Introduction
Gene therapy for brain disorders is one of the most promising frontiers in the practice of restorative neurosurgery. There are significant experimental gene therapy initiatives underway that have led to currently active clinical trials employing direct intracerebral delivery of viral vectors, and these treatments have been reported as safe and well tolerated. These studies include putaminal delivery of human aromatic L-amino acid decarboxylase (hAADC) 2, putaminal delivery of the neurotrophin neurturin 11, and glutamic acid decarboxylase gene transfer to the subthalamic nucleus for Parkinson’s Disease 7. In the future, other clinical trials will likely employ viral vectors to transfer genes that bestow upon recipient tissue a desired enzymatic or neurotrophic activity relevant to the treatment of other neurodegenerative diseases, stroke, and traumatic brain injury.
The blood–brain barrier prevents significant amounts of most systemically administered agents from reaching therapeutic parenchymal levels without producing systemic toxicity. Although traditional direct local delivery of therapeutic agents into the brain relies on diffusion, resulting in non-homogeneous distribution restricted to a few millimeters from the source, convection-enhanced delivery (CED) uses a pressure gradient established at the tip of an infusion catheter to distribute the therapeutic agent by bulk flow. This method produces an even distribution of highly concentrated agent over considerable distances, the volume of which is dependent on the structural properties of the tissue and the parameters of the infusion procedure.
Over the past decade, this laboratory has spearheaded non-clinical work in developing direct parenchymal CED of viral vectors for treating Parkinson’s disease. Multiple studies have demonstrated the efficacy of CED for distributing adeno-associated virus serotype 2 (AAV2) to specified brain regions, and an infusion cannula was designed to prevent vector infused under pressure from refluxing around the cannula. More recently, we have investigated the use of intraoperative MRI (iMRI) to guide infusions in real time, which provides the neurosurgeon with rapid feedback on the physical and anatomical diffusion parameters important for optimizing gene transfer and reducing the potential for adverse effects.
In Vivo Gene Therapy: AAV2 Vectors
There are two broad categories of gene therapy: the direct transfer of a gene into the patient’s own cells (in vivo), or the transplantation of cells genetically modified to perform a specific function (ex vivo). Clinical trials for Parkinson’s disease have used in vivo approaches, and these have shared in common the type of vector used for gene transfer: recombinant vectors derived from human adeno-associated virus serotype 2 (hAAV2). These vectors have been the most attractive of the candidate vector systems for gene transfer into neurons, because of the lack of any human disease associated with wild-type virus and the ability of AAV to transduce both dividing and nondividing cells 4. Additionally, a consistently observed property of AAV2 is the extensive trafficking of vector from the site of infusion to distal anatomic locations, such as rapid transport of vector to globus pallidus after putaminal infusion.
Two key factors affect AAV2 trafficking, particularly relevant to iMRI imaging: the vasculature and axonal projections. CED studies in recipient rodents with and without a heartbeat demonstrated that cerebral fluid circulation through the perivascular space, powered by cardiac contractions, is the primary mechanism by which both AAV2 and liposomes are spread within the brain 5. Axonal distribution may occur through anterograde or retrograde transport, or via the periaxonal space in a manner similar to perivascular transport (Varenika et al, in press). Additionally, distribution of infusate increases more rapidly in white matter, like the corona radiata, than in the putamen and other grey matter 8. Thus, monitoring and control of viral vector trafficking presents a complex clinical challenge.
In our laboratory, extensive preclinical work in primates demonstrated that human aromatic L-amino acid decarboxylase (hAADC) function, the generation of dopamine from L-dopa, is restored in the striatum following AAV2-hAADC delivery. AAV2 hAADC-treated monkeys showed increased responsiveness to L-dopa, as measured by PET, histology and dopamine levels 1. This work provided proof of principle for initiating clinical trials to deliver the hAADC gene to the human striatum in patients with moderately advanced Parkinson’s disease. In a clinical trial involving our institution (NCT00229736), CED was used to infuse the post-commissural putamen bilaterally. In the first 5 patients treated in the lowest dose cohort, PET imaging 6 months after the procedure showed a mean 30% increase in AADC activity 2. Although post-operative T2-MRI and PET data obtained from 10 patients in the phase I AAV2-hAADC trial showed good overlap between the volume of distribution suggested by T2-MRI and the increase in PET signal, the overall infusion coverage of the putamen was quite variable from patient to patient (Valles, in press). In the absence of real-time visualization by iMRI, however, the extent to which this variation reflects actual target coverage cannot be determined.
Real Time iMRI-based Imaging of CED
The multifactorial nature of AAV2 trafficking alone suggests the need for real-time assessment of vector distribution. However, when the variables of the infusion procedure itself are also considered, real-time imaging becomes essential for optimizing clinical efficacy and safety. The goal is accurate delivery of therapeutic agents into target sites while minimizing exposure of untargeted tissue. Adequate visualization should confirm target coverage as well as detect reflux, leakage, anatomic deformation, and aberrant delivery. With these factors in mind, an iMRI-based method to visualize CED in real-time was developed.
CED is an effective method for local delivery, not only of AAV, but also of liposomes that are nanoscale carriers consisting of a phospholipid membrane shell surrounding a hollow core. Because liposomes can encapsulate a broad range of therapeutic agents and other small molecules, they were used to develop a real-time MR imaging method to track infused liposomes containing Gadoteridol (GDL). MRI of liposomal GDL was found to be highly predictive in determining liposomal tissue distribution, as confirmed by histological comparison with concomitant administration of fluorescent liposomes 13.
Liposomal infusion studies demonstrate that differences in cyto-architecture between structures infused, particularly between grey matter and white matter, are an anatomical determinant of infusate distribution. For instance, corona radiata infusions clearly show distribution following white matter fibers, whereas the limited size of the putamen restricts the allowable volume of infusion in that nucleus. In addition, the real time liposomal infusion protocol has been used to identify important potential pitfalls for putaminal infusion. Anterior lateral leakage can be visualized by signal enhancement that follows the perivascular space of lateral striate arteries connecting to perivascular space of the medial cerebral artery, terminating in the Sylvian fissure and insular cortex 9. Despite this potential for leakage, the real-time monitoring technique allows us to stop the infusion at any point, thereby permitting the filling of the putamen or similar structures with some precision.
An additional, related challenge is the leakage of infusate out of targeted parenchyma into adjacent sulci and ventricles. In a retrospective non-human primate study comprised of 54 CED sessions monitored with real-time iMRI imaging, leakage occurred in approximately 20% of infusions 16. Consequently, the distribution of liposomes within the target structure ceased to increase or was significantly attenuated after the onset of leakage. Escape of the agent from the targeted area, especially into the CSF space, potentially increases the risk of adverse and unexpected clinical events. This phenomenon may be reflected in mixed results reported from some clinical trials in which CED was used to infuse therapeutic agents, in the absence of real-time visual guidance. For example, neutralizing antibodies have been reported in the CSF of some subjects after GDNF infusion into the putamen 3,14.
Strategies for real-time iMRI-based imaging of AAV distribution during CED include co-infusion of nanoscale imaging agents or labeling of the viral capsid. Lonser’s group 15 has demonstrated the co-infusion of radiolabeled AAV1 serotype capsids with ferumoxtran-10, a similarly sized (24 nm) super-paramagnetic iron oxide nanoparticle. This method was used to define clear hypo-intense regions of distribution visualized with real-time MRI, which were easily distinguishable from surrounding tissue in white and gray matter. The true volume of distribution of the viral capsid was confirmed by autoradiography, and the concentration of ferumoxtran-10 that most closely predicted AAV1 volume of distribution was determined. Similarly, recent studies in our laboratory have employed co-infusion of GDL with AAV2 vector, where GDL distribution observed with real-time MRI corresponded well with the volume AAV2 transduction, as detected by immunohistochemistry in both the corona radiata (Fig 1) and thalamus (Fig 2). These efforts are the first to demonstrate real-time imaging of viral vector infusions in primate models, a critical step in bringing this technology into clinical practice.
Figure 1.
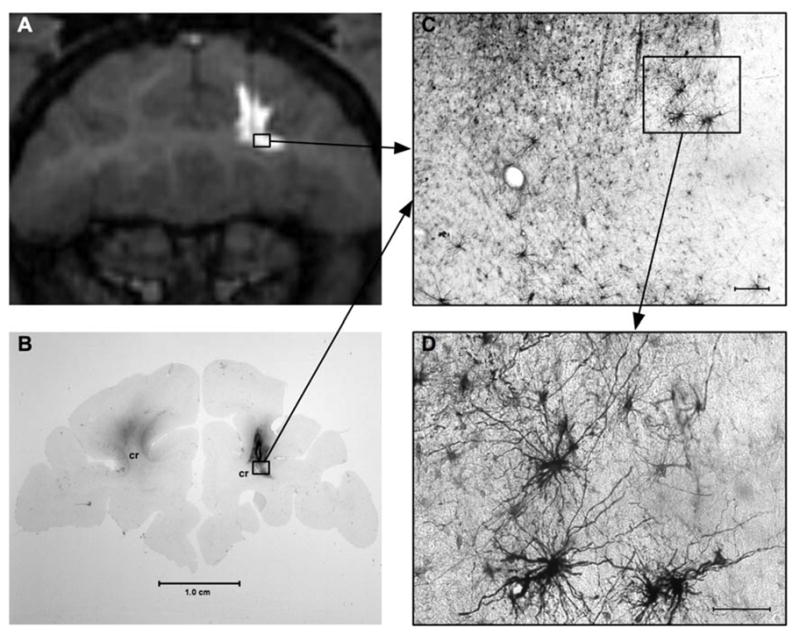
Co-infusion of GDL (GD liposomes) and AAV1-GFP vector into the corona radiata. Successful distribution is demonstrated by MRI during vector administration (A) and 5 weeks post-infusion using GFP-IR staining (B). GFP-expressing glia cells are well visualized at higher magnification (C, D).
Figure 2.
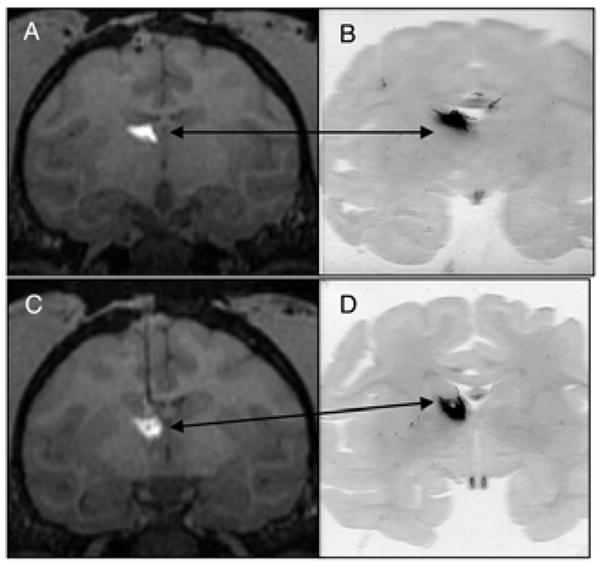
Successful targeting of thalamus using GDL/AAV2-GFP. Two coronal MRI levels of thalamus during GDL/AAV-2-GFP administration (A, C) with corresponding histological demonstration of neuronal GFP-expression in the thalamus (B, D).
Results from a double-blind, sham controlled Phase 2 trial of CERE-120 (AAV2-NTN, driving expression of human Neurturin, a neurotrophic factor, were recently released 6. This trial in 58 patients with advanced Parkinson’s disease did not employ CED for infusion of the viral vector, and did not demonstrate an appreciable difference between patients treated with AAV2-NTN versus those in the control group. Careful analysis of these data will be required to ascertain whether a variable extent of target transduction, due to manual rather than convection-enhanced delivery, may have contributed to the negative results. Eventual employment of iMRI-guided CED in all gene therapy clinical trials will be critical for separating issues related to agent delivery from those related to agent efficacy.
iMRI for Clinical Gene Therapy
Real-time iMRI imaging of CED in the human brain has thus far not been utilized in gene therapy trials, although one case has been reported for the infusion of glucocerebrosidase into the frontal lobe and brainstem of a patient with neuronopathic Gaucher disease 10. A guide cannula was implanted for the procedure, the imaging and infusion were completed outside of the operating room, and the patient was returned to the operating room for cannula removal under a single session of general anesthesia. Whereas T2 and FLAIR sequences could not distinguish infusate distribution in the initial frontal lobe infusion, when GDL was co-administered for the brainstem infusion, the infused anatomic region was clearly distinguished from the surrounding non-infused tissue. Although obtaining MR imaging without removing the patient from the operating room would be ideal, this report did validate the use of real-time imaging of CED with GDL co-infusion in humans.
At our institution, prospective stereotaxy has been used for iMRI-guided placement of DBS electrodes (Chapter 9), where surgery is performed within the MR bore. The intubated patient is immobilized within the intra-operative MR scanner and imaging is performed to define the desired trajectory, with the electrode placed through a skull-mounted trajectory guide. We envision a modification of this technique for gene therapy, by adapting the CED infusion and cannula set-up used in the AAV2-hAADC clinical trial for use with the skull-mounted trajectory guide.
Continuing Translational Research
The refinement of non-clinical studies in non-human primates remains critical for driving subsequent CED gene therapy clinical trials that employ iMRI. Primates studies have several advantages over rodent studies: greater anatomical, metabolic and electrophysiological similarity to the human brain, ability to model stereotactic and computer-assisted surgical techniques, ability to model parenchymal infusion characteristics such as perivascular transport, analogous postoperative functional imaging, and ability for long-term (years) follow-up 12. Primary problems that remain to be addressed in future studies include optimizing contrast agents for co-infusion with AAV vectors and verifying lack of neuronal damage with long-term follow up, versus developing tagging methods for direct capsid labeling that do not alter vector function or receptor binding. As AAV infusion imaging improves, further studies will better elucidate mechanisms involved in leakage or spread of infusate from the intended target.
Additional disease paradigms should also be pursued. The structural anatomy that defines AAV trafficking in one disease may be altered in others. Eventual gene therapy strategies for neuroprotection, pertinent to treating stroke and traumatic brain injury, will have to overcome major macro- and microscopic structural alterations in brain tissue that will vary with injury mechanism, location and acuity. Likewise, application of gene therapy to demyelinating diseases will have to consider the effect of demeylination on convection of the infusate through white matter tracts. Gene therapy targets such as the subventricular zone and hippocampal dentate gyrus, areas of endogenous neurogenesis that harbor neural progenitor cells with the potential for therapeutic genetic reprogramming, offer a unique challenge due to the potential for vector leakage through neighboring ependyma and into the ventricular space.
Summary and Future Clinical Application
Gene therapy for Parkinson’s disease is progressing well as an experimental therapy, due to an effective, safe vector system and a growing understanding of vector trafficking within the brain. The next major step forward will be to translate experience with iMRI for DBS placement into protocols for real-time monitoring of the CED system for AAV2 infusion. Parkinson’s disease is the disease entity with which the field has entered the era of in vivo gene therapy, and efforts have initially been targeted toward alleviation of symptoms. Continued development of iMRI-CED will allow finer resolution of infused agent and better control of target coverage, which in turn will expand the potential clinical targets for in vivo gene therapy. Reliable visualization of viral infusate or surrogate, and subsequent ability to control the volume of distribution, will be absolute requirements for applying this technology in more severely damaged brain parenchyma, as occurs in stroke, traumatic brain injury or demyelinating diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bankiewicz KS, Forsayeth J, Eberling JL, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Eberling JL, Jagust WJ, Christine CW, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70:1980. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- 3.Gill SS, Patel NK, Hotton GR, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 4.Hadaczek P, Kohutnicka M, Krauze MT, et al. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum Gene Ther. 2006;17:291. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- 5.Hadaczek P, Yamashita Y, Mirek H, et al. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther. 2006;14:69. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceregene Announces Clinical Data from Phase 2 Clinical Trial of CERE-120 for Parkinson’s Disease. [accessed December 13, 2008]; http://www.ceregene.com/press_112608.asp.
- 7.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 8.Krauze MT, McKnight TR, Yamashita Y, et al. Real-time visualization and characterization of liposomal delivery into the monkey brain by magnetic resonance imaging. Brain Res Brain Res Protoc. 2005;16:20. doi: 10.1016/j.brainresprot.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Krauze MT, Saito R, Noble C, et al. Effects of the perivascular space on convection-enhanced delivery of liposomes in primate putamen. Exp Neurol. 2005;196:104. doi: 10.1016/j.expneurol.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Lonser RR, Schiffman R, Robison RA, et al. Image-guided, direct convective delivery of glucocerebrosidase for neuronopathic Gaucher disease. Neurology. 2007;68:254. doi: 10.1212/01.wnl.0000247744.10990.e6. [DOI] [PubMed] [Google Scholar]
- 11.Marks WJ, Jr, Ostrem JL, Verhagen L, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 12.Richardson RM, Larson PS, Bankiewicz KS. Gene and cell delivery to the degenerated striatum: status of preclinical efforts in primate models. Neurosurgery. 2008;63:629. doi: 10.1227/01.NEU.0000325491.89984.CE. [DOI] [PubMed] [Google Scholar]
- 13.Saito R, Krauze MT, Bringas JR, et al. Gadolinium-loaded liposomes allow for real-time magnetic resonance imaging of convection-enhanced delivery in the primate brain. Exp Neurol. 2005;196:381. doi: 10.1016/j.expneurol.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Slevin JT, Gerhardt GA, Smith CD, et al. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102:216. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- 15.Szerlip NJ, Walbridge S, Yang L, et al. Real-time imaging of convection-enhanced delivery of viruses and virus-sized particles. J Neurosurg. 2007;107:560. doi: 10.3171/JNS-07/09/0560. [DOI] [PubMed] [Google Scholar]
- 16.Varenika V, Dickinson P, Bringas J, et al. Detection of infusate leakage in the brain using real-time imaging of convection-enhanced delivery. J Neurosurg. 2008;109:874. doi: 10.3171/JNS/2008/109/11/0874. [DOI] [PMC free article] [PubMed] [Google Scholar]


