Abstract
The development of scoliosis in animal models after inducing asymmetric rib growth suggests the possible role of asymmetric rib growth in the etiopathogenesis of adolescent idiopathic scoliosis (AIS). Asymmetric rib length is well recognized in idiopathic scoliosis; however, whether this rib asymmetry is primary or secondary has not been clearly documented. The objectives of this study were to investigate any rib length asymmetry in patients with AIS and compare those with scoliosis with syringomyelia (SS) with the intention of elucidating any relationship between rib growth and pathogenesis of AIS. Forty-eight AIS and 29 SS with apical vertebrae located between T7 and T9 were recruited. The average age was 13.5 ± 2.3 versus 12.5 ± 3.4 years, and the average Cobb angle of thoracic curve was 43.3° ± 16.4° versus 45.6° ± 22.6° in patients with AIS or SS, respectively. The length of all ribs was measured from the tip of costal head to the end of the same rib by built-in software on spiral computed tomography. At the levels of the apical vertebrae, the vertebrae above and below the apex, the mean discrepancy in rib length (concave minus convex rib) was 7, 4 and 7 mm, respectively, in AIS group (p < 0.01), and 6, 5 and 7 mm in SS group, respectively (p < 0.01). The rib length discrepancy between concave and convex sides was significantly correlated with the magnitude of the Cobb angle of thoracic curve in both AIS and SS groups (p < 0.01). Similar findings of the asymmetry of rib length in both AIS and SS patients pointed strongly to the fact that the rib length asymmetry in apical region is most likely secondary to the scoliosis deformity rather than playing a primary role in the etiopathogenesis.
Keywords: Idiopathic scoliosis, Rib length, Asymmetry
Introduction
Adolescent idiopathic scoliosis (AIS) is the most common type of spinal deformity arising during peripubertal period. Scoliosis may deteriorate progressively leading to significant cosmetic problems and functional disabilities. A number of hypotheses concerning the etiology of AIS have been proposed, including neuromuscular, genetic, mechanical, growth-related, and developmental factors, but the exact mechanism of development of AIS remains unclear so far [1, 2, 12, 15, 24].
The stability of the thoracic spine is maintained and supported equally by the ribs from both sides [23]. Experimental, clinical, and biomechanical observations have suggested possible role of rib length asymmetry in the development of spinal deformity [9, 10, 14, 16–18, 20]. Langenskiold [9] found that unilateral resection of the posterior ends of ribs could induce progressive scoliosis in young rabbits. Shortening or elongating the ribs has also been shown to affect the development of the spinal column and subsequently resulted in scoliosis deformity [18]. Similar phenomenon was also observed in clinical studies of human [7, 10]. Loynes [10] reviewed the results of 243 patients treated with thoracoplasty for pulmonary tuberculosis and found that scoliosis developed in over 99% of the cases.
Despite the many reports on the development of scoliosis following iatrogenically induced rib asymmetry, few have focused on the association of rib length asymmetry with idiopathic scoliosis. Furthermore, it has not been clearly documented whether the presence of rib asymmetry in idiopathic scoliosis was primary or secondary [5, 6]. In the present study, the lengths of the 1st to 12th ribs (both the concave and convex side) in patients with AIS and scoliosis with syringomyelia (SS) were measured by computed tomography (CT) using multi-projection volume reconstruction method. The objective of this study was to elucidate the possible role of rib asymmetry in the pathogenesis of AIS by comparing the convex–concave rib length discrepancy between two groups of scoliotic patients with different etiology but with similar curve pattern and curve magnitude.
Patients and methods
Clinical data of patients
From 2007 to 2008, 77 adolescent scoliosis patients with age less than 18 were enrolled into this study and subgrouped into AIS and syringomyelia groups following detail clinical and radiological assessments. The inclusion criteria were: main thoracic curve with apical vertebra between T7 and T9, Cobb angle less than 110°. All patients had spiral CT scans under a strict protocol. The exclusion criteria were scoliosis of congenital, metabolic etiology, skeletal dysplasia and known endocrine or connective tissue abnormalities; history of previous spinal or thoracic surgery or any surgery which might affect the rib and spinal growth. Ethical approval was obtained from the University and Hospital Research Ethics Committee. Informed consent was obtained from all study subjects and their parents or guardians before the CT investigation and measurements were conducted.
The AIS group consisted of 48 patients with right side thoracic scoliosis. All subjects underwent full clinical and radiological examination to rule out other causes of scoliosis and to ascertain the diagnosis of AIS. There were 8 males and 40 females with a mean age of 13.5 ± 2.3 years (range 10–18 years) and an average Cobb angle of 43.3° ± 16.4° (range 25°–100°).
Twenty-nine age-matched patients with scoliosis associated with syringomyelia and Chiari I malformation were selected as control for comparison (SS group). Among them, 16 patients had right sided and 13 with left sided thoracic scoliosis. The diagnosis was based on whole spine MRI scanning study. There were 19 males and 10 females in SS group with a mean age of 12.5 ± 3.4 years (range 9–17 years) and an average Cobb angle of 45.6° ± 22.6° (range 25°–105°).
Rib length measurement
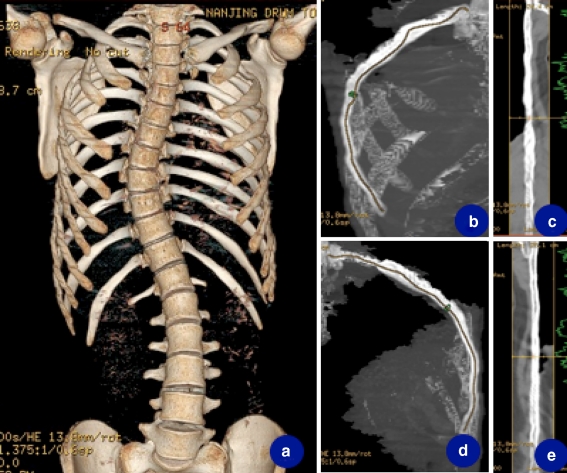
All subjects underwent spiral CT from T1 to T12. The CT examinations were performed by a spiral CT scanner (LightSpeed®16, GE Healthcare) with the following parameters: 400 mA s, 120 kVp, 0.625 mm thicknesses, with 5 mm gap between slices. The lengths of all ribs were measured by multi-projection volume reconstruction method using Volume Viewer (GE Medical system) workstation (Fig. 1). Images of three-dimensional (3D) reconstructions were correlated for the exact vertebral level of each rib. The tip of costal head and the osseous end of the same rib were selected on the 3D reconstruction image.
Fig. 1.
Measurement of rib length by multi-projection volume reconstruction method. a 3D reconstruction of thoracic cage in a 14-year-old right thoracic AIS patient with Cobb’s angle of 45°. b, c Curve reformat of the apical rib on the convex side. The length measured on the reformatted image = 24.4 cm. d, e Similar measurement of the apical rib on the concave side. The rib length = 25.1 cm
Each rib was traced and reformatted into a straight line after which the length of the rib was measured. The value of rib length on the convex and concave side was calculated by the mean value of the rib lengths measured by two observers. The discrepancy of the rib length (as defined by laterality) was calculated from the concave rib length minus the convex rib length at the same level. The equation for laterality (%) is defined as follows: laterality (%) = laterality/[rib length (L + R)/2] × 100%.
The inter-observer variability of the rib length measurement was 11.4% and the reliability was 96% with a kappa value of 0.938 (Landis and Koch’s [8] interpretation of kappa: <0.00 = poor agreement, 0.00–0.20 = slight agreement, 0.21–0.40 = fair agreement, 0.41–0.60 = moderate agreement, 0.61–0.80 = substantial agreement and 0.81–1.00 = almost perfect agreement).
Statistical analysis
All data were expressed as mean ± standard deviation (SD). SPSS/PC 13.0 (SPSS Inc., Chicago, USA) was used on all statistical computation and analysis. Comparison of rib lengths between concave and convex sides was performed by paired Student’s t test. The Pearson correlation analysis was used to evaluate the relationship between the extent of discrepancy of the rib length and the Cobb’s angle in AIS and SS, respectively. A value of p < 0.05 was considered statistically significant.
Results
No significant difference was found between AIS and SS groups in comparing the age and thoracic curve magnitude. In AIS group, laterality of rib length was statistically significant from 8th rib to 12th rib. No significant difference in the rib lengths between two sides could be found for the upper seven ribs (p > 0.05; Table 1).
Table 1.
Rib lengths and laterality between two sides in AIS (n = 48)
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left side length (cm) | 10.1 ± 2 | 19.6 ± 1.9 | 24.2 ± 1.8 | 26.4 ± 1.8 | 27.7 ± 1.8 | 28.1 ± 2.0 | 27.7 ± 2.1 | 26.4 ± 2.2 | 23.9 ± 2.2 | 20.3 ± 2.1 | 15.0 ± 2.4 | 7.8 ± 3.2 |
| Right side length (cm) | 10.1 ± 2 | 19.7 ± 1.8 | 24.1 ± 1.8 | 26.3 ± 1.8 | 27.6 ± 2.0 | 28.1 ± 2.0 | 27.7 ± 2.1 | 26.7 ± 2.3* | 24.5 ± 2.2* | 20.9 ± 2.3* | 15.4 ± 2.3* | 8.0 ± 3.1* |
| Laterality | −0.07 ± 0.27 | 0.07 ± 0.44 | −0.03 ± 0.51 | −0.19 ± 0.54 | −0.13 ± 0.59 | −0.09 ± 0.57 | −0.05 ± 0.52 | 0.30 ± 0.65 | 0.56 ± 0.69 | 0.56 ± 0.74 | 0.19 ± 0.89 | −0.02 ± 1.17 |
| Laterality (%) | −0.65 ± 2.81 | 0.41 ± 2.31 | −0.13 ± 2.07 | −0.71 ± 2.06 | −0.49 ± 2.12 | −0.30 ± 2.03 | −0.15 ± 1.87 | 1.14 ± 2.54 | 2.42 ± 2.97 | 2.75 ± 3.66 | 0.25 ± 12.41 | 4.55 ± 61.63 |
| p value | 0.548 | 0.148 | 0.274 | 0.213 | 0.423 | 0.178 | 0.378 | <0.001 | <0.001 | <0.001 | 0.015 | <0.001 |
* Rib length discrepancy reaches statistical significance (p < 0.05)
The rib lengths on the concave side were significantly longer than those on the convex side in the apical region in both AIS and SS groups (p < 0.01). At the levels of the apical vertebrae, the vertebrae above and below the apex, the mean rib length discrepancy (concave minus convex rib length) was 7, 4 and 7 mm, respectively, in AIS group (p < 0.01), and 6, 5 and 7 mm in SS group, respectively (p < 0.01). However, there was no significant difference in rib lengths between two sides for upper and lower end vertebrae in both AIS and SS groups (Table 2).
Table 2.
Rib length of upper end vertebrae, lower end vertebrae and apical vertebral region (mean ± SD, cm)
| Group | N | Segment | Convex rib length | Concave rib length | p value |
|---|---|---|---|---|---|
| AIS | 48 | Upper end vertebra | 27.5 ± 2.3 | 27.4 ± 2.3 | 0.135 |
| Apex−1 | 26.3 ± 2.2 | 26.7 ± 2.4 | 0.001* | ||
| Apex vertebra | 24.4 ± 3.0 | 25.1 ± 3.0 | 0.000* | ||
| Apex+1 | 21.7 ± 4.2 | 22.4 ± 4.1 | 0.000* | ||
| Lower end vertebrae | 21.3 ± 2.5 | 21.4 ± 2.5 | 0.176 | ||
| SS | 29 | Upper end vertebrae | 23.8 ± 3.7 | 23.9 ± 3.8 | 0.201 |
| Apex−1 | 23.4 ± 2.6 | 23.9 ± 2.5 | 0.014* | ||
| Apex vertebra | 22.2 ± 3.4 | 22.8 ± 3.3 | 0.000* | ||
| Apex+1 | 21.0 ± 4.3 | 21.7 ± 4.2 | 0.000* | ||
| Lower end vertebrae | 21.5 ± 4.5 | 21.8 ± 4.8 | 0.179 |
* Rib length discrepancy reaches statistical significance (p < 0.05)
In both AIS and SS groups, the mean rib length discrepancy of apical vertebrae was positively correlated with the Cobb angle (for AIS group, r = 0.429, p = 0.003; for SS group, r = 0.556, p = 0.001).
Discussion
AIS is considered primarily a deformity of the spine, although it is associated with asymmetries of the entire trunk. Though the etiology is still not clear, its association with abnormal growth during the adolescent growth spurt has been widely reported. This included anomalous growth of the vertebrae, muscular imbalance, central or peripheral nerve impairment or asymmetric growth of the ribs [3, 4, 9, 11, 14].
The stability of the thoracic spine is maintained by equal support through the ribs from both sides. Previous studies demonstrated that the rib length of bilateral sides was isometric in subjects without scoliosis [6, 13]. The observation suggested that a balanced rib cage with symmetrical rib length might be an important factor for maintaining a straight spine. The load from a rib is likely to be transmitted to the transverse process through the costotransverse articulation and ligament. From there the load is transferred onto the lamina, and then the body of the lower vertebra through the facet joint and its pedicle. Asymmetric growth of the ribs, therefore, might play an apparent mechanical role in the development of scoliosis [17, 21, 23]. This is supported by animal models with induced scoliosis after rib resection or rib elongation [14, 17, 20], as well as clinical observation of development of scoliosis after rib resection [7] or thoracoplasty [10].
Previous studies have attempted to demonstrate the asymmetry of rib or rib cage in scoliosis. Normelli [13] compared cadaveric specimens of rib cage in patients with scoliosis and that of the control group. It was found that the ribs on the concave side have an average of 4 mm longer than those on the convex side but the difference was not statistically significant due to the relatively small sample size. Stokes studied the asymmetry of rib cage shape in scoliosis patients by stereoradiographic 3D reconstruction. Rib length asymmetry was observed in different curve types when compared with normal controls. The mean rib length differences in patients with single thoracic curve, single lumbar curve and double curve were 1.39, 3.57 and 3.18%, respectively [22]. Kasai [6] measured the lengths of ribs from 7th to 12th of patients with idiopathic scoliosis using the multi-projection volume reconstruction method on CT. A significant difference was observed in the 11th and 12th ribs but not in 7th, 8th, 9th, and 10th ribs. Kasai [6] speculated that the laterality of rib length was not true in scoliosis as the 11th and 12th ribs were floating ribs.
In the current study, significant discrepancy of rib length was observed at three levels including the apical vertebrae, the vertebrae above and below the apex in both the AIS and SS groups. The rib lengths on the concave side were significantly longer than those on the convex side. It was also interesting to note that significant positive correlation existed between the rib length difference and Cobb angle in both groups. However, no significant rib length discrepancy could be found in other levels and the non-scoliotic vertebral levels.
Similar findings of the asymmetry of rib length in both AIS and SS patients pointed strongly to the fact that the rib length asymmetry in peri-apical region is most likely secondary to the scoliosis deformity rather than playing a significant primary role during the initiation of the curve. First, unlike AIS, the etiopathogenesis of scoliosis associated with syringomyelia is relatively clear [25], which is explained by alteration in the innervations of the trunk musculature. Second, there was a significant positive correlation between the rib length difference and Cobb angle in both groups from the results of current study. The asymmetric load on bilateral side of the ribs could lead to asymmetrical growth in the ribs. The resulting rib length discrepancy in the apex vertebral region could, by itself, also affect the mechanical stability and growth of the thoracic spine and thus create a vicious cycle contributing in part to the progressive deformity; however, this is not unique in idiopathic scoliosis but a general principle applicable to other kind of scoliosis including SS as aforementioned. One limitation of this study, of course, is that it is a cross-sectional study; therefore, we cannot confidently state that those patients with AIS do not have the rib discrepancy to start with, and current study also does not rule out rib-muscle as a causative factor; however, as we notice there is a positive correlation of mean rib length discrepancy at apical vertebra with the Cobb angle, even there is pre-existing rib length asymmetry in AIS (which is minimal before the curve starts to develop), the further progression of asymmetry is likely explained by secondary remodeling of the ribs which is caused by asymmetrical lateral forces from the spine. Another possible explanation of current finding was that the pathogenesis of the scoliotic deformity in AIS and SS groups is the same and that the SS group only presents a variation of the AIS group (with the syringomyelia adding some other characteristics like more kyphosis and other atypical curve patterns). But such hypothesis deserves further exploration to validate. A recent theory for the etiology of idiopathic scoliosis suggested that AIS might be related to the disturbance of the underlying autonomous nerve system [2, 19].
In conclusion, rib length asymmetry was found around the apical vertebral levels in both groups of scoliotic patients with idiopathic cause and those associated with syringomyelia. The findings suggested that the rib length asymmetry is most likely secondary to the spinal deformity rather than causative factor of AIS. As the rib length discrepancy is positively correlated with Cobb’s angle, it might be an associated feature relating to progression of scoliotic curve. However, the above speculations need to be substantiated by further longitudinal studies.
Acknowledgments
Conflict of interest None.
Footnotes
F. Zhu and W. C. Chu contributed equally to this manuscript.
This work was supported by the Provincial Natural Science Foundation of Jiangsu, China (Grant No. BK2009001).
References
- 1.Ahn UM, Ahn NU, Nallamshetty L, Buchowski JM, Rose PS, Miller NH, Kostuik JP, Sponseller PD. The etiology of adolescent idiopathic scoliosis. Am J Orthop (Belle Mead, NJ) 2002;31:387–395. [PubMed] [Google Scholar]
- 2.Burwell RG, Dangerfield PH, Moulton A, Anderson SI. Etiologic theories of idiopathic scoliosis: autonomic nervous system and the leptin-sympathetic nervous system concept for the pathogenesis of adolescent idiopathic scoliosis. Stud Health Technol Inform. 2008;140:197–207. [PubMed] [Google Scholar]
- 3.Fidler MW, Jowett RL. Muscle imbalance in the aetiology of scoliosis. J Bone Joint Surg Br. 1976;58:200–201. doi: 10.1302/0301-620X.58B2.932082. [DOI] [PubMed] [Google Scholar]
- 4.Huynh AM, Aubin CE, Mathieu PA, Labelle H. Simulation of progressive spinal deformities in Duchenne muscular dystrophy using a biomechanical model integrating muscles and vertebral growth modulation. Clin Biomech. 2007;22:392–399. doi: 10.1016/j.clinbiomech.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Iliopoulos P, Korovessis P, Koureas G, Zacharatos S, Stergiou P. Asymmetric evolution of anterior chest wall blood supply in female adolescents with progressive right-convex thoracic idiopathic scoliosis. Eur Spine J. 2007;16:1343–1347. doi: 10.1007/s00586-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasai Y, Takegami K, Uchida A. Length of the ribs in patients with idiopathic scoliosis. Arch Orthop Trauma Surg. 2002;122:161–162. doi: 10.1007/s00402-001-0357-4. [DOI] [PubMed] [Google Scholar]
- 7.Kawakami N, Winter RB, Lonstein JE, Denis F. Scoliosis secondary to rib resection. J Spinal Disord. 1994;7:522–527. doi: 10.1097/00002517-199412000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 9.Langenskiold A, Michelsson JE (1961) Experimental progressive scoliosis in the rabbit. J Bone Joint Surg Br B 43:116–120 [DOI] [PubMed]
- 10.Loynes RD. Scoliosis after thoracoplasty. J Bone Joint Surg Br. 1972;54:484–498. [PubMed] [Google Scholar]
- 11.Masharawi YM, Peleg S, Albert HB, Dar G, Steingberg N, Medlej B, Abbas J, Salame K, Mirovski Y, Peled N, Hershkovitz I. Facet asymmetry in normal vertebral growth: characterization and etiologic theory of scoliosis. Spine (Philadelphia, PA, 1976) 2008;33:898–902. doi: 10.1097/BRS.0b013e31816b1f83. [DOI] [PubMed] [Google Scholar]
- 12.Moreau A, Wang DS, Forget S, Azeddine B, Angeloni D, Fraschini F, Labelle H, Poitras B, Rivard CH, Grimard G. Melatonin signaling dysfunction in adolescent idiopathic scoliosis. Spine (Philadelphia, PA, 1976) 2004;29:1772–1781. doi: 10.1097/01.brs.0000134567.52303.1a. [DOI] [PubMed] [Google Scholar]
- 13.Normelli H, Sevastik J, Akrivos J. The length and ash weight of the ribs of normal and scoliotic persons. Spine (Philadelphia, PA, 1976) 1985;10:590–592. doi: 10.1097/00007632-198507000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Pal GP, Bhatt RH, Patel VS. Mechanism of production of experimental scoliosis in rabbits. Spine (Philadelphia, PA, 1976) 1991;16:137–142. [PubMed] [Google Scholar]
- 15.Qiu XS, Tang NL, Yeung HY, Lee KM, Hung VW, Ng BK, Ma SL, Kwok RH, Qin L, Qiu Y, Cheng JC. Melatonin receptor 1B (MTNR1B) gene polymorphism is associated with the occurrence of adolescent idiopathic scoliosis. Spine (Philadelphia, PA, 1976) 2007;32:1748–1753. doi: 10.1097/BRS.0b013e3180b9f0ff. [DOI] [PubMed] [Google Scholar]
- 16.Sevastik B, Willers U, Hedlund R, Sevastik J, Kristjansson S. Scoliosis induced immediately after mechanical medial rib elongation in the rabbit. Spine. 1993;18:923–926. doi: 10.1097/00007632-199306000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Sevastik J, Agadir M, Sevastik B. Effects of rib elongation on the spine. I. Distortion of the vertebral alignment in the rabbit. Spine (Philadelphia, PA, 1976) 1990;15:822–825. [PubMed] [Google Scholar]
- 18.Sevastik J, Agadir M, Sevastik B. Effects of rib elongation on the spine. II. Correction of scoliosis in the rabbit. Spine. 1990;15:826–829. [PubMed] [Google Scholar]
- 19.Sevastik J, Burwell RG, Dangerfield PH. A new concept for the etiopathogenesis of the thoracospinal deformity of idiopathic scoliosis: summary of an electronic focus group debate of the IBSE. Eur Spine J. 2003;12:440–450. doi: 10.1007/s00586-002-0489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sevastikoglou JA, Aaro S, Lindholm TS, Dahlborn M. Experimental scoliosis in growing rabbits by operations on the rib cage. Clin Orthop Relat Res. 1978;136:282–286. [PubMed] [Google Scholar]
- 21.Sham ML, Zander T, Rohlmann A, Bergmann G. Effects of the rib cage on thoracic spine flexibility. Biomed Tech (Berlin) 2005;50:361–365. doi: 10.1515/BMT.2005.051. [DOI] [PubMed] [Google Scholar]
- 22.Stokes IA, Dansereau J, Moreland MS. Rib cage asymmetry in idiopathic scoliosis. J Orthop Res. 1989;7:599–606. doi: 10.1002/jor.1100070419. [DOI] [PubMed] [Google Scholar]
- 23.Watkins R, 4th, Watkins R, 3rd, Williams L, Ahlbrand S, Garcia R, Karamanian A, Sharp L, Vo C, Hedman T. Stability provided by the sternum and rib cage in the thoracic spine. Spine (Philadelphia, PA, 1976) 2005;30:1283–1286. doi: 10.1097/01.brs.0000164257.69354.bb. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. 2008;371:1527–1537. doi: 10.1016/S0140-6736(08)60658-3. [DOI] [PubMed] [Google Scholar]
- 25.Zhu ZZ, Qiu Y, Wang B, Yang Y, Qian BP, Zhu F. Abnormal spreading and subunit expression of junctional acetylcholine receptors of paraspinal muscles in scoliosis associated with syringomyelia. Spine. 2007;32:2449–2454. doi: 10.1097/BRS.0b013e3181573d01. [DOI] [PubMed] [Google Scholar]