Abstract
Electromyographic (EMG) activity from voluntarily contracting hand muscles undergoes transient suppression following nociceptive fingertip stimulation. This suppression is mediated by a spinal inhibitory reflex designated the cutaneous silent period (CSP). The CSP is abolished or altered in a variety of myelopathic conditions. However, before the CSP can gain acceptance as an aid in the diagnosis of myelopathy, the contribution of non-myelopathic conditions that can interrupt the afferent pathways responsible for the CSP needs to be considered. Accordingly, we examined the effect of radiculopathy on the CSP. Nociceptive stimulation was applied to thumb (C6 dermatome), middle (C7) and little (C8) fingers of 23 patients with cervical radiculopathy. Four or more CSP responses were recorded in abductor pollicis brevis muscle following digital stimulation. The patients had C6 (n = 10), C7 (n = 7), or C8 (n = 6) radiculopathy documented by EMG. A complete CSP was elicited in 21 of 23 patients with comparable latencies and durations irrespective of digit stimulated. We conclude that the CSP is preserved in radiculopathy, probably because afferent impulses are carried by smaller, slower conducting ‘injury-resistant’ A-delta fibers. These results provide important missing evidence that ensures specificity of CSP alterations in the diagnosis of cervical myelopathy. The finding that the CSP is spared in radiculopathy should open the door for investigators and clinicians to adopt this simple spinal inhibitory reflex as a physiologic aid in the diagnosis of spinal cord dysfunction.
Keywords: Spinal reflex, A-delta fibers, Cutaneous silent period, Cervical radiculopathy
Introduction
Most investigators agree that the afferent impulses that generate the cutaneous silent period (CSP) are carried primarily by the smaller, slower conducting A-delta fibers [3, 7, 11, 15]. The afferent impulses enter the dorsal horn and suppress activity in motor nuclei of intrinsic hand muscles via a spinal inhibitory reflex [3, 7, 15]. Conditions that interrupt this reflex pathway should be associated with the absence or delay of the CSP. Indeed, the CSP shows a high sensitivity for detecting spinal cord lesions, including syringomyelia [13], intramedullary spinal cord lesions [6], and myelopathy due to spondylosis or other structural abnormalities of the spine [8, 10, 12]. These myelopathic conditions abolish or alter the CSP [6, 8, 10, 12, 13]. In spite of these data, the clinical acceptance of the CSP as an aid in the diagnosis of cervical myelopathy has faltered. One reason for the apparent widespread lack of acceptance of the CSP in the evaluation of myelopathy is the possibility that spondylosis and root compression may independently alter the CSP. This potentially confounding effect needs to be investigated since it is currently unknown if A-delta fibers are affected by cervical radiculopathy.
The CSP technique can be used to quickly determine whether afferent impulses in A-delta fibers pass through individual cervical roots to enter the spinal cord. This is because the impulses that originate in the fingertips to produce the CSP ascend along their respective nerves and pass through the corresponding C6, C7 or C8 roots [4]. Accordingly, we sought to determine the relationship between transmission along A-delta fibers from different digits and the level of cervical radiculopathy. We hypothesized that the CSP would be abolished or altered if A-delta fibers are affected by cervical radiculopathy. Alternatively, a normal CSP would suggest preserved conduction along A-delta fibers through involved cervical roots.
Materials and methods
The CSP was evoked by applying high-intensity electrical stimulation to the thumb (C6 dermatome), middle finger (C7), and little finger (C8) of 23 patients (18 men, mean age 49 years; 5 women, mean age 51 years) with clinical and electrodiagnostic evidence of cervical radiculopathy while the subjects maintained voluntary contraction of the abductor pollicis brevis (APB) muscle. Single shocks (≤0.2 ms square waves) were delivered to individual fingertips using ring electrodes at intensity at or above the pain threshold. Four or more CSPs were recorded with surface electrodes placed over the APB following stimulation to each digit.
Each patient underwent a problem-focused neurological examination (e.g., assessment of muscle tone, manual muscle testing, deep tendon reflexes, sensory testing), including searching for evidence of myelopathy (spasticity, hyperreflexia, Babinski or Hoffmann’s signs, clonus, sensory level or hemisensory deficits). In addition, all patients were questioned about bowel or bladder dysfunction, gait difficulty, or lower limb symptoms. If patients had signs or symptoms of myelopathy, they were excluded from this study, since myelopathic conditions are known to abolish or alter the CSP [6, 8, 10, 12, 13]. The diagnosis of C6 radiculopathy was made if a patient had radicular pain or altered sensation involving the thumb, ±decreased biceps reflex, ±weakness in muscles supplied by C6, and EMG that showed fibrillation potentials limited to muscles supplied by the C6 root (shoulder girdle muscles, pronator teres or flexor carpi radialis). A diagnosis of C7 radiculopathy was made if symptoms included radicular pain or altered sensation involving the middle or index finger, ±decreased triceps reflex, ±weakness in muscles supplied by C7, and EMG showing fibrillation potentials in muscles supplied by C7 (triceps, pronator teres or flexor carpi radialis). A C8 radiculopathy was diagnosed if radicular symptoms involved the little finger, ±weakness in muscles supplied by C8 (intrinsic hand muscles or forearm digital extensors and flexors) and EMG showing fibrillation potentials in muscles sharing C8 innervation. EMG of cervical paraspinal muscles was not used to localize the level of root involvement. Correlation with imaging studies was not performed because imaging studies were not always available and the focus of this study was on physiological rather than structural abnormalities. Moreover, an extensive literature review on the utility of EMG in cervical radiculopathy indicated only a ‘moderate’ correlation between EMG and imaging findings [1].
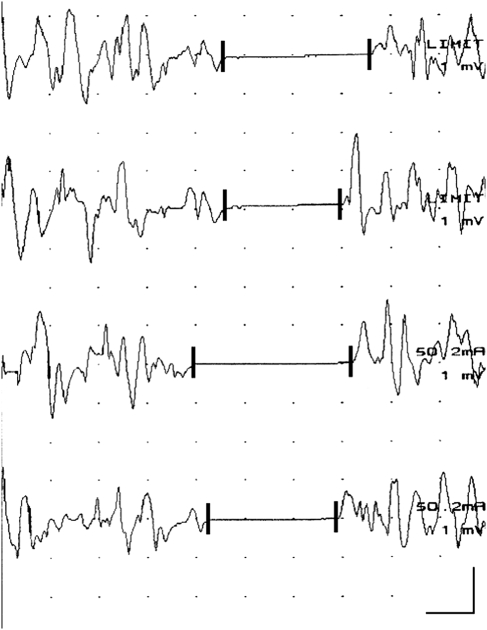
The CSP was analyzed by visual inspection of the individual tracings (Fig. 1). This approach was applied to all data for consistency since some records were only available in the print form. The CSP was defined as the portion of tracing after stimulus artifact with no visible EMG at the display gain ranging from 500 to 1 mV per division. The CSP onset and endpoint for each tracing were marked and their latencies were measured based on the calibration grid (Fig. 1). The CSP duration was calculated as the difference between the endpoint and onset latencies. The mean values for each digit were used for analysis. Multivariate analysis of variance (MANOVA) was used to test whether CSP parameters differed among patients with radiculopathy depending on the digit stimulated. For that purpose, the stimulated digit (thumb, middle finger, little finger) and CSP parameter (onset latency, end latency, duration) were the within-subject factors, whereas the radiculopathy level (C6, C7, C8) was the between-subject factor. The Institutional Review Board approved the clinical research.
Fig. 1.
Sequential cutaneous silent period (CSP) responses recorded from the voluntarily contracting abductor pollicis brevis (APB) muscle following high-intensity digital electrical stimulation. In this case, single shocks (0.2 ms square waves) were delivered to middle fingertip. The CSP was defined as the portion of tracing with no visible EMG activity. CSP onset and endpoint for each tracing was marked (vertical latency markers) and latencies were measured. The CSP duration was calculated as the difference between endpoint and onset latencies. Mean values for each digit were used for analysis. Calibration bar: 20 ms, 1 mV
Results
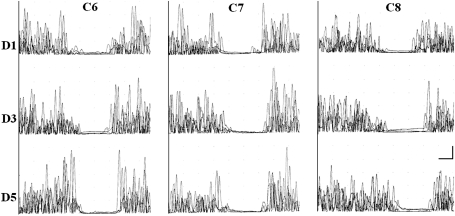
Ten patients had C6, seven had C7, and six had C8 radiculopathy. A CSP defined by complete EMG silence was elicited in 21 of 23 patients from each stimulated digit. In the remaining two patients, no clear CSP responses were elicited regardless of the digit stimulated. One of these patients had a C7 radiculopathy and a remote history of electrocution injury in 1990 while the other had a C8 radiculopathy but no other available history or clinical examination findings of coexisting cervical cord or root injury. Figure 2 shows representative CSP responses following stimulation to the thumb (C6 dermatome), middle finger (C7), and little finger (C8) of patients with C6, C7, and C8 radiculopathies, respectively. MANOVA revealed no significant main effects or interaction. In other words, the CSP onset latency, endpoint latency, and duration did not differ significantly between thumb, middle finger, and little finger stimulation in patients with C6, C7, and C8 radiculopathies (Table 1).
Fig. 2.
Representative cutaneous silent period (CSP) responses following stimulation to digit 1 (D1, top row of tracings, four superimposed rectified trials), digit 3 (D3, middle row), and digit 5 (D5, bottom row) of patients with C6 (left column of tracings), C7 (middle column), and C8 radiculopathy (right column). Radiculopathies were documented by electromyography. CSP responses were recorded from the voluntarily contracting abductor pollicis brevis (APB) muscle. Note the complete CSP responses in all patients irrespective of level of radiculopathy or digit stimulated. Calibration bar: 20 ms, 1 mV
Table 1.
Cutaneous silent period onset latency, end latency, and duration in the abductor pollicis brevis muscle of patients with C6, C7, and C8 radiculopathy
| Onset latency (ms) | End latency (ms) | Duration (ms) | |
|---|---|---|---|
| C6 (n = 10) | |||
| Thumb | 77 ± 6 | 139 ± 17 | 62 ± 18 |
| Middle finger | 81 ± 11 | 139 ± 16 | 57 ± 13 |
| Little finger | 88 ± 16 | 138 ± 16 | 50 ± 11 |
| C7 (n = 7) | |||
| Thumb | 76 ± 10 | 124 ± 9 | 48 ± 16 |
| Middle finger | 82 ± 7 | 127 ± 5 | 45 ± 7 |
| Little finger | 83 ± 10 | 126 ± 5 | 43 ± 12 |
| C8 (n = 6) | |||
| Thumb | 80 ± 11 | 138 ± 16 | 57 ± 16 |
| Middle finger | 80 ± 10 | 145 ± 32 | 65 ± 36 |
| Little finger | 85 ± 10 | 144 ± 21 | 59 ± 26 |
Mean values for each digit per group
Discussion
The main finding of this study is that cervical radiculopathy is not associated with the absence or delay of the CSP. Hence, the findings refute our working hypothesis that afferent fibers responsible for eliciting the CSP are affected by cervical radiculopathies. One reason for this is that the smaller, slower conducting A-delta fibers appear to be relatively less affected by injury or disease that may significantly impair conduction along large afferent fibers. Indeed, the main results of this study are supported by previous findings that indicate differential involvement of small versus large fibers in several related peripheral nervous system conditions. For example, Leis et al. [7] reported preserved CSPs in patients with pure sensory neuropathies who had absent routine sensory nerve action potentials and somatosensory evoked potentials. Aurora et al. [2] reported normal CSP onset latencies in mild to moderate carpal tunnel syndrome, and concluded that small fiber dysfunction occurred late in the course of this compressive neuropathy. Kofler et al. [5] found that preserved CSPs may serve to document residual nerve continuity in severe entrapment neuropathies when fast-conducting fibers were so compromised that their continuity could not be detected by standard electrodiagnostic techniques. Similarly, Svilpauskaite et al. [14] showed that only transection of a peripheral nerve, but not severe entrapment, abolished the CSP. In agreement with previous reports that demonstrated relative preservation of CSP responses in other peripheral nerve syndromes [2, 5, 7, 14], we now provide evidence that the CSP is not affected by cervical radiculopathies. Our findings are also congruous with the observation that many patients seeking medical treatment for cervical radiculopathy do not manifest features of overt axonal loss. For example, in one of the largest population-based studies of cervical radiculopathy, focal weakness or atrophy in a myotomal distribution was an inconsistent finding that was absent in over one-third of patients, and even when present was usually mild in degree [9]. Hence, a substantial number of patients with cervical radiculopathy have sparing of even the larger presumably more vulnerable axons. Although we did not grade severity of the root lesion, all patients in the current series had EMG evidence of loss of motor axons (based on the presence of fibrillation potentials). Consequently, our observations on CSP reported here suggest that even in more severe root lesions, the majority of radiculopathies are not severe enough to alter conduction in the smaller, slower conducting A-delta fibers. Another possible contributing factor to the preserved CSP in radiculopathy is that volleys in A-delta fibers are transmitted to an interneuronal network in the spinal cord that amplifies the incoming signal, thereby still leading to profound inhibition of motoneuron pools. However, cervical spinal cord lesions, including small lesions in the centromedullary region of the cervical cord, may completely abolish the CSP [6, 12, 13], making this possibility less likely. It also remains possible that by averaging a substantially greater number of trials and calculating the degree of relative suppression, a significant difference in CSP parameters may have emerged. However, no study thus far has demonstrated how the number of recorded and averaged trials affects CSP parameters, which is warranted before applying averaging technique in patients. Moreover, any neurophysiologic study that requires a high number of painful stimuli is unlikely to be accepted into clinical practice.
In conclusion, conduction in A-delta fibers is relatively unaffected by cervical radiculopathy. These results provide the missing evidence that ensures specificity of CSP alterations in the diagnosis of cervical myelopathy. The current findings should open the door for investigators and clinicians alike to adopt this quick and simple spinal inhibitory reflex as a physiologic aid in the diagnosis of spinal cord dysfunction.
Acknowledgments
The authors are grateful to the Wilson Research Foundation, Jackson, MS, for their support of clinical research.
Conflict of interest None.
References
- 1.American Association of Electrodiagnostic Medicine. So YT. Guidelines in electrodiagnostic medicine. Practice parameter for needle electromyographic evaluation of patients with suspected cervical radiculopathy. Muscle Nerve. 1999;Suppl 8:S209–S221. [PubMed] [Google Scholar]
- 2.Aurora SK, Ahmad BK, Aurora TK. Silent period abnormalities in carpal tunnel syndrome. Muscle Nerve. 1998;21:1213–1215. doi: 10.1002/(SICI)1097-4598(199809)21:9<1213::AID-MUS16>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Caccia MR, McComas AJ, Upton ARM, Blogg T. Cutaneous reflexes in small muscles of the hand. J Neurol Neurosurg Psychiatry. 1973;36:960–977. doi: 10.1136/jnnp.36.6.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kofler M. Functional organization of exteroceptive inhibition following nociceptive electrical fingertip stimulation in humans. Clin Neurophysiol. 2003;114:973–980. doi: 10.1016/S1388-2457(03)00060-9. [DOI] [PubMed] [Google Scholar]
- 5.Kofler M, Fröhlich K, Saltuari L. Preserved cutaneous silent periods in severe entrapment neuropathies. Muscle Nerve. 2003;28:711–714. doi: 10.1002/mus.10595. [DOI] [PubMed] [Google Scholar]
- 6.Kofler M, Kronenberg MF, Brenneis C, Felber A, Saltuari L. Cutaneous silent periods in intramedullary spinal cord lesions. J Neurol Sci. 2003;216:67–79. doi: 10.1016/S0022-510X(03)00211-9. [DOI] [PubMed] [Google Scholar]
- 7.Leis AA, Kofler M, Ross MA. The silent period in pure sensory neuronopathy. Muscle Nerve. 1992;15:1345–1348. doi: 10.1002/mus.880151209. [DOI] [PubMed] [Google Scholar]
- 8.Lo YL, Tan YE, Dan YF, Leoh TH, Tan SB, Tan CT, Chan LL. Cutaneous silent periods in the evaluation of cord compression in cervical spondylosis. J Neurol. 2007;254:14–19. doi: 10.1007/s00415-007-0142-6. [DOI] [PubMed] [Google Scholar]
- 9.Radhakrishnan K, Litchy WJ, O’Fallon WM, Kurland LT. Epidemiology of cervical radiculopathy. A population-based study from Rochester, Minnesota, 1976 through 1990. Brain. 1994;117(Pt 2):325–335. doi: 10.1093/brain/117.2.325. [DOI] [PubMed] [Google Scholar]
- 10.Roser F, Ebner FH, Liebsch M, Dietz K, Tatagiba M. A new concept in the electrophysiological evaluation of syringomyelia. J Neurosurg Spine. 2008;8:517–523. doi: 10.3171/SPI/2008/8/6/517. [DOI] [PubMed] [Google Scholar]
- 11.Shefner JM, Logigian EL. Relationship between stimulus strength and the cutaneous silent period. Muscle Nerve. 1993;16:278–282. doi: 10.1002/mus.880160306. [DOI] [PubMed] [Google Scholar]
- 12.Štetkárová I, Kofler M (2009) Cutaneous silent periods in the assessment of mild cervical spondylotic myelopathy. Spine 34:34–42 [DOI] [PubMed]
- 13.Štetkárová I, Kofler M, Leis AA. Cutaneous and mixed nerve silent periods in syringomyelia. Clin Neurophysiol. 2001;112:78–85. doi: 10.1016/S1388-2457(00)00486-7. [DOI] [PubMed] [Google Scholar]
- 14.Svilpauskaite J, Truffert A, Vaiciene N, Magistris MR. Cutaneous silent period in carpal tunnel syndrome. Muscle Nerve. 2006;33:487–493. doi: 10.1002/mus.20496. [DOI] [PubMed] [Google Scholar]
- 15.Uncini A, Kujirai T, Gluck B, Pullman S. Silent period induced by cutaneous stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:344–352. doi: 10.1016/0168-5597(91)90023-Q. [DOI] [PubMed] [Google Scholar]