Abstract
The traditional method of thoracoabdominal retroperitoneal approach requires dissection of diaphragm which bears potential complications such as postoperatively weakened abdominal breathing and dysfunction of diaphragm. Mini-open anterior instrumentation with diaphragm sparing is designed to minimize the damage to diaphragm and improve cosmesis. This study compared the traditional anterior instrumentation and mini-open anterior instrumentation under the hypothesis that both results in similar surgical outcomes in treating thoracolumbar scoliosis. In Group A, 38 patients with an average age of 16.5 years underwent mini-open anterior instrumentation with diaphragm sparing. The average standing coronal Cobb angle was 56.4° in Group A. Thirty-eight patients with average age of 16.7 years in Group B received traditional open approach. The preoperative average Cobb angle was 55.8° in Group B. The average correction rate of coronal curve was 78% in group A while 75% in group B. No statistical difference between the two groups in terms of coronal curve correction, sagittal profile restoration and estimated blood loss was observed. The operation time was significantly higher in Group A than that in Group B. All patients in the two groups had good healing of incisions without neurological and instrumental complications during minimal 2 year follow-up. In Groups A and B, two patients suffered from pleural effusion, respectively. The wedging of the vertebral discs distal to the lowest fused level occurred in three and four patients in Group A and B, respectively. One case in group B was found to be suspicious pseudoarthrosis without loss of correction. Mini-open anterior instrumentation with diaphragm sparing could minimize the surgical invasion as well as achieve similar clinical outcomes compared with classical anterior approach.
Keywords: Minimal invasive, Thoracolumbar, Scoliosis, Anterior instrumentation, Diaphragm
Introduction
The advantage of anterior instrumentation for scoliosis such as direct derotation and saving the fusion levels have been reported in various comparative and retrospective studies [3, 17, 18], though these advantages have been weakened with application of the direct vertebral derotation (DVD) maneuver in pedicle-screw-based construct [1, 13]. For anterior thoracolumbar corrective surgery, the traditional retroperitoneal approach, in addition to transpleural or extrapleural approach, calls for dissection of the diaphragm. This technique has the advantages of thorough exposure of the thoracolumbar spine and being more convenient for instrumentation and correction while also carries the disadvantages of longer incision and greater damage to the diaphragm [2, 15, 19, 20, 25]. The diaphragm as an important organ standing between thoracic and abdominal cavity influences pulmonary function substantially. Iatrogenic injuries of the diaphragm have been found to be responsible for declined activities of diaphragm, which can lead to the atelectasis, reduced vital capacity, and hypoxemia in postoperative patients [7]. Decrease of postoperative pulmonary function has also been observed in patients who received anterior instrumentations [9]. We hypothesized that two separate small incisions and a mini hole at the attachment of the diaphragm to the spine would allow access to and instrumentation of the thoracolumbar spine, and would benefit patients’ postoperative recovery and pulmonary function, while not sacrificing the correction effect. To our knowledge this is the first report addressing this technique and comparing the surgical outcomes with the traditional open approach. In this study, two groups with similar ages, curve magnitude and curve patterns were set up prospectively. One group utilized the anterior mini-open approach without dissecting the diaphragm while the other used the traditional open approach.
Materials and methods
General data
Between June 2002 and June 2005, 76 patients diagnosed with idiopathic thoracolumbar scoliosis were recruited in this prospective study in the author’s spinal center. The inclusion criteria were: (1) idiopathic scoliosis with Lenke 5C classification as confirmed by two experienced spine surgeons, (2) apex between T12 and L1, (3) age between 12 and 25 years, (4) Cobb angle less than 75°. Exclusion criteria were: (1) any neurological abnormality on clinical examination or MRI imaging, (2) previous spinal surgery or abdominal surgery, (3) right thoracolumbar curve.
The 76 female patients were enrolled into two subgroups according to the order of hospitalization. Based on the principle of first come first enroll, patients admitted on an odd number were assorted into group one (the mini-open and diaphragm-sparing approach) and those admitted on an even number were enrolled in the other group (the traditional open approach). The surgeries were performed by the same team. Ethical approval was obtained from the University and Hospital Research Ethics Committee. Informed consent was obtained from all study subjects and their parents or guardians before the CT investigation or anterior surgery.
Radiographic measurements
Accurate Cobb measurements of identifiable anatomic landmarks were made on coronal and lateral digital radiographs of the whole spine with the patient standing. All radiographic measurements were made by one of the authors, a senior spine surgeon independent of the operative team. Complete radiographic follow-up required standing whole spine AP and lateral radiographs consisting of preoperative, immediate postoperative (within 4 weeks), then at intervals of 6, 12, and 24 months postoperatively and at final follow-up. Curve flexibility was determined by preoperative supine side bending films. Apical translation and lower instrumented vertebra tilt were also measured on standing PA X-film. Rotation at the apex vertebra was measured by the rotational angle to sacrum (RAsac), using preoperative and postoperative CT scans [16]. Postoperative CT scanning was taken to evaluate the accuracy of vertebra screw insertion in case of suspicious screw malposition on postoperative X-ray. Another rational for postoperative CT scanning was that some patients, we found, may have suffered from asymptomatic pleural effusion after anterior spinal surgery, and CT scanning can help to make the diagnosis and take appropriate intervention timely.
Surgical techniques
Positioning
The patient was placed in a stable lateral position on the right side because all the patients had the convexity of the curve to the left side and fixed with a four-point support at the symphysis, sacrum, and scapula, as well as with arm rests. The affected vertebra was identified with the image intensifier and its projection was marked laterally on the flank.
Mini-open approach
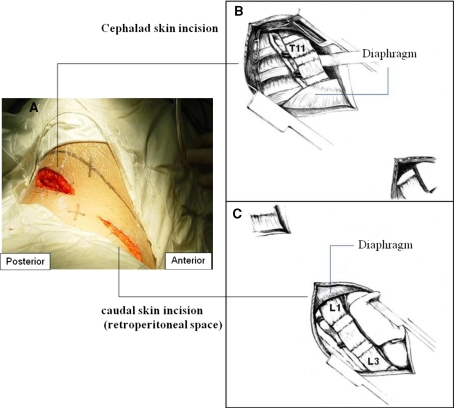
The mini-open approach included two small skin incisions, cephalad one and the caudal one, which were intermittent but still curvilinear for the sake of cosmesis. The two incisions were both made on the mid-line between the skin surface of the 10th and 11th rib, unlike the traditional open approach in which the skin incision is just on the surface of corresponding ribs.
Retroperitoneal approach was first made with a caudal skin incision from middle axillary line extended to lateral flank with a length of 7–8 cm and the distal part of 11th rib was resected at the same length and was followed by dissection of the three layers of the abdominal wall along their fibers until the retroperitoneal space is reached after penetrating the transversus abdominis fascia and muscle. The peritoneal sac was dissected off the fascia bluntly by a finger or a wet sponge mounted on a stick until the psoas muscle is reached. A special retractor was used to hold back the diaphragm to expose the retroperitoneal space and the anterolateral part of the spine. Retroperitoneal fat tissue was then exposed and mobilized from the anterior surface of the psoas insertions. The psoas muscle was dissected from the anterolateral surfaces of the convex vertebrae. A flap of periosteum and the anterior longitudinal ligament was then elevated after the ligation of segmental vessels.
Next the cephalad skin incision with 8 cm in length was made from middle to posterior axillary line and followed by the resection of the posterior part of the 10th rib with equivalent length, which allowed exposure between T10 and L2. It is necessary to meticulously peel off the parietal pleural from the diaphragm and access the thoracic spine through the extrapleural approach to minimize the disturbance to the respiratory function. If this failed, the parietal pleura was cut and the thoracic cavity entered (3 cases whose uppermost level was T10). A 2–3 cm small hole was made on the diaphragm at its attachment to the spine (diaphragmatic crura and arcuate ligaments) which was prepared for the rod insertion during curve correction (Figs. 1, 2).
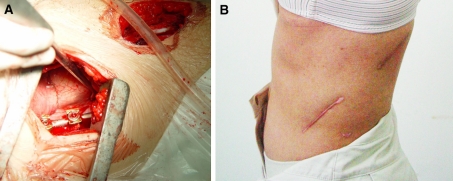
Fig. 1.
Instrumentation and correction done without dissecting diaphragm (a); post-op skin appearance of one patient with mini-open diaphragm-sparing approach (b)
Fig. 2.
Diagram of mini-open approach. a Two separate skin incision. b Cephalad skin incision and the exposure of lower thoracic spine. c Caudal skin incision and the exposure of upper lumbar spine. b and c Drawn by Zhu Yintao from Nanjing Normal University Academy of Fine Art
Conventional open approach
The standard transthoracic or extrapleural thoracoabdominal retroperitoneal approach was used for Group B, as described in literature [4, 11].
Instrumentation
All patients underwent one-stage anterior correction with one rod instrumentation (CDH Legacy, Medtronic, USA), rib graft, and titanium mesh cages (Medtronic, USA). The fusion levels were decided from the upper to the lower end vertebra. Cages were placed at every disc level below L1, and occasionally at T11–T12 and T12–L1 disc level, depending on the sagittal profile and disc space available. Disectomies performed in the thoracolumbar and lumbar regions made the spine more flexible. Titanium screws (6.5 mm in diameter and 3.0–4.0 cm in length) were introduced in both groups through a staple on the lateral surface of the vertebral body. For the vertebrae body screw from T10 to T12, the ventral excursion angle was 15°, and the entry point should shift 5–10 mm ventrally from the rib head. The accuracy of screw insertion was confirmed by AP and lateral X-ray intraoperatively. A rod is measured and contoured with gentle lordosis at the lumbar segment and straight at the T11–L1 segment. A 6.35-mm rod was used in both groups for correction.
Titanium mesh cages packed with autologous bone graft were inserted into the intervertebral disc space to restore or maintain lordosis. Correction was achieved through the derotation maneuver. For the mini-open group the diaphragm was now incised at the thoracolumbar junction for 2 cm to allow passage of the fixation rod. The contoured rod was passed through the diaphragm to fit into the receiving top loading vertebral screws. With adequate intervertebral disc excision, the instrumented segments were mobile and the fixation rod could be easily sequentially fitted into the screws. The set screws were loosely placed and not tightened. Once the rod was in place, anterior rotation of this pre-contoured rod would turn the frontal “scoliotic curve” into lordosis and derotate the spine. The nuts were first tightened in apical vertebrae and each segment above and below apex could be compressed sequentially and the fixation was completed. Compression force could be applied during correction with the apical vertebrae in center position in order to improve the correction, but distal disc wedging should be avoided. The correction and instrumentation were checked under C-arm X-ray. Somatosensory evoked potentials (SSEPs) and motor evoked potential (MEP) were used for intra-operative neurological monitoring.
Statistical analysis
All variables were compared between the two groups by independent samples t test and the preoperative and postoperative data were compared in each group by paired samples t test. Differences were considered statistically significant when P < 0.05.
Results
No statistical difference was found between the two groups in terms of age, Risser sign, preoperative Cobb angle and curve flexibility (Table 1). The levels of instrumentation and average number of levels instrumented were similar in both groups. Mean postoperative VAS was significantly lower in Group A (5.5) than in Group B (7.0) and mean post-op hospitalization (days) was less in Group A (7.8) than in Group B (9.2) as shown in Table 1.
Table 1.
Demographics and surgical details of the study groups
| Parameter | Group A | Group B |
|---|---|---|
| Number of patients | 38 | 38 |
| Age (years) | 16.5 (12–25) | 16.7 (12–24) |
| Risser sign | 3.6 (1–5) | 3.4 (1–5) |
| Instrumented level | 5.4 (4–6) | 5.5 (4–6) |
| Flexibility (%) | 65.0 (55–70) | 62.6 (50–70) |
| Operation time (min) | 240 (180–280) | 200 (180–250)* |
| EBL (cc) | 390 (300–470) | 370 (280–460) |
| VAS | 5.5 (3–8) | 7.0 (4–10)* |
| Post-op hospitalization (days) | 7.8 (5–10) | 9.2 (6–13)* |
* P < 0.05
In Group A, the instrumented levels were from T10 to L3 in 17 cases, T11 to L3 in 9 cases, T11 to L2 in 7 cases, T11 to L4 in 5 cases, while in Group B the instrumented levels were 19 cases from T10 to L3, 9 cases from T11 to L3, 5 cases from T11 to L2 and 5 cases from T11 to L4. The operation time was significantly higher in Group A than that in Group B (240 vs. 200 min), while estimated blood loss and instrumented levels showed no significant difference between two groups, as shown in Table 1. In Group A the mean value of operation time was 270 min (255–280 min) for the first ten cases which gradually decreased to 210 min (180–250 min) in the last ten cases.
Postoperative coronal and sagittal data were also identical in two groups (Table 2). The postoperative coronal TL Cobb angle, apical translation, lower instrumented vertebra tilt, apical vertebrae rotation all improved significantly compared with preoperative value in both groups (P < 0.05) (Table 2). The TL curve was corrected to 12.7° with 78% correction rate in Group A and was reduced to 14.6° with 75% correction rate in group B. The TL curve at final follow-up was 14.3° in Group A and 17.5° in Group B with 4 and 5% loss of correction, respectively. The normal TL sagittal profile were restored after surgery and maintained during the follow-up period in both groups. Those with a preoperative Cobb angle larger than 10° all decreased to normal value. There were no significant changes in preoperative versus postoperative lumbar lordosis in either group (Table 2; Fig. 3).
Table 2.
Surgical results comparing mini-open (Group A) and traditional open (Group B) anterior corrective surgery (mean value and range)
| Group A | Group B | |||||
|---|---|---|---|---|---|---|
| Pre-op | Post-op | Final follow-up | Pre-op | Post-op | Final follow-up | |
| Coronal TL curve (°) | 56.4 | 12.7* | 14.3 | 55.8 | 14.6* | 17.5 |
| 42 to 75 | 5 to 17 | 6 to 19 | 40 to 75 | 4 to 18 | 7 to 20 | |
| Apical translation (mm)a | 48 | 16* | 17 | 50 | 15* | 17 |
| 35 to 65 | 10 to 35 | 12 to 35 | 33 to 68 | 10 to 33 | 13 to 35 | |
| Lower instrumented vertebra tilt (°)b | 28 | 9* | 10 | 25 | 8* | 10 |
| 14 to 42 | −11 to 20 | −10 to 25 | 11 to 40 | −12 to 20 | −13 to 24 | |
| Apical vertebrae rotation (°) | 29.5 | 14.5 * | 32.4 | 15.7 * | ||
| 20 to 46 | 6 to 20 | 25 to 45 | 7 to 22 | |||
| Sagittal TL kyphosis (T10–L2) (°) | 10.7 | 6.5* | 7.3 | 9.8 | 5.2* | 6.4 |
| 0 to 18 | 2 to 8 | 2 to 9 | −2 to 16 | 2 to 7 | 2 to 10 | |
| Lumbar lordosis T12–S1 (°) | 55 | 58 | 56 | 54 | 59 | 59 |
| 25 to 70 | 27 to 75 | 27 to 72 | 28 to 70 | 35 to 75 | 36 to 70 | |
TL thoracolumbar
* Pre-op versus post-op comparison P < 0.05
aApical Translation: the center of the apical vertebral body or disc of the thoracolumbar curve to the vertical center sacral line
bLower instrumented vertebra tilt: lower instrumented vertebra angle from horizontal line
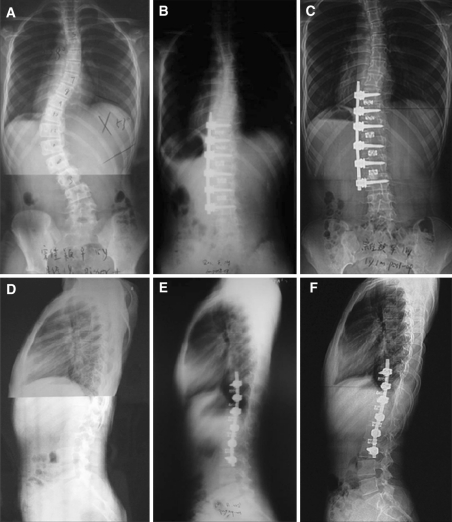
Fig. 3.
A 16-year-old girl with IS Lenke Type 5C was operated by mini-open anterior approach. a A 53° left thoracolumbar scoliosis and significant trunk shift. b 3 months postoperative coronal radiograph shows the scoliosis corrected to 5° with single solid-rod instrumentation mesh cages from T10–L3. c Three-year follow-up coronal radiographs show maintenance of scoliosis correction at 5°. d Sagittal plane showed thoracolumbar kyphosis measuring 5° (T10–L2). e 3 months postoperative lateral radiograph demonstrates improvement in thoracolumbar and lumbar lordosis over the instrumented levels. f Three-year follow-up sagittal radiograph shows maintenance of thoracolumbar and lumbar sagittal alignment
The patients were followed up for an average of 35 months (range 24–48) in Group A and for an average of 38 months (range 24–55) in Group B. All patients in both groups had good healing of incisions without pulmonary and neurological complications. In group A, two patients suffered from pleural effusion, one of whom needed the treatment of chest tube drainage and recovered in 2 weeks. In group B, two patients suffered from pleural effusion. All patients in both groups had no instrumentation complications, such as screw breakage or dislodgement during follow-up. The wedging of the vertebral discs distal to the lowest fused level occurred in three and four patients in Group A and B, respectively. One case in group B was found to be suspicious of pseudoarthrosis on the 12 months post-op X-film, but there was no loss of correction and implant failure during a total of 40 months follow-up.
Discussion
There are currently two approaching options for correction of single structural thoracolumbar scoliosis, the anterior approach and the posterior approach, though debates on which one is superior always exist. Correction of idiopathic scoliosis has traditionally been accomplished by posterior spinal fusion and instrumentation. Good three dimensional control of the deformity were reported with techniques like the direct vertebral derotation (DVD) maneuver in pedicle-only construct recently [1, 13]. Anterior instrumentation techniques for spinal fusion have been used for several decades [5, 10, 11, 26]. The relatively high morbidity, when compared with that in a purely posterior approach, has deterred most surgeons from using this technique. Though currently many surgeons tend to use pedicle screws to correct thoracolumbar scoliosis from a posterior approach, anterior instrumentation is still one viable option because review of retrospective and comparative studies illustrated that the anterior approach can also achieve good coronal correction with a direct derotation effect and shorter fusion levels [8, 17, 18, 23].
The standard anterior surgical approach to the thoracolumbar spine has been through an open thoracoabdominal retroperitoneal approach which necessitates dissecting to dissect the diaphragm. The disadvantages of this approach include a large dissection of diaphragm and long skin scar. In the year 2002, we developed a mini-open anterior approach and diaphragm-sparing approach in an attempt to minimize surgical morbidity, while at the same time achieve the same clinical outcomes and better patient satisfaction. The difference between the mini-open and open approach for thoracolumbar spine is not only the length of the incision. The former one has two separate 7–8 cm skin incisions while the latter has an incision around 25 cm in length. The more notable difference between these two approaches is the extent of the damage to diaphragm. The diaphragm is innervated by the phrenic nerve [6]. The phrenic nerve joins the diaphragm adjacent to the fibrous pericardium, then divides into three major branches that extend peripherally in an anterior, lateral and posterior direction. In addition, there are numerous interconnections that loop between these branches. Damaging any of these major branches or intercommunications interferes with diaphragmatic function and reduces respiratory reserves [28]. Some complications such as postoperatively weakened abdominal breathing, paralysis of diaphragm and atelectasis may emerge as the results of iatrogenic injury of the diaphragm [21, 22].
Concern about such side effects inspired the authors to explore the possibility of a mini-open approach to access the thoracolumbar spine without cutting the whole diaphragm. Since both the crura and arcuate ligaments of the diaphragm are inserted below the T12–L1 disc space, the discectomy and the vertebra screw insertion for T10–T12 could be performed from an extrapleural or transpleural approach without dividing the diaphragm. Meanwhile, the costodiaphragmatic recess is just above the inferior endplate of the second lumbar vertebra. Thus, the entire L1 vertebral body can be exposed with a small hole of about 2–3 cm in diameter on the diaphragm rather than dissecting the whole diaphragm as used in the traditional open techniques. So anatomically, it is feasible to apply the mini-open approach to access the thoracolumbar spine, but difficulties come out in preparing the disc space, screwing and correcting the scoliosis. First, as the T12–L1 disc and L1 vertebrae body are covered by diaphragmatic crura and arcuate ligaments [6, 24], the T12–L1 discectomy, ligation of T12 and L1 segmental vessels, L1 screw insertion were relatively difficult in mini-open approach. Secondly, with two separate incisions, the rod insertion and derotation may not be as convenient as in the whole vision under open approach. Such difficulties were reflected by the relative increase of operation time in the mini-open group compared with open group (240 vs. 200 min). But the inconvenience can be overcome with the accumulation of surgeons’ experience and skills. In the current series, the T12–L1 discectomy were performed with the assistance of thoracoscopic instruments which have longer handle and angled curettes and rongeurs. The L1 upper endplate can be prepared with an upper incision while the T12 lower endplate can be treated with a lower skin incision. The L1 segmental vessel should be dissected meticulously and coagulated with diathermy. Since the vision is blocked by the diaphragm, the accuracy of screw insertion should be confirmed by AP and lateral X-ray intraoperatively just as in endoscopic procedure. Such techniques were easy to apprehend during the slack learning curve of mastering this diaphragm-sparing approach. In these 36 cases in Group A, the surgical time gradually decreased in the last 10 patients compared with that in first 10 cases (210 vs. 270 min) as the surgeon became more and more familiar with the technique.
In this study while the order of hospitalization was used to sort patients into two different groups, the limitation of this method is that it is not a randomized study. But the only difference between the two groups is the approaching methods to the thoracolumbar spine and the surgical results were all objectively and blindly collected by the author who was not involved in the surgery. Equivalent surgical results in terms of coronal and sagittal corrections were observed after anterior solid rod with interbody structural supporting though different approaching method was used. The mean correction rate of thoracolumbar curve in mini-open group was 78% which was comparable to 75% in the traditional open group and the restoration of normal sagittal alignment were achieved in both groups. During follow-up, the loss of coronal correction was 4 and 5%, respectively, in mini-open and open group. The lumbar lordosis and optimal thoracolumbar sagittal profile were maintained during follow-up in both groups. The significant improvement of other parameters such as apical translation, lower instrumented vertebra tilt and apical vertebrae rotation found in both groups also further validate the similar correction effect through these two approaches. Such clinical outcomes described in the current study are comparable to literature reports on anterior instrumentation for Lenke 5C patients. In Lowe’s study, 21 patients with thoracolumbar adolescent idiopathic scoliosis underwent anterior single rod instruments and structural interbody support. The preoperative primary thoracolumbar curves averaged 47° and the curves at final follow-up was reduced to 13° with a mean loss of 2° over the minimum 3 years follow-up [18]. Sweet treated 20 patients with thoracolumbar idiopathic scoliosis using anterior single solid-rod instrumentation and titanium mesh cages. A correction rate of 77% (from 48°–11°) on coronal Cobb angle was achieved and no obvious loss of correction was observed at final the follow-up. The physical lumbar lordosis was also preserved without instrumentation failure [27]. In Lenke’s study with the same treatment method, the coronal Cobb angle was corrected from 50° to 15° without any loss of correction during a 2-year follow-up period [17].
One possible concern in using this mini-open technique was the control of bleeding, but neither major vascular complication nor increase of estimated blood loss was seen in Group A. No increase of the other complications related to the approach was found. The reported complications (pleural effusion, LIV tilting and disc wedging) in Groups A and B were also not uncommon in other anterior correction for thoracolumbar scoliosis in literature [12, 14] and certainly not approach related. The majority of AIS patients who received anterior surgery in the author’s center were female and to ensure the study was carried out in a homogeneous population, only female patients were selected. While in this study the order of hospitalization was used to sort patients into two different groups, the limitation of this method is that it is not a randomized study. However, the difference between the mini-open approach and conventional open approach lies mainly in the surgical approach but not the instrumentation techniques. The major advantage of mini-open approach is to protect the diaphragm and improve cosmesis. As for the patients fitting into our inclusion criteria, both surgical approaches are optional. Thus, concerning the correction of skeletal deformity, there is no such viewpoint that one is superior to the other. What we did only changed the surgical approach, but not the treatment strategy. Besides, the surgical results were all objectively and blindly collected by the author who is not related to the surgery. As a result, our technique of patient selection may not directly lead to bias on results.
Compared to traditional anterior instrumentation, the diaphragm-sparing approach presented similar correction effect and comparable complication occurrence besides the slight increase of operation time. It is undeniable that this mini-open procedure is really more complex than the traditional anterior technique. The authors definitely know that the lack of the data on pulmonary function is one of the limitations of this study. But with less extensive dissection and minimal damage to the diaphragm, the mini-open approach could reasonably result in less pain and faster post-op recovery.
Conflict of interest
None.
References
- 1.Asghar J, Samdani AF, Pahys JM, D’Andrea LP, Guille JT, Clements DH, Betz RR. Computed tomography evaluation of rotation correction in adolescent idiopathic scoliosis: a comparison of an all pedicle screw construct versus a hook-rod system. Spine (Phila Pa 1976) 2009;34:804–807. doi: 10.1097/BRS.0b013e3181996c1b. [DOI] [PubMed] [Google Scholar]
- 2.Betz RR, Harms J, Clements DH, 3rd, Lenke LG, Lowe TG, Shufflebarger HL, Jeszenszky D, Beele B. Comparison of anterior and posterior instrumentation for correction of adolescent thoracic idiopathic scoliosis. Spine (Phila Pa 1976) 1999;24:225–239. doi: 10.1097/00007632-199902010-00007. [DOI] [PubMed] [Google Scholar]
- 3.Bitan FD, Neuwirth MG, Kuflik PL, Casden A, Bloom N, Siddiqui S. The use of short and rigid anterior instrumentation in the treatment of idiopathic thoracolumbar scoliosis: a retrospective review of 24 cases. Spine (Phila Pa 1976) 2002;27:1553–1557. doi: 10.1097/00007632-200207150-00014. [DOI] [PubMed] [Google Scholar]
- 4.Bridwell KH. Surgical treatment of adolescent idiopathic scoliosis: the basics and the controversies. Spine (Phila Pa 1976) 1994;19:1095–1100. doi: 10.1097/00007632-199405000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Dwyer AP, Schafer MF (1974) Anterior approach to scoliosis: results of treatment in 51 cases. J Bone Jt Surg Br 56:218–224 [PubMed]
- 6.Fell SC. Surgical anatomy of the diaphragm and the phrenic nerve. Chest Surg Clin N Am. 1998;8:281–294. [PubMed] [Google Scholar]
- 7.Ford GT, Whitelaw WA, Rosenal TW, Cruse PJ, Guenter CA. Diaphragm function after upper abdominal surgery in humans. Am Rev Respir Dis. 1983;127:431–436. doi: 10.1164/arrd.1983.127.4.431. [DOI] [PubMed] [Google Scholar]
- 8.Geck MJ, Rinella A, Hawthorne D, Macagno A, Koester L, Sides B, Bridwell K, Lenke L, Shufflebarger H. Comparison of surgical treatment in Lenke 5C adolescent idiopathic scoliosis: anterior dual rod versus posterior pedicle fixation surgery: a comparison of two practices. Spine (Phila Pa 1976) 2009;34:1942–1951. doi: 10.1097/BRS.0b013e3181a3c777. [DOI] [PubMed] [Google Scholar]
- 9.Graham EJ, Lenke LG, Lowe TG, Betz RR, Bridwell KH, Kong Y, Blanke K. Prospective pulmonary function evaluation following open thoracotomy for anterior spinal fusion in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2000;25:2319–2325. doi: 10.1097/00007632-200009150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Gupta M, Punu R, Mehta S (1984) Zielke instrumentation for the treatment of thoracolumbar and lumbar curves in idiopathic scoliosis: revisited. Orthop Trans 18:119
- 11.Hammerberg KW, Rodts MF, DeWald RL. Zielke instrumentation. Orthopedics. 1988;11:1365–1371. doi: 10.3928/0147-7447-19881001-05. [DOI] [PubMed] [Google Scholar]
- 12.Johnston CE II, Turi M, Richards BS (1994) Treatment of idiopathic scoliosis with anterior TSRH instrumentation. Orthop Trans 18:18–19 [PubMed]
- 13.Kadoury S, Cheriet F, Beausejour M, Stokes IA, Parent S, Labelle H. A three-dimensional retrospective analysis of the evolution of spinal instrumentation for the correction of adolescent idiopathic scoliosis. Eur Spine J. 2009;18:23–37. doi: 10.1007/s00586-008-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaneda K, Shono Y, Satoh S, Abumi K. New anterior instrumentation for the management of thoracolumbar and lumbar scoliosis. Application of the Kaneda two-rod system. Spine (Phila Pa 1976) 1996;21:1250–1261. doi: 10.1097/00007632-199605150-00021. [DOI] [PubMed] [Google Scholar]
- 15.Kohler R, Galland O, Mechin H, Michel CR, Onimus M. The Dwyer procedure in the treatment of idiopathic scoliosis. A 10-year follow-up review of 21 patients. Spine (Phila Pa 1976) 1990;15:75–80. doi: 10.1097/00007632-199002000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Lee SM, Suk SI, Chung ER. Direct vertebral rotation: a new technique of three-dimensional deformity correction with segmental pedicle screw fixation in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2004;29:343–349. doi: 10.1097/01.brs.0000109991.88149.19. [DOI] [PubMed] [Google Scholar]
- 17.Lenke LG, Bridwell KH (2002) Mesh cages in idiopathic scoliosis in adolescents. Clin Orthop Relat Res 98–108 [DOI] [PubMed]
- 18.Lowe TG, Alongi PR, Smith DA, O’Brien MF, Mitchell SL, Pinteric RJ. Anterior single rod instrumentation for thoracolumbar adolescent idiopathic scoliosis with and without the use of structural interbody support. Spine (Phila Pa 1976) 2003;28:2232–2241. doi: 10.1097/01.BRS.0000085028.70985.39. [DOI] [PubMed] [Google Scholar]
- 19.Lowe TG, Peters JD. Anterior spinal fusion with Zielke instrumentation for idiopathic scoliosis. A frontal and sagittal curve analysis in 36 patients. Spine (Phila Pa 1976) 1993;18:423–426. [PubMed] [Google Scholar]
- 20.Luk KD, Leong JC, Reyes L, Hsu LC. The comparative results of treatment in idiopathic thoracolumbar and lumbar scoliosis using the Harrington, Dwyer, and Zielke instrumentations. Spine (Phila Pa 1976) 1989;14:275–280. doi: 10.1097/00007632-198903000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Majd ME, Castro FP, Jr, Holt RT. Anterior fusion for idiopathic scoliosis. Spine (Phila Pa 1976) 2000;25:696–702. doi: 10.1097/00007632-200003150-00008. [DOI] [PubMed] [Google Scholar]
- 22.McDonnell MF, Glassman SD, Dimar JR, 2nd, Puno RM, Johnson JR. Perioperative complications of anterior procedures on the spine. J Bone Jt Surg Am. 1996;78:839–847. doi: 10.2106/00004623-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Rhee JM, Bridwell KH, Won DS, Lenke LG, Chotigavanichaya C, Hanson DS. Sagittal plane analysis of adolescent idiopathic scoliosis: the effect of anterior versus posterior instrumentation. Spine (Phila Pa 1976) 2002;27:2350–2356. doi: 10.1097/00007632-200211010-00008. [DOI] [PubMed] [Google Scholar]
- 24.Schumpelick V, Steinau G, Schluper I, Prescher A. Surgical embryology and anatomy of the diaphragm with surgical applications. Surg Clin N Am. 2000;80:213–239. doi: 10.1016/s0039-6109(05)70403-5. [DOI] [PubMed] [Google Scholar]
- 25.Segel MH, Betz RR, Bridwell KH (1992) A critical analysis comparing Zielke instrumentation alone, Cotrel-Dubousset instrumentation alone and a combination of the two in the treatment of adolescent thoracolumbar and lumbar idiopathic scoliosis. Orthop Trans 153–154
- 26.Suk SI, Lee CK, Chung SS. Comparison of Zielke ventral derotation system and Cotrel-Dubousset instrumentation in the treatment of idiopathic lumbar and thoracolumbar scoliosis. Spine (Phila Pa 1976) 1994;19:419–429. doi: 10.1097/00007632-199402001-00007. [DOI] [PubMed] [Google Scholar]
- 27.Sweet FA, Lenke LG, Bridwell KH, Blanke KM. Maintaining lumbar lordosis with anterior single solid-rod instrumentation in thoracolumbar and lumbar adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 1999;24:1655–1662. doi: 10.1097/00007632-199908150-00006. [DOI] [PubMed] [Google Scholar]
- 28.Tripp HF, Bolton JW. Phrenic nerve injury following cardiac surgery: a review. J Card Surg. 1998;13:218–223. doi: 10.1111/j.1540-8191.1998.tb01265.x. [DOI] [PubMed] [Google Scholar]