Abstract
Treatment of chronic low back pain due to degenerative lumbar spine conditions often involves fusion of the symptomatic level. A known risk of this procedure is accelerated adjacent level degeneration. Motion preservation devices have been designed to provide stabilization to the symptomatic motion segment while preserving some physiologic motion. The aim of this study was to compare the changes in relative range of motion caused as a result of application of two non-fusion, dynamic stabilization devices: the Universal Clamp (UC) and the Wallis device. Nine fresh, frozen human lumbar spines (L1–Sacrum) were tested in flexion–extension, lateral bending, and axial rotation with a custom spine simulator. Specimens were tested in four conditions: (1) intact, (2) the Universal Clamp implanted at L3–4 (UC), (3) the UC with a transverse rod added (UCTR), and (4) the Wallis device implanted at L3–4. Total range of motion at 7.5 N-m was determined for each device and compared to intact condition. The UC device (with or without a transverse rod) restricted motion in all planes more than the Wallis. The greatest restriction was observed in flexion. The neutral position of the L3–4 motion segment shifted toward extension with the UC and UCTR. Motion at the adjacent levels remained similar to that observed in the intact spine for all three constructs. These results suggest that the UC device may be an appropriate dynamic stabilization device for degenerative lumbar disorders.
Keywords: Posterior dynamic stabilization, Universal Clamp, Wallis, Biomechanics, Range of motion
Introduction
Chronic back pain due to intervertebral disc degeneration that has not responded to conservative measures is often treated by fusing the affected levels. Despite the reported benefits of spinal fusion surgery, it is not without risk and many complications may arise. These include pseudarthrosis, donor site pain, infection, and instrumentation failure. Another potential problem is increased motion at adjacent motion segments, with possible increased risk of disc degeneration [1]. Thus, many investigators are seeking less invasive, motion sparing alternatives to fusion for degenerative conditions. “Dynamic” or “semi-rigid” stabilization are terms used to describe instrumentation systems designed to restore stiffness to lumbar motion segments with degenerative instability that are associated with chronic low back pain. A number of dynamic stabilization implants applied via a posterior approach have been developed since the 1980s [2–7]. The most common designs are pedicle screw systems and interspinous implants. The Wallis implant (Zimmer SAS, Bordeaux, France) is an interspinous spacer composed of polyetheretherketone (PEEK), which limits extension and polyethylene bands that limit flexion. It has low morbidity and provided satisfactory clinical outcome at 13-year follow-up [8].
Recently, an alternative fixation technique has been proposed in the treatment of scoliosis and spinal fractures [9–11]. The Universal Clamp system (UC) uses a polyester band that is passed under the lamina and anchored to a rod by a titanium clamp. With little bulk, the UC evenly distributes forces over large areas of cortical bone permitting application of increased forces for spinal deformity reduction. This system allows shorter operating time and easier postoperative investigation of the spinal canal, especially with MRI. Preliminary clinical outcomes in adolescent idiopathic scoliosis have been encouraging with low complication rates and high rates of fusion at 2-year follow up [11]. The simplicity of the system and its theoretical ability to permit motion and avoid fusion make it an appealing candidate implant for dynamic stabilization. The goal of the present study was to compare the effect of the Universal Clamp to that of the Wallis device on intervertebral mobility at the instrumented and adjacent levels.
Materials and methods
Cadaveric specimens and preparation
Nine fresh human lumbar cadaveric spines (L1–Sacrum) were obtained from the Mayo Anatomical Bequest program after IRB approval. Screening radiographs were used to exclude specimens with post-traumatic deformity or significant anatomical anomaly. After removal of all the non-ligamentous soft tissues, the specimens were kept frozen at −20°C. The spines were thawed overnight at room temperature (65° F) just prior to use and kept moist with normal saline-soaked toweling throughout preparation and testing. After thawing, the spines were potted in fixtures that integrated with a custom spine testing apparatus.
Polymethylmethacrylate (PMMA) orthodontic resin was used to pot the upper-most vertebral body in a circular acrylic fixture with k-wires and screws passed through the osseous tissue for secure fixation. The sacrum was embedded in resin and maintained in neutral position with respect to the global coordinate system. Testing was performed at room temperature.
Spine testing apparatus
The potted specimens were attached to a custom spine-testing device previously described [12]. The apparatus has two passive axes of translation in the transverse plane and a third distal axial stage under load control using pneumatic methods. Three-axis gimbals and stepper motors generate motions in flexion–extension, lateral bending, and axial rotation. Forces and moments are measured with a 6-component load cell (JR3, Woodland, CA, USA). Pure moment loading was achieved by the elimination of shear loads with linear slides, as validated by observing the force and moment profiles during testing.
Implants, constructs, and operative procedures
Universal Clamp
On each side of the two instrumented vertebrae, the polyester band of a UC was passed under the lamina and through the clamp, which was connected to 5.5 mm titanium rod and then tightened using a custom pistol previously described [11]. Once the appropriate tension was obtained, the belt was locked to the rod using a screw torqued to 6 N-m. The pliable polyester belt posed little compromise to the canal while providing increased contact with the bone surface compared to sublaminar wiring, yet maintained a low profile.
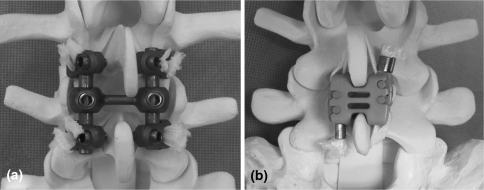
Universal Clamp with transverse connector (Fig. 1a)
Fig. 1.
Implants tested in this study; a Universal Clamp with transverse rod; b Wallis
A transverse connecting rod (52 mm) was added to the UC system to determine if it provided additional restriction of motion (UCTR).
Wallis (Fig. 1b)
The second-generation Wallis implant has an interspinous spacer made of PEEK, which restricts intervertebral extension and two woven Dacron ribbons, which are wrapped around the spinous processes and fixed under tension to limit intervertebral flexion. This dynamic stabilization device is extraosseous, i.e., it does not require the insertion of pedicle screws. In addition to limiting extension, the interspinous block is intended to maintain neuroforaminal height and unload the posterior disc and zygapophyseal joints.
The Wallis implant was inserted using established surgical techniques [13]. The spacer was placed between the anterior spinous processes and secured with the two dacron ligaments that were wrapped around the spinous processes under tension. One of two implant sizes (10 and 12 mm) was used to fit each individual interspinous distance. As recommended, the smallest size that provided sufficient stability on the two spinous processes was chosen to avoid reduction of segmental lordosis.
After being mounted in the testing apparatus, all specimens were tested following the recommended testing criteria for spinal implants [14]. Five conditions were performed in a fixed order: (1) intact; (2) L3–4 instrumented with the UC (two bands on each side); (3) L3–4 instrumented with UC with transverse rod (UCTR); (4) intact; (5) L3–4 instrumented with the Wallis device. During conditions 1, 2, 3, and 4 the polyester bands of the UC were attached to the specimens. In condition 5 the UC bands were removed and the L3–4 interspinous and supraspinous ligaments were transected to allow for Wallis implant placement.
Experimental setup
Kinematic
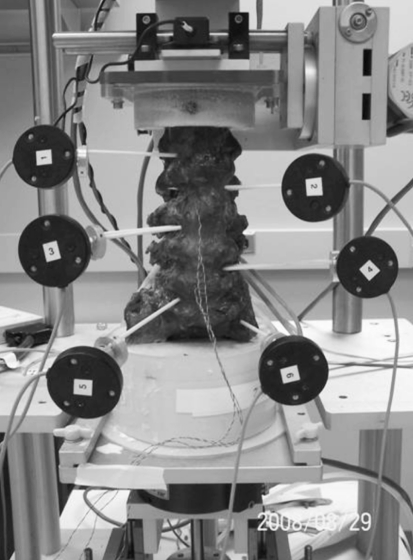
Three-dimensional kinematic measurements were obtained using an Optotrak Certus optoelectric data acquisition system (Northern Digital Inc., ON, Canada) with a far-focus motion sensor (3 camera unit) and accompanying software (The MotionMonitor, Innovative Sports Training Inc., Chicago, IL, USA). Active marker sensors (Innovative Sports Training, Inc.) were rigidly fixed to the bodies of L1, L2, L3, L4, L5, and sacrum (Fig. 2). Two fiberglass pins were inserted into each vertebral body and Optotrak sensors were rigidly secured to the pins with aluminum connecting elements. These fixtures allowed the sensors to be removed and replaced in the identical position and orientation relative to the vertebral body.
Fig. 2.
Specimen mounted in the spine testing apparatus
Data collection
Motion was induced in flexion–extension, lateral bending, and axial rotation with continuous cycles under pure moments until reaching ±7.5 N-m at 4° per second. The range of motion (ROM) of the intact specimen was determined in each plane with no external preload. The load–displacement data was collected until two reproducible load–displacement loops were obtained (3–5 cycles). The position of the sensors in the global coordinate system and the moments measured from the load cell were recorded simultaneously at a frequency of 60 Hz and stored with MotionMonitor software.
Data analysis
The total angular ranges of motion (flexion–extension, lateral bending, and axial rotation) were determined at the instrumented level (L3–4) and adjacent levels (L2–3 and L4–5) in each condition. The neutral position in the sagittal plane was identified in each specimen (mid-point of the region of zero load during flexion and extension). Changes in neutral position were calculated by comparing the experimental condition data to the intact condition. Additionally, the amount of flexion and extension separately (beginning from the neutral position) was determined.
Statistical analysis
Data were compared with one-way repeated measures analysis of variance (ANOVA) (P < 0.05). Pair-wise comparisons were made between levels of instrumentation, when indicated, using Tukey’s Honest Significance test (95% confidence). P values less than 0.05 were considered statistically significant.
Results
Reproducibility
To ensure reproducibility of results, the two intact conditions were recorded and compared. The first intact recording was prior to testing (condition 1) and the second intact was subsequent to instrumentation with the UC but before ligament transaction (condition 4). There were no statistically significant differences between the two intact conditions in regard to ROM observed at instrumented level (L3–4) or at the adjacent levels.
Dynamic stabilization
Global ROM
The mean ROM (L1–S1) across all nine specimens for the intact condition was 34.9° ± 11.4° in flexion–extension, 33.2° ± 11.7° in lateral bending, and 19.9° ± 10.2° in axial rotation (Table 1). There were no statistically significant changes in global ROM due to any instrumentation.
Table 1.
Average total range of motion at the instrumented level, L3–4, and adjacent levels L2–3 and L4–5 for the intact, Universal Clamp (UC), Universal Clamp with transverse connector (UCTR), and Wallis conditions
| Total ROM (mean and SD) (degrees) | Global ROM (mean and SD) (degrees) | |||
|---|---|---|---|---|
| L2–L3 | L3–L4 | L4–L5 | L1–S1 | |
| Flexion–extension | ||||
| Intact | 6.8 (2.5) | 6.6 (3.5) | 8.0 (3.5) | 34.9 (11.4) |
| UC | 6.7 (2.6) | 4.1 (2.2) | 8.0 (3.8) | 32.5 (10.6) |
| −1.3% | −38.3% | −0.7% | −6.8% | |
| UCTR | 7.0 (2.8) | 3.3 (1.4)* | 8.4 (4.4) | 32.5 (10.5) |
| 3.4% | −50.8% | 5.1% | −6.8% | |
| Wallis | 7.3 (2.9) | 5.7 (2.6) | 8.3 (3.7) | 35.1 (11.0) |
| 6.9% | −13.8% | 3.5% | 0.6% | |
| Lateral bending | ||||
| Intact | 7.3 (2.3) | 7.9 (4.5) | 6.8 (3.5) | 33.2 (11.7) |
| UC | 7.6 (2.5) | 7.6 (4.4) | 7.0 (3.8) | 33.6 (12.3) |
| 3.1% | −4.2% | 3.1% | 1.1% | |
| UCTR | 7.9 (3.0) | 6.3 (5.9) | 7.2 (4.0) | 34.0 (13.4) |
| 7.9% | −20.3% | 5.9% | 2.5% | |
| Wallis | 8.1 (3.0) | 8.4 (4.8) | 7.3 (4.0) | 35.4 (13.3) |
| 10.5% | 6.2% | 6.9% | 6.6% | |
| Axial rotation | ||||
| Intact | 4.7 (2.7) | 5.5 (3.9) | 3.8 (2.1) | 19.9 (10.2) |
| UC | 4.9 (2.8) | 4.9 (3.6) | 4.0 (2.3) | 19.6 (10.3) |
| 3.2% | −10.4% | 3.4% | −1.2% | |
| UCTR | 5.0 (3.0) | 4.8 (3.5) | 4.2 (2.4) | 20.0 (10.6) |
| 7.0% | −13.0% | 8.3% | 0.7% | |
| Wallis | 5.1 (3.0) | 5.5 (4.5) | 4.5 (2.5) | 21.0 (11.1) |
| 7.4% | 0.4% | 16.9% | 5.5% | |
Percent change from intact ROM is included. A negative number represents a decrease in ROM as compared to the intact condition. Global total range of motion for the L1–S1 spinal segment is also included
* P < 0.05 for comparisons between intact and instrumented conditions over the entire range of motion
Instrumented level (L3–4) ROM
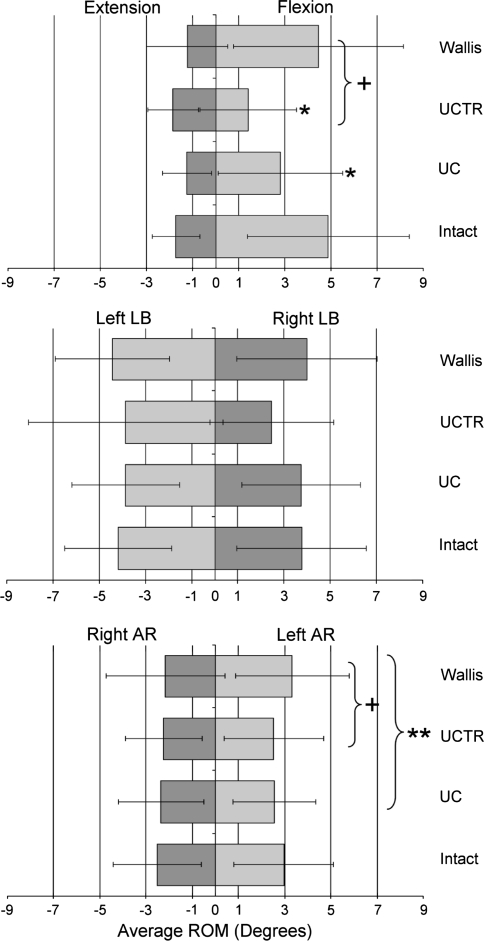
Both the UC and UCTR significantly reduced L3–4 ROM in flexion–extension compared to the intact condition; UC reduced ROM 38.3% while UCTR decreased it by 50.8% (both P < 0.05). Non-significant reductions were observed in other planes with the UC and UCTR (Table 1; Fig. 4). The Wallis reduced flexion–extension at L3–4 by 13.8% (NS) but increased lateral bending and axial rotation ROM by 6.2 and 0.4%, respectively.
Fig. 4.
Average range of motion at the instrumented level (L3–4) for the intact, Universal Clamp (UC), Universal Clamp with transverse connector (UCTR), and Wallis conditions. AR axial rotation, LB lateral bending. *P < 0.05 for comparisons between intact and instrumented conditions; +P < 0.05 for comparisons between UCTR and Wallis; **P < 0.05 for comparisons between UC and Wallis
When observing flexion and extension at L3–4 separately (from the neutral position), the UC and UCTR devices reduced motion in flexion as compared to intact by 44.0% (P < 0.05) and 75.8% (P = 0.0001), respectively. There was no difference in L3–4 ROM when comparing the intact condition to the UC or UCTR conditions in extension, left and right lateral bending, or left and right axial rotation (Fig. 4). Both the UC and UCTR conditions reduced motion at the instrumented level more than the Wallis device. The UC had significantly less angular motion in left axial rotation than the Wallis (P < 0.05). The UCTR had less flexion and left axial rotation than the Wallis (P = 0.0004 and P < 0.05, respectively).
Adjacent level ROM
Adjacent level total ROM data is shown in Table 1. There were no statistically significant changes in angular motion at L2–3 or L4–5 compared to the intact condition when the UC, UCTR, or Wallis devices were implanted. The greatest change at L2–3 was observed with the Wallis device; lateral bending increased 10.5% compared to intact. The greatest change in L4–5 motion was again due to the Wallis device; axial rotation increased 16.9% compared to the intact condition.
Changes in motion segment neutral position
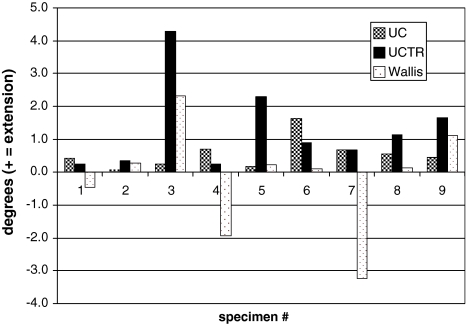
The neutral position of the L3–4 motion segment was consistently shifted toward extension in all specimens when instrumented with the UC (mean shift 0.55 ± 0.46 degrees) and UCTR (mean shift 1.31 ± 1.30 degrees). In contrast, the Wallis device did not predictably shift the neutral position into flexion or extension (Fig. 3). No consistent change in the neutral position was observed at either L2–3 or L4–5.
Fig. 3.
Shift in L3–4 neutral position after application of the Universal Clamp (UC), Universal Clamp with transverse rod (UCTR), and Wallis device for flexion–extension. The UC and UCTR shift to further extension was significantly different than intact (P < 0.05)
Discussion
This study is the first biomechanical comparison between the Wallis and UC devices. Results confirm that both implants restrict motion at the instrumented level without significant changes in the ROM of the adjacent segments.
Spinal fusion has been the standard procedure for the treatment of degenerative instability for many years. However, adjacent segment degeneration (ASD) has been reported as a long-term complication with an incidence ranging from 10 to 100% [15, 16]. The overall revision rate for symptomatic ASD ranges from 2.7 to 20% [17]. Since the 1980s, non-rigid, dynamic implants have been studied in an attempt to reduce ASD. The most commonly used dynamic stabilization systems are either fixed in the pedicles, or secured between the spinous processes [2–7]. The first pedicle screw-based system was likely the Graf ligamentoplasty, which used knitted Dacron bands (INVISTA, Wichita, KS, USA) to lock the facet joints in extension and thus limit flexion [18, 19]. Although positive short-term results were obtained in carefully selected young patients, long-term follow-up results were not encouraging [20, 21]. The Dynesys (Zimmer, Winterthur, Switzerland) is a popular device using titanium alloy pedicle screws and polycarbonate urethane spacers that surround tensioned polyethylene terephtalate (PET) cords on each side in order to reduce facet joint loading [22]. Recent studies have reported significant drawbacks and implant-related complications in pedicle screw-based instrumentation [23]. This has led to the development of implants with non-bony fixation.
The Wallis implant is an interspinous spacer with a load-sharing effect that is expected to restore foraminal height. During extension, the spacer limits the amplitude of the movement without eliminating it. A recent finite-element analysis suggested that the Wallis lowers stress in the disc fibers and annulus matrix, which may contribute to its ability to relieve pain [24]. Recent studies showed that Wallis not only stabilized and reduced intradiscal pressure during extension, but also restricted flexion [7, 25]. Results of the current study confirm its effect on motion, and extension was the most affected. A study by Korovessis et al. found that the Wallis implant could change the natural history of disc degeneration, with significant reduction of clinical and radiological incidence of ASD at 5-year follow-up [26]. The procedure to implant the Wallis is safe and associated with low morbidity, but requires the resection of the supraspinous and interspinous ligaments [13].
The UC is an innovative implant recently described for the surgical correction of spinal deformity and fractures [9, 11]. Initially developed to replace hooks at thoracic levels in scoliosis surgery [10], it can be used in the lumbar spine and might have potential as a non-rigid implant for dynamic stabilization. In addition, the surface area of contact between the polyester band and the lamina is large, and the pull-out strength is not influenced by the patient’s bone mineral density, providing a safe anchor to the spine in most elderly patients [10]. Results of the present study describe its effect on motion; motion is limited but substantial motion persisted at the instrumented level (61.7% of the intact ROM) in flexion–extension without instrumentation failure. Flexion was reduced most (44%), but extension was also reduced in a range comparable to Wallis (Fig. 4). The motion restriction obtained with the UC affected all three planes, even though this reduction failed to achieve significance in lateral bending and axial rotation. This finding, not observed with the Wallis, is of great interest since Fujiwara et al. showed that axial rotation was most affected by disc degeneration, leading to increased ROM with mild and intermediate degeneration [27]. No significant additional motion was observed with either of the implants at the adjacent segments, suggesting a possibly reduced risk of ASD compared to fusion. The transverse connector added significant stability, with 50.8% reduction in flexion–extension, but the UCTR construct might be too rigid (75% reduction in flexion) to be useful as a dynamic stabilization system.
The main drawbacks of the UC techniques is the need for ligamentum flavum resection and potential neurological risk during the sublaminar insertion, although this complication has not been reported [9, 11, 28, 29]. Neither of these implants can be used if decompressive laminectomy is required. Another drawback shared by both of these devices is that neither can be implanted at the L5–S1 level because of anatomical differences between S1 and the lumbar vertebrae.
Two roadblocks to clinical trials of spinal implants are safety concerns and what to do if a device fails to relieve symptoms. These are not great issues with the UC. Its safety has been documented in scoliotic children [11, 28, 29]. It should be easier and safer to use in adults, especially in the lumbar spine where vertebrae are larger. Removal of a UC by section of the bands is straightforward [29] with no reported complications. Moreover, sublaminar polyethylene bands have been shown to have no adverse interactions with the dura [30] and several other groups have reported clinical use of soft sublaminar bands with no observed complications [31–34].
There are several limitations to our study. First, a fixed order of testing was used rather than randomized conditions. This was somewhat unavoidable. We chose to test the Wallis condition last because it required interspinous ligaments resection, but the ligamentum flavum had been previously removed for UC testing. Second, no injury condition was used to test the ability of the implants to restore physiologic motion. Both implants were compared to the intact condition. Third, we were not able to investigate the effect of these constructs on sagittal balance, which is likely of importance for long-term clinical outcomes. There was a consistent shift of the neutral position of the L3–4 motion segment toward extension with both the UC and UCTR, but no consistent change was observed with the Wallis device. We were unable to accurately assess the effect of the devices on the overall lumbar curvature in the sagittal plane as we did not take radiographs before and after device implantation. Although the data from the Optotrak system suggests that any effect on lordosis is not likely great, our data does not allow us to be certain of this. Future studies should include imaging to appropriately assess changes in static lumbar posture. Finally, pure moment testing was performed without additional preload. Although this does not represent ideal physiologic conditions, it is reproducible and in accordance with international recommendations and guidelines for biomechanical testing [14, 35].
In conclusion, the UC restricts motion in all three planes of motion more than the Wallis implant. The greatest difference was observed in the restriction of flexion. This device may warrant preliminary clinical trials as a dynamic stabilization system for degenerative lumbar disorders.
Footnotes
All work were performed at the Mayo Clinic campus in Rochester, MN, USA.
References
- 1.Throckmorton TW, Hilibrand AS, Mencio GA, Hodge A, Spengler DM. The impact of adjacent level disc degeneration on health status outcomes following lumbar fusion. Spine. 2003;28:2546–2550. doi: 10.1097/01.BRS.0000092340.24070.F3. [DOI] [PubMed] [Google Scholar]
- 2.Dubois B, Germay B, Schaerer NS, Fennema P. Dynamic neutralization: a new concept for restabilization of the spine. In: Szpalski M, Gunzburg R, Pope MH, editors. Lumbar segmental instability. Philadelphia: Lippincott/Williams & Wilkins; 1999. pp. 233–240. [Google Scholar]
- 3.Korovessis P, Papazisis Z, Koureas G, Lambiris E. Rigid, semirigid versus dynamic instrumentation for degenerative lumbar spinal stenosis: a correlative radiological and clinical analysis of short-term results. Spine. 2004;29:735–742. doi: 10.1097/01.brs.0000112072.83196.0f. [DOI] [PubMed] [Google Scholar]
- 4.Senegas J, Vital JM, Pointillart V, Mangione P. Long-term actuarial survivorship analysis of an interspinous stabilization system. Eur Spine J. 2007;16:1279–1287. doi: 10.1007/s00586-007-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shim CS, Park SW, Lee SH, Lim TJ, Chun K, Kim DH. Biomechanical evaluation of an interspinous stabilizing device, locker. Spine. 2008;33:E820–E827. doi: 10.1097/BRS.0b013e3181894fb1. [DOI] [PubMed] [Google Scholar]
- 6.Strempel A, et al. Cosmic: dynamic stabilization of the degenerated lumbar spine. In: Yue JJ, et al., editors. Motion preservation surgery of the spine: advanced techniques and controversies. Philadelphia: Elsevier; 2008. pp. 490–499. [Google Scholar]
- 7.Wilke HJ, Drumm J, Haussler K, Mack C, Steudel WI, Kettler A. Biomechanical effect of different lumbar interspinous implants on flexibility and intradiscal pressure. Eur Spine J. 2008;17:1049–1056. doi: 10.1007/s00586-008-0657-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senegas J, Vital JM, Pointillart V, Mangione P. Clinical evaluation of a lumbar interspinous dynamic stabilization device (the wallis system) with a 13-year mean follow-up. Neurosurg Rev. 2009;32:335–341. doi: 10.1007/s10143-009-0199-z. [DOI] [PubMed] [Google Scholar]
- 9.Gazzeri R, Faiola A, Galarza M, Tamorri M. Universal clamp system in thoracolumbar spinal fixation: technical note. Acta Neurochir (Wien) 2009;151:1673–1680. doi: 10.1007/s00701-009-0495-y. [DOI] [PubMed] [Google Scholar]
- 10.Hongo M, Ilharreborde B, Gay RE, Zhao C, Zhao KD, Berglund LJ, Zobitz M, An KN. Biomechanical evaluation of a new fixation device for the thoracic spine. Eur Spine J. 2009;18:1213–1219. doi: 10.1007/s00586-009-0999-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazda K, Ilharreborde B, Even J, Lefevre Y, Fitoussi F, Pennecot GF. Efficacy and safety of posteromedial translation for correction of thoracic curves in adolescent idiopathic scoliosis using a new connection to the spine: the universal clamp. Eur Spine J. 2009;18:158–169. doi: 10.1007/s00586-008-0839-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hongo M, Gay RE, Zhao KD, Ilharreborde B, Huddleston PM, Berglund LJ, An KN, Zhao C. Junction kinematics between proximal mobile and distal fused lumbar segments: biomechanical analysis of pedicle and hook constructs. Spine J. 2009;9:846–853. doi: 10.1016/j.spinee.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Senegas J. Mechanical supplementation by non-rigid fixation in degenerative intervertebral lumbar segments: the Wallis system. Eur Spine J. 2002;11(Suppl 2):S164–S169. doi: 10.1007/s00586-002-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilke HJ, Wenger K, Claes L. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J. 1998;7:148–154. doi: 10.1007/s005860050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Booth KC, Bridwell KH, Eisenberg BA, Baldus CR, Lenke LG. Minimum 5-year results of degenerative spondylolisthesis treated with decompression and instrumented posterior fusion. Spine. 1999;24:1721–1727. doi: 10.1097/00007632-199908150-00014. [DOI] [PubMed] [Google Scholar]
- 16.Kumar MN, Jacquot F, Hall H. Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. Eur Spine J. 2001;10:309–313. doi: 10.1007/s005860000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umehara S, Zindrick MR, Patwardhan AG, Havey RM, Vrbos LA, Knight GW, Miyano S, Kirincic M, Kaneda K, Lorenz MA. The biomechanical effect of postoperative hypolordosis in instrumented lumbar fusion on instrumented and adjacent spinal segments. Spine. 2000;25:1617–1624. doi: 10.1097/00007632-200007010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Guigui P, Chopin D. Assessment of the use of the graf ligamentoplasty in the surgical treatment of lumbar spinal stenosis. Apropos of a series of 26 patients. Rev Chir Orthop Reparatrice Appar Mot. 1994;80:681–688. [PubMed] [Google Scholar]
- 19.Markwalder TM, Dubach R, Braun M. Soft system stabilization of the lumbar spine as an alternative surgical modality to lumbar arthrodesis in the facet syndrome. Preliminary results. Acta Neurochir (Wien) 1995;134:1–4. doi: 10.1007/BF01428494. [DOI] [PubMed] [Google Scholar]
- 20.Grevitt MP, Gardner AD, Spilsbury J, Shackleford IM, Baskerville R, Pursell LM, Hassaan A, Mulholland RC. The graf stabilisation system: Early results in 50 patients. Eur Spine J. 1995;4:169–175. doi: 10.1007/BF00298241. [DOI] [PubMed] [Google Scholar]
- 21.Onda A, Otani K, Konno S, Kikuchi S. Mid-term and long-term follow-up data after placement of the graf stabilization system for lumbar degenerative disorders. J Neurosurg Spine. 2006;5:26–32. doi: 10.3171/spi.2006.5.1.26. [DOI] [PubMed] [Google Scholar]
- 22.Niosi CA, Zhu QA, Wilson DC, Keynan O, Wilson DR, Oxland TR. Biomechanical characterization of the three-dimensional kinematic behaviour of the Dynesys dynamic stabilization system: an in vitro study. Eur Spine J. 2006;15:913–922. doi: 10.1007/s00586-005-0948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grob D, Benini A, Junge A, Mannion AF. Clinical experience with the dynesys semirigid fixation system for the lumbar spine: surgical and patient-oriented outcome in 50 cases after an average of 2 years. Spine. 2005;30:324–331. doi: 10.1097/01.brs.0000152584.46266.25. [DOI] [PubMed] [Google Scholar]
- 24.Lafage V, Gangnet N, Senegas J, Lavaste F, Skalli W. New interspinous implant evaluation using an in vitro biomechanical study combined with a finite-element analysis. Spine. 2007;32:1706–1713. doi: 10.1097/BRS.0b013e3180b9f429. [DOI] [PubMed] [Google Scholar]
- 25.Schulte TL, Hurschler C, Haversath M, Liljenqvist U, Bullmann V, Filler TJ, Osada N, Fallenberg EM, Hackenberg L. The effect of dynamic, semi-rigid implants on the range of motion of lumbar motion segments after decompression. Eur Spine J. 2008;17:1057–1065. doi: 10.1007/s00586-008-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korovessis P, Repantis T, Zacharatos S, Zafiropoulos A. Does wallis implant reduce adjacent segment degeneration above lumbosacral instrumented fusion? Eur Spine J. 2009;18:830–840. doi: 10.1007/s00586-009-0976-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujiwara A, Lim TH, An HS, Tanaka N, Jeon CH, Andersson GB, Haughton VM. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine. 2000;25:3036–3044. doi: 10.1097/00007632-200012010-00011. [DOI] [PubMed] [Google Scholar]
- 28.Ilharreborde B, Even J, Lefevre Y, Fitoussi F, Presedo A, Souchet P, Pennecot GF, Mazda K. How to determine the upper level of instrumentation in lenke types 1 and 2 adolescent idiopathic scoliosis: a prospective study of 132 patients. J Pediatr Orthop. 2008;28:733–739. doi: 10.1097/BPO.0b013e318185a36b. [DOI] [PubMed] [Google Scholar]
- 29.Jouve JL, de Gauzy JS, Blondel B, Launay F, Accadbled F, Bollini G (2010) Use of the universal clamp for deformity correction and as an adjunct to fusion: preliminary results in scoliosis. J Child Orthop 4:73–80 [DOI] [PMC free article] [PubMed]
- 30.Grobler LJ, Gaines RW, Kempff PG. Comparing mersilene* tape and stainless steel wire as sublaminar spinal fixation in the chagma baboon (papio ursinus) Iowa Orthop J. 1997;17:20–31. [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita M, Diab M, Xu Z, Puttlitz CM. A biomechanical analysis of sublaminar and subtransverse process fixation using metal wires and polyethylene cables. Spine. 2006;31:2202–2208. doi: 10.1097/01.brs.0000232831.63589.22. [DOI] [PubMed] [Google Scholar]
- 32.Gaines RW, Jr, Abernathie DL. Mersilene tapes as a substitute for wire in segmental spinal instrumentation for children. Spine. 1986;11:907–913. doi: 10.1097/00007632-198611000-00011. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien JP, Stephens MM, Prickett CF, Wilcox A, Evans JH (1986) Nylon sublaminar straps in segmental instrumentation for spinal disorders. Clin Orthop Relat Res Feb:168-71 [PubMed]
- 34.Takahata M, Ito M, Abumi K, Kotani Y, Sudo H, Ohshima S, Minami A. Comparison of novel ultra-high molecular weight polyethylene tape versus conventional metal wire for sublaminar segmental fixation in the treatment of adolescent idiopathic scoliosis. J Spinal Disord Tech. 2007;20:449–455. doi: 10.1097/bsd.0b013e318030d30e. [DOI] [PubMed] [Google Scholar]
- 35.Goel VK, Panjabi MM, Patwardhan AG, Dooris AP, Serhan H. Test protocols for evaluation of spinal implants. J Bone Joint Surg Am. 2006;88(Suppl 2):103–109. doi: 10.2106/JBJS.E.01363. [DOI] [PubMed] [Google Scholar]