CA, USA). THP-1 cells were seeded in tissue culture plates and treated with phorbol-ester (PMA 5 µM) for 4 h to induce maturation. The monolayers were then washed and RPMI-1640 medium replenished. The monolayers were then used after 24 h. Murine Raw 264.7 macrophages were obtained from ATCC (Manassas VA, USA) and cultured in Dulbecco's Modified Eagle Medium (DMEM, Gibco, Carlsbad CA, USA) supplemented with 10% FBS. The number of cells was adjusted to 1 × 106 cells/0.2 mL.
Macrophage monolayers were infected with 5 × 106 MAC 101 for 1 h then extra-cellular bacteria were removed by washing with Hank’s balanced salt solution (HBSS, Gibco, Carlsbad CA, USA), and the cell culture media was replenished. Monolayers were lysed with sterile water for 30 min and the lysate plated onto 7H10 agar plates for 10 days to obtain the number of CFU. The percent viability for intracellular MAC was determined by lysing attached macrophages, as well as apoptotic (floating) macrophages, and performing the Live-Dead assay, in three hundred bacteria per sample (Molecular Probes, Eugene OR, USA). The methods have been published [21]. Apoptotic macrophages were collected from the tissue culture well 5 days after infection, as described [21, 22, 40].
4.3 Video Microscopy
Human macrophage monolayers established on Lab-Tek chamber slides (Nunc, Inc., Naperville IL, USA) were infected with MAC 101 at a MOI of 10. Four and 5 days after infection, the chambers were prepared for time-lapse video microscopy on a Nikon microscope, and the video images were collected with an Optronics DEI-750 camera. The experiment was repeated at least four times and at least 50 infected macrophages/events were observed each time.
4.4 Electron Microscopy
Uninfected human macrophages, MAC 101-infected but non-apoptotic macrophages, infected apoptotic macrophages, and macrophages apoptotic/bodies were fixed in 2% gluteraldehyde, 1% osmium tetroxide overnight and post-fixed with 0.5% uranyl acetate overnight at 4°C as previously reported [21]. Transmission electron microscopy (TEM) was then performed as reported [21].
4.5 Apoptotic Macrophages
MAC 104 was used to infect fresh THP-1 monolayers in Lab-Tek slide chambers at a 10 MOI. The percent apoptosis of both the detached and attached phagocytes was then quantified, 4 days post-infection using the TUNEL assay (Molecular Probes, Eugene OR, USA). Three hundred cells were evaluated under a fluorescent microscope using the FITC filter. Apoptotic macrophages were also confirmed by flow cytometry and microscopy, after labeling the macrophages with FITC-labeled anti-annexin V antibody (BD Pharmingen, CA). Inhibition of apoptosis was achieved by 5 µM of Z-VAD-FMK (R & D Systems, Inc, MN).
4.6 Supernatant Assay
MAC 104 was grown in Middlebrook 7H9 broth supplemented with OADC for 3–5 days, then used to infect Raw 264.7 macrophages in a 24-well plate at an MOI of 10 for 1 h. Extra-cellular bacteria was washed with HBSS and then fresh media was added to the wells. Four days later, the supernatant was removed, centrifuged at 500 × g for 5 min in a microcentrifuge and an aliquot of the supernatant was plated on 7H10 agar to determine CFU.
4.7 Fluorescent microscopy
MAC 104, grown for 3–5 days in 7H9 broth, was diluted in HBSS to approximately 6 × 108 bacteria/mL. Wheat germ agglutinin, Texas Red-X (Molecular Probes) at 0.15 mg/mL was added to this bacterial culture, and allowed to stain the bacteria for 2 h in the dark at room temperature. Bacteria were then washed three times in HBSS and FBS was added to the labeled bacteria for 30 min at room temperature in the dark. After HBSS washing, the MAC was used to infect Raw 264.7 cell monolayers in chamber slides at an MOI of 10 for 1 h in media containing 5 mM 8-hydroxypyrene-1,3,6-trisulfonic acid, trisodium salt (HPTS) (Molecular Probes). HPTS is a membrane-impermeable dye that concentrates in slightly acidic vacuoles of mycobacteria and other pathogens [41]. The excess HPTS and bacteria were washed away with HBSS, and the media without HPTS was added to the cell culture. Four days later, any extra-cellular bacterium was killed by adding 200 µg/mL amikacin for 2 h at 37°C. The macrophages that remained attached to the plastic, as well as the detached macrophages, were, separately, rinsed three times in HBSS then fixed in 4% paraformaldehyde for 30 min at room temperature. After three more washes in HBSS, the detached and attached cells were separately analyzed to determine the percent of bacteria that co-localize with HPTS (which means that bacteria are still inside intact vacuoles) on a Leica fluorescent microscope. As a control, fluorescent red polystyrene microspheres (0.1 µM, Molecular Probes) were diluted in HBSS, opsonized as described above then passed through a 23-gauge needle before being inoculated to macrophages. Three days later, staurosporine (0.5 µM) was added to half the wells containing beads to induce apoptosis and detachment for 20 h.
4.8 Uptake of apoptotic macrophages
To examine the fate of intracellular bacteria in apoptotic macrophages when the macrophages are ingested by a monolayer of fresh macrophages, THP-1 cells were infected at MOI of 10, and at day 4, detached cells (apoptotic) were collected, counted, and the CFU of viable intracellular bacteria determined. Then 103 or 104 apoptotic macrophages were added to monolayers (1 × 105 THP-1 cells) and allowed to stay for 2 h, after which, monolayers were washed to remove the non-attached macrophages. Monolayers were lysed at 2 h, 48 h and 5 days following the addition of apoptotic cells, and the lysate was plated to quantify the viable bacteria.
In some assays, the percentage of fresh macrophages with apoptotic bodies was determined at 2 h, by microscopic counting. As controls, fresh monolayers were infected with MAC 101 and MAC 104 at MOI of 1 and some monolayers were lysed at 2 h, 48 h and 5 days, for quantitation of viable intracellular bacteria.
4.9 Autophagy
Raw 264.7 cells in chamber slides were infected with MAC 104 or heat-killed (70°C for 1 h) MAC 104 at an MOI of 100. One day and 4 days later, the cells were fixed in 2% paraformaldehyde (Sigma) for 30 min then permeabilized with 0.1% triton X-100 (J.T. Baker) for 5 min. Slides were prepared for immunoflourescence using 3% bovine serum albumin as a blocking agent (Sigma), the MAPLC3 primary antibody (Santa Cruz), and a FITC-conjugated secondary antibody (Santa Cruz). For the positive control, autophagy was induced by 20 µg/mL rapamycin (Calbiochem, San Diego CA, USA) for 7 h, then replenishing the cell culture media overnight before fixing. At least 300 cells were counted per treatment group each time, with cells that had LC3 recruitment to the autophagosome counting as positive. In other experiments, MAC 104 was stained with 100 µg/mL 5-(and-6)-carboxytetramethylrhodamine, succinimidyl ester (rhodamine) (Molecular Probes, Eugene OR, USA) for 1 h in the dark, washed three times, then used to infected Raw 264.7 cells in chamber slides for 1 h at an MOI of ~100. Four days later, the extra-cellular bacteria were killed with 200 µg/mL amikacin (Sigma) for 2 h then the Raw 264.7 cells were treated for LC3 visualization as described above. MAC-infected Raw 264.7 cells were also treated with rapamycin, as above. Control monolayers were left untreated. The extra-cellular bacteria were then killed in half of the samples by adding 200 µg/mL amikacin (Sigma) for 2 h, and washing three times with HBSS. Raw 264.7 cells were lysed with water as before, and plated onto 7H10 and incubated for 7–10 days at 37°C to determine CFU.
4.10 Statistical Analysis
The assays were repeated at least three times. The study samples were compared to the control using the two-sided Student’s t-test at a level of significance (P value) of <0.05.
Table 3.
Percentage of autophagic macrophages indicated by the recruitment of LC3 to vacuoles in Raw.
| Days post-infection | Rapamycin-treated | Uninfected | Heat-Killed MAC 104 | MAC 104 |
|---|---|---|---|---|
| 1 | 84 ± 1.1 | 6.6 ± 4.1 | No Data | 5.4 ± 1.4 |
| 2 | 91 ± 2.4 | 4.8 ± 1.3 | 4.5 ± 0.1 | 4.3 ± 0.2 |
| 4 | 93 ± 4.5 | 4.6 ± 0.4 | 12 ± 0.8 a | 8.1 ± 1.2 a |
p value < 0.05 for the comparison between days 2 and 4.
Acknowledgements
We thank the Daniel D. Rockey Laboratory for use of their fluorescence microscope, and Denny Weber for manuscript preparation. This work was supported by the NIH grant # AI043199.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship
Julie Early performed some of the work, wrote the paper.
Kay Fischer performed the EM.
Luiz Bermudez designed the studies, performed some experiments, participated in the assembly of the paper, and is senior author.
REFERENCES
- 1.Berks BC. A common export pathway for proteins binding complex redox cofactors? Mol Microbiol. 1996;22:393–404. doi: 10.1046/j.1365-2958.1996.00114.x. [DOI] [PubMed] [Google Scholar]
- 2.Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 3.Via LE, Deretic D, Ulmer RJ, Hibler NS, Huber LA, Deretic V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem. 1997;272:13326–13331. doi: 10.1074/jbc.272.20.13326. [DOI] [PubMed] [Google Scholar]
- 4.de Chastellier C, Thilo L. Pathogenic Mycobacterium avium remodels the phagosome membrane in macrophages within days after infection. Eur J Cell Biol. 2002;81:17–25. doi: 10.1078/0171-9335-00220. [DOI] [PubMed] [Google Scholar]
- 5.de Chastellier C, Thilo L. Cholesterol depletion in Mycobacterium avium-infected macrophages overcomes the block in phagosome maturation and leads to the reversible sequestration of viable mycobacteria in phagolysosome-derived autophagic vacuoles. Cell Microbiol. 2006;8:242–256. doi: 10.1111/j.1462-5822.2005.00617.x. [DOI] [PubMed] [Google Scholar]
- 6.Stewart GR, Patel J, Robertson BD, Rae A, Young DB. Mycobacterial mutants with defective control of phagosomal acidification. PLoS Pathog. 2005;1:269–278. doi: 10.1371/journal.ppat.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gan H, Newman GW, Remold HG. Plasminogen activator inhibitor type 2 prevents programmed cell death of human macrophages infected with Mycobacterium avium, serovar 4. J Immunol. 1995;155:1304–1315. [PubMed] [Google Scholar]
- 8.Fratazzi C, Arbeit RD, Carini C, Remold HG. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J Immunol. 1997;158:4320–4327. [PubMed] [Google Scholar]
- 9.Caldelari I, Mann S, Crooks C, Palmer T. The Tat pathway of the plant pathogen Pseudomonas syringae is required for optimal virulence. Mol Plant Microbe Interact. 2006;19:200–212. doi: 10.1094/MPMI-19-0200. [DOI] [PubMed] [Google Scholar]
- 10.Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci U S A. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 12.Monack DM, Mecsas J, Bouley D, Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J Exp Med. 1998;188:2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato J, Schorey J, Ploplis VA, Haalboom E, Krahule L, Castellino FJ. The fibrinolytic system in dissemination and matrix protein deposition during a mycobacterium infection. Am J Pathol. 2003;163:517–531. doi: 10.1016/S0002-9440(10)63680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appelberg R, Castro AG, Gomes S, Pedrosa J, Silva MT. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect Immun. 1995;63:3381–3387. doi: 10.1128/iai.63.9.3381-3387.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orme IM, Furney SK, Roberts AD. Dissemination of enteric Mycobacterium avium infections in mice rendered immunodeficient by thymectomy and CD4 depletion or by prior infection with murine AIDS retroviruses. Infect Immun. 1992;60:4747–4753. doi: 10.1128/iai.60.11.4747-4753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrofsky M, Bermudez LE. CD4+ T cells but Not CD8+ or gammadelta+ lymphocytes are required for host protection against Mycobacterium avium infection and dissemination through the intestinal route. Infect Immun. 2005;73:2621–2627. doi: 10.1128/IAI.73.5.2621-2627.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao LY, Guo S, McLaughlin B, Morisaki H, Engel JN, Brown EJ. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol Microbiol. 2004;53:1677–1693. doi: 10.1111/j.1365-2958.2004.04261.x. [DOI] [PubMed] [Google Scholar]
- 18.Pethe K, Alonso S, Biet F, Delogu G, Brennan MJ, Locht C, et al. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature. 2001;412:190–194. doi: 10.1038/35084083. [DOI] [PubMed] [Google Scholar]
- 19.Mueller-Ortiz SL, Wanger AR, Norris SJ. Mycobacterial protein HbhA binds human complement component C3. Infect Immun. 2001;69:7501–7511. doi: 10.1128/IAI.69.12.7501-7511.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bronstein PA, Marrichi M, Cartinhour S, Schneider DJ, DeLisa MP. Identification of a twin-arginine translocation system in Pseudomonas syringae pv. tomato DC3000 and its contribution to pathogenicity and fitness. J Bacteriol. 2005;187:8450–8461. doi: 10.1128/JB.187.24.8450-8461.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bermudez LE, Parker A, Goodman JR. Growth within macrophages increases the efficiency of Mycobacterium avium in invading other macrophages by a complement receptor-independent pathway. Infect Immun. 1997;65:1916–1925. doi: 10.1128/iai.65.5.1916-1925.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bermudez LE, Petrofsky M, Sangari F. Intracellular phenotype of Mycobacterium avium enters macrophages primarily by a macropinocytosis-like mechanism and survives in a compartment that differs from that with extracellular phenotype. Cell Biol Int. 2004;28:411–419. doi: 10.1016/j.cellbi.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 24.De Buck E, Vranckx L, Meyen E, Maes L, Vandersmissen L, Anne J, et al. The twin-arginine translocation pathway is necessary for correct membrane insertion of the Rieske Fe/S protein in Legionella pneumophila. FEBS Lett. 2007;581:259–264. doi: 10.1016/j.febslet.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 25.DiGiuseppe Champion PA, Cox JS. Protein secretion systems in Mycobacteria. Cell Microbiol. 2007;9:1376–1384. doi: 10.1111/j.1462-5822.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- 26.DeLisa MP, Tullman D, Georgiou G. Folding quality control in the export of proteins by the bacterial twin-arginine translocation pathway. Proc Natl Acad Sci U S A. 2003;100:6115–6120. doi: 10.1073/pnas.0937838100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pais TF, Appelberg R. Induction of Mycobacterium avium growth restriction and inhibition of phagosome-endosome interactions during macrophage activation and apoptosis induction by picolinic acid plus IFNgamma. Microbiology. 2004;150:1507–1518. doi: 10.1099/mic.0.26815-0. [DOI] [PubMed] [Google Scholar]
- 28.Flesselles B, Anand NN, Remani J, Loosmore SM, Klein MH. Disruption of the mycobacterial cell entry gene of Mycobacterium bovis BCG results in a mutant that exhibits a reduced invasiveness for epithelial cells. FEMS Microbiol Lett. 1999;177:237–242. doi: 10.1111/j.1574-6968.1999.tb13738.x. [DOI] [PubMed] [Google Scholar]
- 29.Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Grotzke JE, Lewinsohn DM, et al. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;51:359–370. doi: 10.1046/j.1365-2958.2003.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stamm LM, Morisaki JH, Gao LY, Jeng RL, McDonald KL, Roth R, et al. Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. J Exp Med. 2003;198:1361–1368. doi: 10.1084/jem.20031072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker LP, Porcella SF, Wyatt RG, Small PL. The Mycobacterium marinum G13 promoter is a strong sigma 70-like promoter that is expressed in Escherichia coli and mycobacteria species. FEMS Microbiol Lett. 1999;175:79–85. doi: 10.1111/j.1574-6968.1999.tb13604.x. [DOI] [PubMed] [Google Scholar]
- 32.Guerin I, de Chastellier C. Pathogenic mycobacteria disrupt the macrophage actin filament network. Infect Immun. 2000;68:2655–2662. doi: 10.1128/iai.68.5.2655-2662.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keane J, Balcewicz-Sablinska MK, Remold HG, Chupp GL, Meek BB, Fenton MJ, et al. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi T, Catanzaro A, Rao SP. Apoptosis of human monocytes and macrophages by Mycobacterium avium sonicate. Infect Immun. 1997;65:5262–5271. doi: 10.1128/iai.65.12.5262-5271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinert M, Birkness K, White E, Fields B, Quinn F. Mycobacterium avium bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls. Appl Environ Microbiol. 1998;64:2256–2261. doi: 10.1128/aem.64.6.2256-2261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giusti C, Kosta A, Lam D, Tresse E, Luciani MF, Golstein P. Analysis of autophagic and necrotic cell death in Dictyostelium. Methods Enzymol. 2008;446:1–15. doi: 10.1016/S0076-6879(08)01601-7. [DOI] [PubMed] [Google Scholar]
- 37.Gangadharam PR, Perumal VK, Parikh K, Podapati NR, Taylor R, Farhi DC, et al. Susceptibility of beige mice to Mycobacterium avium complex infections by different routes of challenge. Am Rev Respir Dis. 1989;139:1098–1104. doi: 10.1164/ajrccm/139.5.1098. [DOI] [PubMed] [Google Scholar]
- 38.Torrelles JB, Ellis D, Osborne T, Hoefer A, Orme IM, Chatterjee D, et al. Characterization of virulence, colony morphotype and the glycopeptidolipid of Mycobacterium avium strain 104. Tuberculosis (Edinb) 2002;82:293–300. doi: 10.1054/tube.2002.0373. [DOI] [PubMed] [Google Scholar]
- 39.Bermudez LE, Young LS. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988;140:3006–3013. [PubMed] [Google Scholar]
- 40.Cirillo JD, Falkow S, Tompkins LS, Bermudez LE. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect Immun. 1997;65:3759–3767. doi: 10.1128/iai.65.9.3759-3767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beauregard KE, Lee KD, Collier RJ, Swanson JA. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J Exp Med. 1997;186:1159–1163. doi: 10.1084/jem.186.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]