Abstract
With the aging of the population in developed countries, spine surgeons have recently been more likely to encounter elderly patients in need of treatment. This study investigated whether decompression surgery for cervical spondylotic myelopathy (CSM) in elderly patients aged 80 years or older would likely be a reasonable treatment. We retrospectively reviewed 605 consecutive patients with cervical myelopathy who underwent decompression surgery between 2004 and 2008. Patients with other conditions that could affect functional status or compression factors other than spondylosis were excluded from this study. Of the remaining 189 patients, 161 with CSM whose condition could be evaluated 6 months after surgery were analyzed. The patients were divided into two age groups: 80 years or older (Group A, 37 patients) and younger than 80 years of age (Group B, 124 patients). We evaluated the differences in symptom duration, clinical data, involved levels, surgical outcome, comorbidities, and postoperative complications between the two groups. The symptom duration was significantly shorter in Group A. The average JOA scores preoperatively and 6 months postoperatively were significantly lower in Group A; however, there was no significant difference in the recovery ratio. There were no significant differences in the percentages of patients with comorbidities or those with postoperative complications. Elderly patients aged 80 years or older regained approximately 40% of their function postoperatively, and the incidence of postoperative complication was similar to that in younger patients. Since this age group shows a rapid deterioration after onset, prompt decompression surgery is required.
Keywords: Cervical spine, Myelopathy, Elderly, Surgery, Complication
Introduction
With aging of the population and progressive lengthening of life expectancy in developed countries, spine surgeons have recently been more likely to encounter elderly patients needing treatment. The World Health Organization has reported that eight countries had a life expectancy at birth of 80 years or older in 2000; however, the number of countries meeting that criterion rapidly increased to 28 by 2008 [1]. Due to improved public hygiene, the number of people living beyond 80 years of age and the number of countries with a life expectancy exceeding 80 years are thought to be increasing [2]. Surgical treatment of elderly patients is more difficult than that of younger patients, because the elderly are more likely to have a variety of medical and social problems. Therefore, it is considered important for spine surgeons to clarify the clinical features and surgical outcomes of cervical spondylotic myelopathy (CSM) in patients over 80 years of age.
Many papers describe cervical myelopathy in elderly patients [3–10]; however, definitions of the term elderly have varied from over 65 years [7, 8, 10] to over 70 years [3, 4, 9] and to over 75 years [5, 6]. Some studies have reported that the elderly tended to have a longer duration of symptoms, poorer surgical outcome and more cephalic levels involved [4, 5, 7, 10]. However, other authors have not demonstrated any differences in the duration of symptoms [3, 10] or surgical outcome [4, 8] between elderly and younger patients. These discrepancies may be attributed to their definitions of the term elderly.
To our knowledge, there have been few reports on patients 80 years or older, although all of the previous studies analyzed only limited samples [11–13]. Moreover, there is a possibility that their surgical outcomes may be attributed to other medical conditions, such as joint or other neural diseases, which were not excluded in these studies. To collect a fair-sized sample after strict exclusion of patients with other conditions that may affect surgical outcomes, a multi-center retrospective study was conducted.
When a surgeon plans to perform surgery on an elderly patient, the risks and benefits of that procedure have to be evaluated. A large prospective survey conducted by anesthesiologists from France demonstrated that the rate of complications related to anesthesia increased approximately ten times in patients aged 80 years compared to those aged 30 years [14]. On the other hand, Greenberg et al. [15] and Mohr [16] reported that age was less of a factor in surgical risk than physiologic status. Thus, it remains unclear whether advanced age itself is an independent risk factor [17].
This study investigated whether factors such as preoperative condition, duration of symptoms, involved levels, surgical outcomes, cormobidities and postoperative complications differed between patients aged 80 years or older and younger patients, and then assessed whether decompression surgery for CSM would likely be a reasonable treatment in elderly patients aged 80 years or older.
Materials and methods
This study was approved by the ethical committee of the first author’s institute. We retrospectively reviewed 605 consecutive patients with cervical myelopathy who underwent decompression surgery between 2004 and 2008 at any of the seven institutions where members of the research group were one of the staff. Patients with the following conditions were excluded from this study: patients who had undergone previous cervical surgery; those undergoing surgery for other neurological or locomotor diseases within 1 year before or after cervical decompression surgery; those on hemodialysis; those with palsy attributed to other neurological disorders; those with ossification of the posterior longitudinal ligament, cervical disc herniation, rheumatoid arthritis, spinal or spinal cord tumor, cerebral palsy, cervical myeloradiculopathy, cervical spondylotic amyotrophy, diabetic neuropathy or psychiatric diseases; those with traumatic cervical myelopathy or symptoms aggravated after trauma. Of the remaining 189 patients, 161 with CSM whose condition could be evaluated 6 months after surgery were studied. Among the other 28 patients, 2 patients aged over 80 years died of pneumonia or unknown cause 1 month after surgery (Table 1).
Table 1.
Demographic data of patients who died within the 6 months follow-up
| Parameter | Patient 1 | Patient 2 |
|---|---|---|
| Age (years) | 86 | 80 |
| Gender | Male | Female |
| Symptom duration (months) | 3 | 6 |
| Involved level | C3/4 | C4/5 |
| Preoperative JOA score | 4 | 8 |
| Surgical procedure (level) | French window laminoplasty (C3–6) | French window laminoplasty (C3–6) |
| Comorbidities | Pharyngeal cancer (postoperative) | Hypertension |
| Postoperative complications | None | Pneumonia |
| Cause of death | Unknown | Pneumonia |
There were 110 men and 51 women with an average age of 70.8 years (range, 34–89 years). The duration of follow-up was 6–64 months (average, 19.3 months). The patients were divided into two age groups: 80 years or older (Group A, 37 patients) and under 80 years of age (Group B, 124 patients).
We evaluated differences in symptom duration before surgery, clinical data, involved levels, surgical outcome, comorbidities and postoperative complications between the two groups. The involved level of the spinal cord was determined using magnetic resonance imaging (MRI) by ascertaining signal intensity changes [18]. If the spinal cord showed signal intensity changes at more than one level, the level showing the most severe compression of the spinal cord was identified as the involved level. Clinical data were evaluated using a scoring system for cervical myelopathy by the Japanese Orthopaedic Association (JOA score) [19]. The surgical outcome was evaluated by the recovery ratio calculated using the preoperative and postoperative JOA scores [20]. In elderly patients, there is a greater risk of medical problems; therefore, postoperative clinical data were evaluated 6 months after surgery.
Posterior decompression was performed on 157 patients. Of these, French window laminoplasty was performed on 131, open-door laminoplasty on 25 and laminectomy on 1 patient. The remaining four patients underwent anterior decompression and fusion (Table 2).
Table 2.
Procedures and actual levels operated on
| Procedure and level | Group A | Group B |
|---|---|---|
| Posterior procedure | ||
| French window laminoplasty | ||
| C1–7 | 0 | 1a |
| C3–5 | 1 | 0 |
| C3–6 | 20 | 55 |
| C3–7 | 10b | 36b |
| C4–6 | 2 | 4 |
| C4–7 | 0 | 2 |
| Open-door laminoplasty | ||
| C3–6 | 4 | 9 |
| C3–7 | 0 | 12 |
| Laminectomy | ||
| C4–7 | 0 | 1 |
| Anterior procedure | ||
| C3–4 | 0 | 1 |
| C4–5 | 0 | 2 |
| C4–6 | 0 | 1 |
aThis patient underwent additional posterior arthrodesis
bTwo patients each underwent additional posterior arthrodesis
After excluding patients undergoing anterior procedure, we further studied differences in surgical outcomes using another two sets of subgroups. To evaluate the differences in postoperative JOA scores and recovery ratios between two groups standardized for patient JOA score, we selected patients whose preoperative JOA scores ranged from 8 to 12 points. There were 22 and 71 patients in Groups A and B, respectively. We further studied differences in JOA scores and recovery ratios in patients whose symptoms deteriorated rapidly between the two groups. After the exclusion of patients with a symptom duration over 3 months, there were 16 and 31 patients in Groups A and B, respectively. The percentages of those patients were 43.2 and 25.0% in Groups A and B, respectively.
Differences in gender, comorbidities and postoperative complications between the two groups were statistically analyzed using the chi-square test. Statistical analyses of other data were performed using the Mann–Whitney U test. A probability value less than 0.05 indicated significance. All statistical analyses were performed on SPSS.
Results
Men were dominant in Group B while the percentages of men and women in Group A were nearly equal (Table 3). The symptom duration was significantly shorter in Group A than in Group B (Table 3). The average preoperative JOA score was significantly lower in Group A than in Group B due to poorer motor function in the upper and lower extremities (Tables 3, 4). However, there were no differences in sensory or bladder function (Table 4).
Table 3.
Demographic data of patients
| Parameter | Group A | Group B | p Value |
|---|---|---|---|
| Age (years) | 82.8 ± 2.7 | 67.2 ± 10.4 | <0.001a |
| Gender (men/women) | 17/19 | 94/32 | 0.002b |
| Symptom duration (months) | 10.9 ± 16.9 | 22.2 ± 29.7 | 0.023a |
| Follow-up (months) | 15.9 ± 12.0 | 20.2 ± 12.7 | 0.017a |
| JOA score | |||
| Preoperatively | 8.4 ± 3.0 | 10.2 ± 2.6 | 0.002a |
| Six months postoperatively | 11.6 ± 2.7 | 13.1 ± 2.3 | 0.002a |
| Final follow-up | 11.4 ± 3.3 | 13.2 ± 3.3 | 0.004a |
| Recovery ratio (%) | |||
| Six months postoperatively | 37.5 ± 22.1 | 40.7 ± 29.0 | 0.552a` |
| Final follow-up | 36.0 ± 26.0 | 44.8 ± 55.6 | 0.300a |
Values for age, symptom duration, followup, Japanese Orthopaedic Association score, and recovery ratio are given as mean ± SD
JOA Japanese Orthopaedic Association
aMann–Whitney U test
bChi-square test
Table 4.
Detailed assessment of the preoperative JOA score
| Parameter | Group A | Group B | p Valuea |
|---|---|---|---|
| Motor function | |||
| Upper extremities | 1.3 ± 1.1 | 2.1 ± 0.9 | <0.001 |
| Lower extremities | 1.0 ± 1.0 | 1.8 ± 1.0 | <0.001 |
| Sensory function | |||
| Upper extremities | 1.0 ± 0.5 | 1.1 ± 0.5 | 0.257 |
| Trunk | 1.8 ± 0.4 | 1.8 ± 0.4 | 0.950 |
| Lower extremities | 1.4 ± 0.6 | 1.3 ± 0.6 | 0.822 |
| Bladder function | 2.0 ± 1.0 | 2.2 ± 0.9 | 0.340 |
Values are mean ± SD
JOA Japanese Orthopaedic Association
aMann–Whitney U test
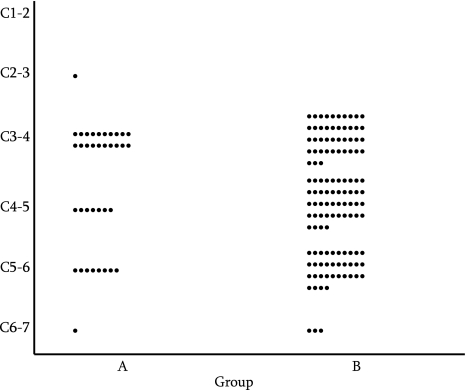
There was no significant difference in the involved levels of the spinal cord between Groups A and B (p = 0.054; Fig. 1).
Fig. 1.
The involved level of the spinal cord. There was no significant difference between Groups A and B (p = 0.054; Mann–Whitney U test)
The postoperative JOA scores 6 months postoperatively and at final follow-up in Group A were significantly lower than those in Group B (p = 0.002, p = 0.004, respectively). However, there were no significant differences in the recovery ratio 6 months postoperatively and at final follow-up between Groups A and B (p = 0.552, p = 0.300, respectively). In Group A, the average recovery ratio 6 months postoperatively was 37.5% (Table 3).
Postoperative JOA scores and recovery ratios were similar between the two age groups in patients with preoperative JOA scores from 8 to 12 points (Table 5). For patients with symptom duration from 0 to 3 months, preoperative and postoperative JOA scores were significantly lower in Group A; however, there were no significant differences in the recovery ratios (Table 6).
Table 5.
Surgical outcomes for patients with preoperative JOA scores from 8 to 12 points
| Parameter | Group A | Group B | p Value |
|---|---|---|---|
| JOA score | |||
| Preoperatively | 9.9 ± 1.2 | 10.1 ± 1.2 | 0.49 |
| Six months postoperatively | 12.7 ± 1.9 | 13.4 ± 1.9 | 0.13 |
| Final follow-up | 12.8 ± 1.8 | 13.3 ± 2.4 | 0.19 |
| Recovery ratio (%) | |||
| Six months postoperatively | 38.5 ± 25.4 | 50.0 ± 26.1 | 0.13 |
| Final follow-up | 40.1 ± 24.6 | 46.0 ± 35.0 | 0.20 |
Values for Japanese Orthopaedic Association score and recovery ratio are given as mean ± SD
Patients undergoing anterior procedure were excluded
JOA Japanese Orthopaedic Association
Table 6.
Surgical outcomes for patients with symptom duration from 0 to 3 months
| Parameter | Group A | Group B | p Value |
|---|---|---|---|
| Symptom duration (months) | 2.1 ± 0.9 | 1.8 ± 0.6 | 0.338 |
| JOA score | |||
| Preoperatively | 8.8 ± 2.6 | 10.5 ± 2.7 | 0.044 |
| Six months postoperatively | 12.0 ± 2.4 | 14.1 ± 1.6 | 0.010 |
| Final follow-up | 11.4 ± 4.3 | 14.3 ± 1.8 | 0.025 |
| Recovery ratio (%) | |||
| Six months postoperatively | 40.7 ± 19.4 | 50.1 ± 30.4 | 0.301 |
| Final follow-up | 37.7 ± 33.0 | 53.7 ± 32.1 | 0.092 |
Values for Japanese Orthopaedic Association score and recovery ratio are given as mean ± SD
Patients undergoing anterior procedure were excluded
JOA Japanese Orthopaedic Association
The percentages of patients with comorbidities were 86.5 and 75.9% in Groups A and B, respectively (Table 7). The incidences of postoperative complications were 8.1 and 12.9% in Groups A and B, respectively (Table 8). However, there were no significant differences in the percentages of patients with comorbidities or the incidence of postoperative complications between Groups A and B (p = 0.167, p = 0.428, respectively). In Group B, one patient died of heart failure 10 months after surgery, and another patient underwent additional anterior decompression and fusion 3 months after laminoplasty due to recurrence of cervical myelopathy accompanied by progression of cervical kyphosis.
Table 7.
Number of patients with comorbidities
| Medical problem | Group A | Group B |
|---|---|---|
| Hypertension | 14 | 46 |
| Diabetes mellitus | 7 | 23 |
| Prostate hypertrophy | 5 | 4 |
| Atrial fibrillation | 4 | 5 |
| Cancer | 3 | 5 |
| Cardiac failure | 2 | 3 |
| Ischemic heart disease | 2 | 3 |
| Bronchial asthma | 2 | 3 |
| Pulmonary emphysema | 0 | 1 |
| Hyperthyroidism | 0 | 1 |
| Hepatic cirrhosis | 0 | 1 |
| Total (%) | 32 (86.5%) | 94 (75.9%) |
Some patients had two or more comorbidities
Table 8.
Postoperative complications
| Complication | Group A | Group B |
|---|---|---|
| Cerebrospinal fluid leakage | 1 | 5 |
| Deep surgical site infection | 0 | 3 |
| Paraparesis (C5) | 0 | 3 |
| Wound problems | 0 | 2 |
| Dislodgement of spacer | 1 | 0 |
| Atrial fibrillation | 1 | 0 |
| Delirium | 0 | 1 |
| Ileus | 0 | 1 |
| Pseudomembranous enterocolitis | 0 | 1 |
| Total (%) | 3 (8.1%) | 16 (12.9%) |
Some patients had two or more complications
Discussion
This study has several limitations. Firstly, we retrospectively reviewed only patients who underwent surgery. Most patients with CSM referred to our hospitals are considered candidates for surgery. Therefore, an adequate number of controls who underwent nonsurgical therapy could not be obtained. In addition, there was a possibility that we did not perform surgery for elderly patients with serious comorbid conditions, or that anesthesiologists refused to anesthetize such patients. Therefore, this study may contain a bias. Secondly, clinical data 6 months postoperatively may not as adequately reflect outcomes as data collected at the 2-year follow-up given the natural course of recovery after surgery. However, there is a possibility of motor deterioration due to aging in patients over 80 years. We consider that it is unreasonable to compare long-term outcomes in patients older than 80 years old to those in younger patients; therefore, postoperative clinical data were evaluated 6 months after surgery. Thirdly, to compare postoperative functions between elderly and younger patients, using a recover ratio calculated with JOA scores may be unfair, because there is a question of whether elderly people without CSM demonstrate full JOA scores. Therefore, it is possible that recovery ratios in the elderly would be undervalued. However, there has not been any reliable data on JOA scores in elderly people without CSM. This concern can arise not only for JOA score, but also for other evaluation methods. Fourthly, patients with several underlying conditions were excluded from the current study; however, the authors checked only medical charts to determine whether patients had these conditions. If electrophysiological studies, brain MRI and other studies had been performed for all of the patients, it is likely that some additional patients would have been excluded. Finally, we excluded 28 patients who did not return to our clinics 6 months after surgery, since their JOA scores at that time could not be obtained. Although we could not confirm the reasons in individual cases, it could be that their homes were far from our hospitals, the patient did not feel the need for follow-up, none of their family members could bring them to our clinic, or that they could not return because of difficulties in walking. Therefore, the exclusion of these 28 patients may have introduced further bias.
The current study showed that men were dominant among CSM patients under 80 years old, while the percentages of men and women over 80 years old were nearly equal. This finding was ascribed to the difference in male-to-female ratios in different age groups. In 2008, female life expectancy in Japan was much higher than that of males (86.05 and 79.29 years, respectively) [21], and the male-to-female ratios in age groups 30–79 years and 80+ years were 1.05 and 0.50, respectively [22].
Although our study did not demonstrate any significant difference in the involved level between patients over 80 years old and those under 80 years old, the most frequently involved level of the spinal cord was the C3–C4 disc level in the elderly group, while the percentages of C3–C4, C4–C5 and C5–C6 as the involved level were nearly equal in the younger group. We previously reported that the involved level of the spinal cord was more cephalic in the older groups, and the most frequently involved level in patients older than 75 years was C3–C4 [6]. Other investigators [7] have reported that the C3–C4 or C4–C5 levels were more frequently involved in elderly patients aged 65 years or older than in younger patients. These data have also been ascertained by electrophysiological studies [9]. These authors [7, 9] indicated that these data could be ascribed to hypermobility at the cephalic cervical levels with compensating stabilization of the caudal cervical levels with age, as supported by the results of other studies [23–25]. Meanwhile, cervical myelopathy in elderly patients was reported to be affected by a relatively static factor (i.e., the development of canal stenosis over a long period of time) [3].
The authors also demonstrated shorter symptom duration in patients aged 80 years or older. Contrarily, Nagata et al. [7] reported that the elderly patients aged 65 years and older showed longer symptom duration, while other authors [6, 10] indicated that there was no significant difference in symptom duration between elderly and younger patients. The previous reports [11, 13] on CSM in patients 80 years or older did not evaluate the difference in symptom duration compared to that in the younger group. Our data indicating shorter symptom duration and poorer preoperative function suggest rapid deterioration after the onset of myelopathy in older patients. This conclusion can also be proven by showing that the preoperative JOA scores in patients who had been deteriorating rapidly were significantly lower in the elderly group (Table 6).
In the current study, patients aged 80 years or older had a tendency to have more comorbidities and less postoperative complications compared to younger patients, although there were no significant differences. Two previous reports [11, 13] regarding this age group showed conflicting results. Nagano et al. [11] reported that 38.5% of the patients had postoperative complications, but Izumi et al. [13] reported none. In Nagano’s series, 23.1% of 13 patients demonstrated delirium, which was reported as the most frequent postoperative complication [5], while there were no patients demonstrating delirium in our or Izumi’s series. Although the mechanism underlying delirium remains unclear, the hypothesis is that a decrease in the oxidative metabolism of the brain, resulting in a decline in neurotransmitter levels within the brain along with an increase in serum cortisol from the stress of surgery or anesthesia, may be responsible for postoperative confusion [17]. However, there was no serious postoperative complication in this age group in the three series described above. This outcome might be attributed to the medical staff’s conscientious postoperative care, supplying even more careful management to the elderly than to younger patients. Another hypothesis is that people surviving over 80 years exert greater control over their health care. Of course, there is a possibility that we did not perform surgery for elderly patients with serious comorbid conditions, or that anesthesiologists refused to anesthetize such patients as indicated above.
This multi-center retrospective study demonstrated that patients aged 80 years or older showed a rapid functional deterioration after the onset of myelopathy. Although there is a possibility that we did not perform surgery for elderly patients with serious comorbid conditions, or that anesthesiologists refused to anesthetize such patients, the benefits and risks of decompression surgery were similar to those in younger patients and elderly patients regained approximately 40% of their function postoperatively. Therefore, prompt decompression surgery is required even in this age group.
Acknowledgments
This work was presented in part at the 39th annual meeting of the Japanese Society for Spine Surgery and Related Research, Kochi, Japan, 22–24 April 2010 and the 26th annual meeting of the Cervical Spine Research Society European Section, Corfu Island, Greece, 26–29 May 2010. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1.World Health Organization. World health statistics 2010 (WHO web site). http://www.who.int/whosis/whostat/2010/en/index.html. Accessed 31 August 2010
- 2.World Health Organization. The world health report 1998: life in the 21st century: a vision for all (WHO web site). http://www.who.int/whr/1998/en/whr98_en.pdf. Accessed 31 August 2010
- 3.Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis: a retrospective comparison with younger patients. J Neurosurg. 2002;96:173–179. doi: 10.3171/spi.2002.96.2.0173. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa K, Homma T, Chiba Y, Hirano T, Watanabe K, Yamazaki A. Effects of surgical treatment for cervical spondylotic myelopathy in patients ≥70 years of age: a retrospective comparative study. J Spinal Disord Tech. 2002;15:458–460. doi: 10.1097/00024720-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda Y, Shibata T, Oki S, Kawatani Y, Mashima N, Oishi H. Outcomes of surgical treatment for cervical myelopathy in patients more than 75 years of age. Spine. 1999;24:529–534. doi: 10.1097/00007632-199903150-00005. [DOI] [PubMed] [Google Scholar]
- 6.Nagashima H, Morio Y, Yamashita H, Yamane K, Teshima R. Clinical features and surgical outcomes of cervical myelopathy in the elderly. Clin Orthop Relat Res. 2006;444:140–145. doi: 10.1097/01.blo.0000201156.21701.86. [DOI] [PubMed] [Google Scholar]
- 7.Nagata K, Ohashi T, Abe J, Morita M, Inoue A. Cervical myelopathy in elderly patients: clinical results and MRI findings before and after decompression surgery. Spinal Cord. 1996;34:220–226. doi: 10.1038/sc.1996.41. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka J, Seki N, Tokimura F, Doi K, Inoue S. Operative results of canal-expansive laminoplasty for cervical spondylotic myelopathy in elderly patients. Spine. 1999;24:2308–2312. doi: 10.1097/00007632-199911150-00004. [DOI] [PubMed] [Google Scholar]
- 9.Tani T, Ushida T, Taniguchi S, Kimura J. Age related shift in the primary sites of involvement in cervical spondylotic myelopathy from lower to upper levels. J Neurol Neurosurg Psychiatry. 2002;73:316–318. doi: 10.1136/jnnp.73.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52:122–126. doi: 10.1097/00006123-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Nagano A, Miyamoto K, Hosoe H, Iinuma N, Nishimoto H, Sakaeda H, Wada E, Shimizu K. Surgical treatment for cervical myelopathy in patients aged >80 years. Orthopedics. 2004;27:45–48. doi: 10.3928/0147-7447-20040101-17. [DOI] [PubMed] [Google Scholar]
- 12.Nagashima H, Yamane K, Nishihata T, Teshima R. Surgical outcome of cervical spondylotic myelopathy in patients aged 80 years and older. Eur Spine J. 2006;15:S481. doi: 10.1007/s00586-010-1672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izumi B, Sumida T, Manabe H, Ito Y, Fujiwara Y, Nakasaki K, Ota R (2008) Surgical result of open door laminoplasty for cervical myelopathy in 80-year or older patients. Rinsho Seikei Geka 43:705–708 (in Japanese)
- 14.Tiret L, Desmonts JM, Hatton F, Vourc’h G. Complications associated with anaesthesia: a prospective survey in France. Can Anaesth Soc J. 1986;33:336–344. doi: 10.1007/BF03010747. [DOI] [PubMed] [Google Scholar]
- 15.Greenburg AG, Saik RP, Pridham D. Influence of age on mortality of colon surgery. Am J Surg. 1985;150:65–70. doi: 10.1016/0002-9610(85)90011-X. [DOI] [PubMed] [Google Scholar]
- 16.Mohr DN. Estimation of surgical risk in the elderly: a correlative review. J Am Geriatr Soc. 1983;31:99–102. doi: 10.1111/j.1532-5415.1983.tb05421.x. [DOI] [PubMed] [Google Scholar]
- 17.American Society of Anesthesiologists. Syllabus on geriatric anesthesiology (American Society of Anesthesiologists web site). http://www.asahq.org/clinical/geriatrics/PDFSyllabus5-011002.pdf. Accessed 31 August 2010
- 18.Morio Y, Teshima R, Nagashima H, Nawata K, Yamasaki D, Nanjo Y. Correlation between operative outcomes of cervical compression myelopathy and MRI of the spinal cord. Spine. 2001;26:1238–1245. doi: 10.1097/00007632-200106010-00012. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi H, Hirabayashi K. Scoring system (17–2) for cervical myelopathy (Japanese Orthopaedic Association) J Jpn Orthop Assoc. 1994;68:490–503. [Google Scholar]
- 20.Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine. 1981;6:354–364. doi: 10.1097/00007632-198107000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Japanese Ministry of Health, Labour and Welfare. White paper on health, labour and welfare 2009 (Japanese Ministry of Health, Labour and Welfare web site). http://www.mhlw.go.jp/za/0825/c05/pdf/21010102.pdf. Accessed 31 August 2010 (in Japanese)
- 22.National Institute of Population and Society Security Research. Population statistics in Japan 2010 (National Institute of Population and Society Security Research web site) Available at http://www.ipss.go.jp/syoushika/tohkei/Popular/Popular2010.asp?chap=2&title1=%87U%81D%94N%97%EE%95%CA%90l%8C%FB. Accessed 31 August 2010 (in Japanese)
- 23.Bohlman HH. Cervical spondylosis with moderate to severe myelopathy: a report of 17 cases treated by Robinson anterior cervical discectomy and fusion. Spine. 1977;2:618–625. doi: 10.1097/00007632-197706000-00008. [DOI] [Google Scholar]
- 24.Braakman R. Management of cervical spondylotic myelopathy and radiculopathy. J Neurol Neurosurg Psychiatry. 1994;57:257–263. doi: 10.1136/jnnp.57.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi H, Okada K, Hamada M, Tada K, Ueno R. Etiologic factors of myelopathy: a radiographic evaluation of the aging changes in the cervical spine. Clin Orthop Relat Res. 1987;214:200–209. [PubMed] [Google Scholar]