Abstract
Mechanisms regulating intestinal T-cell accumulation during inflammation have considerable therapeutic value. In this study, LPS increased Staphylococcus aureus enterotoxin A-specific T cells in the gut through induction of IL-12 family members. Mice deficient in IL-12 (p35−/−) favored Th17 differentiation in lamina propria, whereas mice lacking both IL-12 and IL-23 (p40−/−) produced significantly fewer Th17 cells. However, serum analysis revealed that IL-27p28 was much higher and sustained following LPS injection than other IL-12 family cytokines. Strikingly, WSX-1 (IL-27Rα) deficiency resulted in log-fold increases in lamina propria Th17 cells without affecting Th1 numbers. These results may be explained by increased expression of α4β7 on WSX-1-deficient T cells after immunization. WSX-1-deficient regulatory T cells (Tregs) were also perturbed, producing more IL-17 and less IL-10 than wild-type Tregs. Thus, IL-27 blockade may provide a new pathway to improve mucosal vaccination.
Keywords: differentiation, lamina propria, LPS, superantigen
Introduction
The activation of antigen-presenting cells (APCs) through TLRs leads to their release of IL-12 family members, which substantially impact T-cell responses. IL-12 (p35/p40) preferentially stimulates IFN-γ production from T cells while IL-23 (p19/p40) stimulates IL-17A (IL-17) (1–3). Thus, genetic deficiency of p35, p19 or p40 results in decreased levels of T-cell-derived IFN-γ, IL-17 or both, respectively (1, 4). Accordingly, IL-12 and IL-23 are characterized as Th1 and Th17 cytokines, respectively.
The third member of the IL-12 family, IL-27, is a heterodimer of subunits named p28 and Epstein–Barr virus-induced gene 3 (EBI3) and signals through a receptor complex containing WSX-1/TCCR and gp130 (5, 6). IL-27 is a Th1-type cytokine since it stimulates IFN-γ production from T cells while inhibiting IL-17 (5, 7–9). In addition, IL-27 has anti-inflammatory properties and suppresses T-cell hyperactivity following infection with Toxoplasma gondii, Mycobacterium tuberculosis and Leishmania donovani (10–12), possibly due to its ability to induce IL-10 in Th1 cells (13–15). EBI3 can also associate with p35 to form the anti-inflammatory cytokine IL-35, which is produced by regulatory T cells (Tregs) (16). Since LPS can induce all five genes within the IL-12 family (17, 18), this may explain its ability to support both Th1 and Th17 responses in vivo (19, 20). The balance of cytokines produced upon LPS stimulation, which could impact T-cell polarization, has not been carefully examined in vivo, and tissue microenvironments such as the intestine versus lymphoid tissue could be differentially regulated. Therefore, we studied the contribution of IL-12 family cytokines toward intestinal T-cell priming following peripheral immunization with LPS.
Materials and methods
Mice
C57BL/6, IL-12p35−/− and IL-12/23p40−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and Taconic Farms (Oxnard, CA, USA). Mice maintained in the animal facility at The University of Connecticut Health Center were kept under specific pathogen-free conditions and handled in accordance to National Institutes of Health federal guidelines. WSX1−/− mice [ref. (21); 92% C57BL/6 background] were maintained at Taconic Farms.
Immunization
Reagents were diluted in PBS and injected intra-peritoneally (i.p.) in a total volume of 0.2ml. Staphylococcal enterotoxin A (SEA; Toxin Tech, Sarasota, FL, USA) was injected at 1 μg per mouse, followed by LPS derived from Salmonella typhimurium (45–50 μg; Sigma–Aldrich; St Louis, MO, USA) 18 h later. LPS doses were determined from titration studies on individual batches to find the amount providing maximal T-cell survival.
Tissue processing
Spleens were crushed through nylon mesh cell strainers (Falcon/BD Biosciences, San Jose, CA, USA) and treated with ammonium chloride to lyse RBCs. Isolation of intestinal lamina propria lymphocytes was based on a previous report (22) with some modifications. The small intestine was excised from mice followed by removal of Peyer's patches and fat. The tissue was then flushed with balanced salt solution (BSS), sliced open longitudinally, cut into ∼1 cm pieces and washed with Ca/Mg-free BSS. Tissue was stirred at 37°C in Ca/Mg-free BSS containing 5 mM EDTA and 0.15 mg ml−1 dithioerythritol, with the supernatant removed. Then, tissue was incubated at 37°C in BSS containing 1 mM CaCl2, 1mM MgCl2, 0.3 mg ml−1 collagenase (Sigma–Aldrich) and 0.1 mg ml−1 DNase I (Sigma–Aldrich). Supernatants from collagenase-treated tissue were poured over cell strainers and spun down at 1400 r.p.m. The cells were then fractionated on a 44% and 67% percoll gradient (Amersham Biosciences; Piscataway, NJ, USA), with lymphocytes partitioning at the interface.
Serum cytokines
Blood was taken from tail veins of mice at 1.5, 3, 6 and 9 h following injection of 120 μg LPS. Blood was kept on ice for ≥30 min followed by centrifugation at 13 000 r.p.m. and 4°C for 20 min, with serum partitioning as the upper fraction. Levels of IL-27p28 and IL-23p19/p40 complex were determined with ELISA kits from R&D Systems (Minneapolis, MN, USA) and eBioscience (San Diego, CA, USA).
Cell culturing
For in vitro re-stimulation, 1 million cells were cultured for 5 h at 37°C in 0.2 ml complete tumor medium, consisting of MEM with fetal bovine serum (FBS) , amino acids, salts and antibiotics. Cells were cultured with phorbol myristate acetate (PMA) (50 ng ml−1; Calbiochem, Gibbstown, NJ, USA) plus ionomycin (1 μg ml−1; Invitrogen), SEA (1 μg ml−1) and brefeldin A (BFA; 5 μg ml−1; Calbiochem), as indicated, and stained intracellularly for cytokines.
Cell staining and flow cytometry
The following mAbs were purchased from eBioscience: Allophycocyanin-conjugated α4β7 integrin, IL-17A and rat IgG2a; PE-conjugated CD62L, CD103 and Foxp3; FITC-conjugated CD44; Alexa-700-conjugated IL-10 and rat IgG2a. The following mAbs were purchased from BD Biosciences: biotinylated TCR Vβ3; Pacific Blue-conjugated CD4; PerCP-conjugated CD4, CD90.1 and streptavidin; PE-conjugated TCR Vβ3; FITC-conjugated CD4, CD8b.2, IFN-γ and rat IgG1. Allophycocyanin-conjugated CXCR3 was purchased from Biolegend (San Diego, CA, USA).
Surface and intracellular staining was performed as described previously (23). Briefly, cells were re-suspended in staining buffer consisting of BSS, 3% FBS and 0.1% sodium azide. Non-specific binding was blocked by a solution containing mouse serum, human IgG and the anti-Fc mAb 2.4G2 (24), followed by incubation with fluorescently conjugated mAbs on ice for 30 min. For intracellular staining, the Foxp3 staining buffer set from eBioscience was used. Flow cytometry was conducted on BD LSR II flow cytometers, with data analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Statistical analysis
Two-tailed Student's t-tests were performed with P < 0.05 representing a significant statistical difference. The type of variance was determined by F-tests, with F > 0.05 corresponding to equal variance and F < 0.05 corresponding to unequal variance.
Results
Divergent roles for IL-12p35 and IL-12/23p40 in determining the balance of Th subsets following immunization
Staphylococcus aureus synthesizes enterotoxins that stimulate massive cytokine responses associated with a variety of inflammatory-based diseases in humans (25). SEA induces TCR Vβ3+ T cells to undergo clonal expansion followed by peripheral deletion unless LPS is co-injected (26). This LPS-rescuing effect is observed in lymph nodes, spleen, liver, lung, bone marrow and small intestinal lamina propria (LP) (19, 23, 27). Although Th1 cells predominate in most tissues, Th17 cells are found in LP and the mechanism regulating this process is unknown. We hypothesized the balance of IL-12 family members induced through SEA and LPS immunization determines T-cell polarization and their accumulation into mucosal tissues.
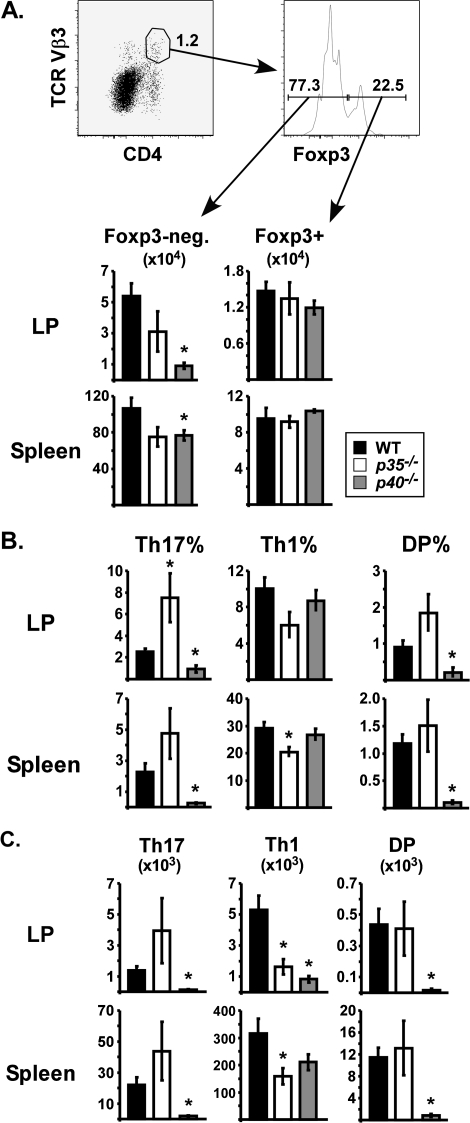
C57BL/6 (wild type, WT), IL-12p35−/− and IL-12/23p40−/− mice obtained from Jackson Laboratory were immunized i.p. with SEA at time 0 and LPS 18 h later. On day 7, the frequency and numbers of CD4+ Vβ3+ T cells were assessed in LP and spleen by flow cytometry. Conventional T cells were distinguished from Tregs by intracellular staining for the transcription factor Foxp3. The total number of Foxp3-negative CD4+ Vβ3+ T cells were >80% lower in the LP of p40−/− mice compared with WT, while a 40% reduction was observed in p35−/− mice (Fig. 1A, left). In spleen, T-cell numbers were modestly reduced by ∼30% in the absence of either IL-12 subunit. Therefore, p40 mediated the accumulation of specific CD4 T cells in the intestine. In contrast, numbers of Foxp3+ CD4+ Vβ3+ Tregs were unaffected by IL-12 deficiency (Fig. 1A, right).
Fig. 1.
Divergent roles for IL-12p35 and IL-12/23p40 in determining the balance of Th subsets following immunization. C57BL/6 (WT; closed bars), IL-12p35−/− (open bars) and IL-12/23p40−/− (gray bars) mice were injected with SEA at time 0 and LPS 18 h later. On day 7, lymphocytes from the small intestinal LP and spleen were analyzed by flow cytometry. (A) Representative dot plots demonstrate the gating. Lymphocytes DP for CD4 and TCR Vβ3 were divided into Foxp3− (left) and Foxp3+ (right) groups, with total numbers shown in bar graphs. (B) Cells were re-stimulated in vitro with SEA for 5 h and stained for intracellular IFN-γ and IL-17A. Charts show the percentage of CD4+ Vβ3+ Foxp3− T cells staining single positive for IL-17 (Th17), single positive for IFN-γ (Th1) or DP. (C) Total numbers of Th17, Th1 and DP cells in each tissue. Data are combined from three experiments with n = 8 and displayed as mean ± SEM. Asterisks represent significant statistical differences compared with WT mice, as determined by two-tailed Student's t-tests (P < 0.05).
To analyze effector cytokine production, cells were re-stimulated with SEA in vitro and stained for intracellular IL-17 and IFN-γ, with Foxp3+ cells excluded from analysis. In the LP of WT mice, ∼2.6% of Foxp3-negative CD4+ Vβ3+ T cells were identified as Th17 (IL-17 single positive; Fig. 1B). In the absence of p35, Th17 levels increased to 7.5%. In contrast, the Th17 percentage was significantly lower in p40−/− mice (Fig. 1B). Th1 differentiation was not affected by p40, but p35−/− mice generated fewer Th1 cells in LP (IFN-γ single positive; P = 0.056; Fig. 1B), demonstrating that p35 influences the Th17:Th1 balance following immunization. A similar trend was observed in spleen with Th17 percentages increased in p35−/− mice and decreased p40−/− mice (Fig. 1B). Total numbers of cytokine-producing cells correlated very well with percentages (Fig. 1C). Although the number of double-positive cells (DP; IL-17+ IFN-γ+) was not affected by p35, significant reductions were observed in the tissues of p40−/− mice.
LPS potently induces IL-27 in vivo
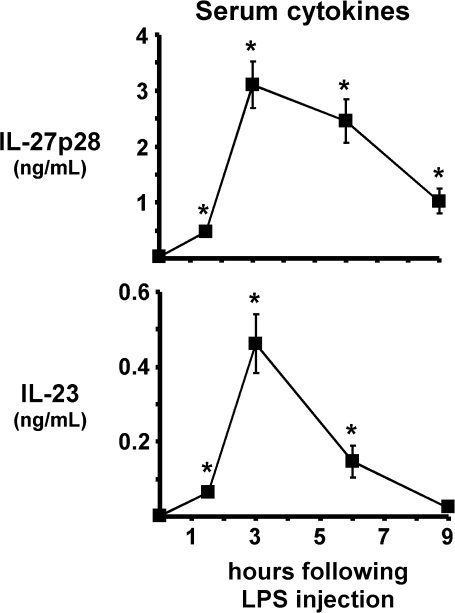
By comparing p35−/− with p40−/− mice, we could distinguish between effects caused by IL-12 versus IL-23. Based on these data, we postulated that the related cytokine IL-27 may also contribute to LPS adjuvanticity and found that i.p. injection of LPS potently increased serum levels of IL-27p28 (Fig. 2). The most significant increase was observed between 1.5 and 3 h, when p28 levels peaked at 3.1 ng ml−1. Interestingly, p28 was sustained for at least 9 h (Fig. 2). By comparison, the kinetics of IL-23 induction covered a much narrower time frame, with levels approaching 0.5 ng ml−1 at 3 h (Fig. 2). Thus, LPS was a much more potent inducer of the Th1 cytokine IL-27 than the Th17 cytokine IL-23.
Fig. 2.
LPS induces IL-27p28 and IL-23 systemically. Mice were injected with 120 μg LPS and serum was collected 1.5, 3, 6 and 9 h later. Line graphs show levels of IL-27p28 and IL-23 as mean ± SEM. Untreated mice were used for time 0. Asterisks represent significant statistical differences compared with time 0. Data are combined from four experiments with 8–11 mice per time point.
The early levels of IL-27p28 were similar to that observed for IL-12p70: at time 0, background levels (146 ± 146 pg ml−1) of IL-12p70 were detected in serum. At 3 h after LPS injection, statistically significant (P = 0.00587) amounts of IL-12p70 were detected (2626 ± 607 pg ml−1), and this declined by 6 h although the levels were still statistically significant compared with time 0 (P = 0.0487, 638 ± 176 pg ml−1). At 9 h, IL-12p70 decreased to 360 ± 288 pg ml−1. In one experiment, we measured IL-12p70 at 90 min after LPS and again found a very high amount (3315 ± 1212 pg ml−1). Therefore, the expansion and contraction kinetics of IL-12p70 in the serum slightly preceded those of IL-27p28.
WSX-1 inhibits Th17 conversion following LPS-based immunization
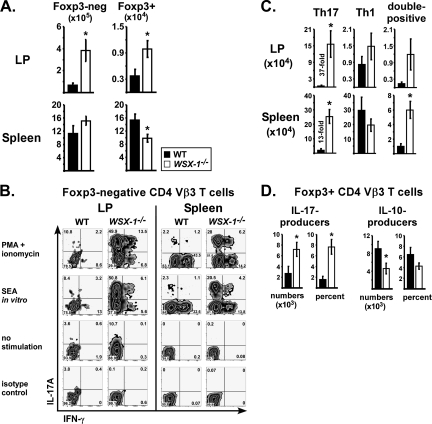
To determine the role of IL-27 signaling in LPS adjuvanticity, WT and WSX-1−/− mice were immunized with SEA and LPS, with tissues examined on day 7. In these experiments, both strains were acquired from Taconic Farms. Significantly more CD4+ Vβ3+ T cells were recovered from the LP of WSX-1−/− mice (Fig. 3A). This increase was selective for the intestinal mucosa since increases were not observed in spleen.
Fig. 3.
WSX-1 inhibits Th17 conversion following LPS-based immunization. WT (closed bars) and WSX-1−/− (open bars) mice were immunized with SEA at time 0 and LPS 18 h later, with tissues analyzed on day 7. (A) Total numbers of CD4+ Vβ3+ Foxp3− (left) or Foxp3+ (right) T cells in small intestinal LP and spleen. (B) Cells re-stimulated in vitro with PMA plus ionomycin, SEA or left unstimulated and were stained for intracellular cytokines. Representative dot plots gated on CD4+ Vβ3+ Foxp3− cells. (C) Total numbers of CD4+ Vβ3+ Foxp3− T cells staining single positive for IL-17A (Th17), single positive for IFN-γ (Th1) or DP in each tissue. (D) Total numbers and percentages of CD4+ Vβ3+ Foxp3+ cells staining single positive for IL-17A or IL-10 following re-stimulation with PMA plus ionomycin as indicated. Data are combined from four experiments with n = 9 and displayed as mean ± SEM, with asterisks representing statistically significant differences between WT and WSX-1−/− mice (P < 0.04).
To determine the balance of Th subsets, cells were re-stimulated in vitro with PMA plus ionomycin, SEA or nothing and then stained for intracellular cytokines. Cells from WSX-1−/− mice had decreased percentages of IFN-γ+ cells and increased percentages of IL-17+ cells compared with WT (Fig. 3B). In the LP of WSX-1−/− mice, over 35% of the Foxp3-negative CD4+ Vβ3+ T cells produced IL-17, versus an average of 5% in WT. When total cell numbers were calculated, WSX-1 deficiency yielded a massive 37-fold increase in Th17 cells compared with WT (Fig. 3C; LP). This effect was very selective since Th1 numbers were not significantly impacted by WSX-1. In spleen, WSX-1 deficiency resulted in 13-fold more Th17 cells. Importantly, LPS was required for this effect since the splenic Th17 frequency was ≤1% in WT and WSX-1−/− mice that were treated with SEA alone or left untreated (data not shown). In spleen, significantly more Foxp3+ Vβ3+ T cells also produced IL-17 in the absence of WSX-1, correlating with fewer IL-10 producers (Fig. 3D). Altogether, LPS-induced Th17 differentiation was potently inhibited by WSX-1. Finally, in the steady state LP, i.e. with no immunization, WSX-1−/− mice had increased IL-17 producers (data not shown).
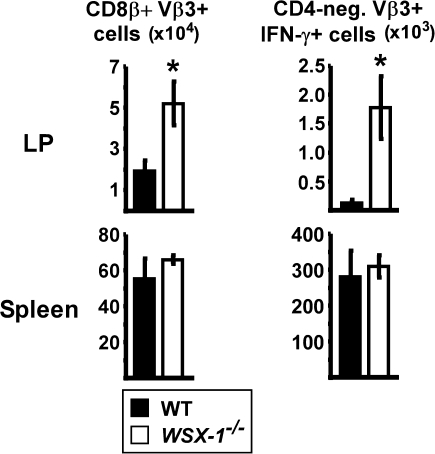
In addition to its effects on CD4 T-cell priming, SEA activates endogenous CD8 T cells expressing Vβ3. The total number of CD8+ Vβ3+ T cells was significantly increased in the LP of WSX-1−/− mice compared with WT (Fig. 4, left). When analyzing cytokine production, gating on the CD4-negative Vβ3+ T cells revealed increased numbers of IFN-γ producers in the absence of WSX-1. In comparison with the spleen, where nearly half of the CD8 T cells produced IFN-γ upon re-stimulation, only 4% were capable of IFN-γ production in the LP. Thus, in comparison with other tissues, the intestinal LP is less conducive for IFN-γ production from both CD4 and CD8 T cells. It is notable that neither WT nor WSX-1−/− CD8 T cells produced IL-17 following immunization (data not shown). Therefore, the inhibitory effect of WSX-1 on IL-17 production was limited to CD4+ T cells.
Fig. 4.
WSX-1 suppresses the accumulation of effector CD8 T cells into small intestinal LP following immunization. WT (closed bars) and WSX-1−/− (open bars) mice were immunized with SEA at time 0 and LPS 18 h later. On day 7, lymphocytes from small intestinal LP and spleen were re-stimulated with SEA in vitro and stained for intracellular cytokines. Charts show total numbers of CD8β+ Vβ3+ (left) or CD4− Vβ3+ IFN-γ+ T cells in each tissue. Data are from the same experiments used for Figs 3 and 5 and displayed as mean ± SEM. Asterisks represent statistically significant differences between WT and WSX-1−/− mice (P < 0.016).
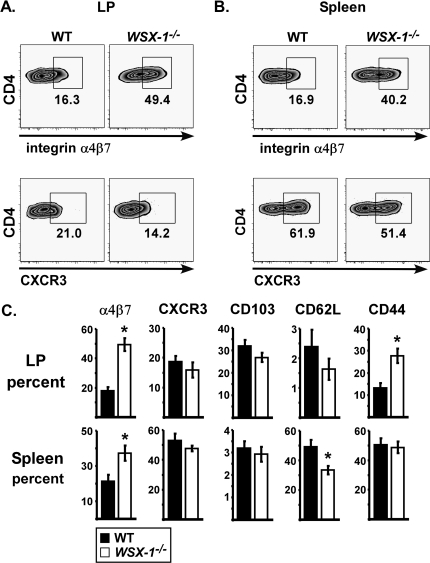
LPS adjuvanticity selectively induces gut homing potential in the absence of WSX-1
T-cell differentiation is inextricably bridged to extra-lymphoid migration, an important consideration for mucosal vaccination (28). To determine if WSX-1 impacted the migratory potential of T cells, we analyzed expression of several integrins by flow cytometry. WSX-1−/− mice contained increased percentages of LP specific CD4 T cells expressing high levels of the gut homing integrin α4β7 following SEA and LPS immunization (Fig. 5A, upper panels). This was consistent with the increased numbers of specific CD4 and CD8 T cells observed in LP (Figs 3A and 4). The spleens of WSX-1−/− mice also contained higher frequencies of CD4+ Vβ3+ T cells expressing α4β7 (Fig. 5B), demonstrating the gut homing phenotype acquired by T cells in lymphoid tissue can be potently inhibited by IL-27. WSX-1 had little effect on expression of the liver homing receptor CXCR3 (Fig. 5A and B, lower panels). Of the markers analyzed, α4β7 expression was most impacted by WSX-1 (Fig. 5C). Other differences included increased expression of CD44 in the LP of WSX-1−/− mice and decreased expression of CD62L, consistent with an activated phenotype (Fig. 5C).
Fig. 5.
WSX-1 suppresses intestinal homing phenotype on T cells following LPS-based immunization. WT (closed bars) and WSX-1−/− (open bars) mice were immunized with SEA at time 0 and LPS 18 h later, with tissues analyzed on day 7. Representative dot plots gated on CD4+ Vβ3+ T cells show expression of integrin α4β7 or CXCR3 in small intestinal LP (A) and spleen (B). (C) Charts show percentage of gated cells staining high for surface markers. Data are from same experiments used for Fig. 3 and displayed as mean ± SEM, with asterisks representing statistically significant differences between WT and WSX-1−/− mice (P < 0.014).
Discussion
Th17 cells contribute to immunity although uncontrolled responses are also associated with autoimmune etiologies [reviewed in ref. (29)]. We show that IL-27 receptor signaling restrains Th17 induction following LPS-based immunization (Fig. 3). This effect was observed in spleen but was most prominent in the intestine where WSX-1 deficiency resulted in a 37-fold increase in Th17 numbers. Perhaps, this is related to the higher steady-state levels of Th17 cells observed in the LP of WSX-1−/− mice [ref. (30) and data not shown]. We favor the hypothesis that WSX-1 inhibits Th17 priming in the spleen and their subsequent migration to intestinal LP. The milieu in the gut appears to be particularly suited for Th17 outgrowth and/or maintenance in the absence of WSX-1 signaling. Although intestinal microbiota may support Th17 differentiation by stimulating IL-23 production from LP-resident dendritic cells (DCs) (31), IL-27 is induced within the intestine upon infection with Trichuris muris or during Crohn's disease (32–34). Our data support earlier findings that one function of IL-27 is to limit IL-17-mediated pathology (7, 8, 10–12). Although we did not study the relative contributions of WSX-1 signaling in T cells versus DCs, another study found that WSX-1−/− DCs were hyperinflammatory to LPS, producing elevated levels of IL-12/23p40, EBI3, IL-12p70, delta4 and tumor necrosis factor (35). Future experiments will be required to determine if IL-27 inhibits Th17 priming in our model through its direct action on T cells or DCs.
LPS induced high serum levels of IL-27p28, which peaked at 3 h and were sustained to 6 h (Fig. 2). The 1 ng ml−1 level observed at 9 h suggests that IL-27 exerts its function over a broad time frame following its induction. In contrast, IL-23 levels were much lower (0.46 ng ml−1) and transient, nearly returning to baseline by 9 h (Fig. 2). The dominant effect of IL-27 over IL-23 could explain why LPS generates robust Th1 responses in most tissues when used as an adjuvant. It is unclear if other pathogen-associated molecular patterns (PAMPs) induce IL-12 family cytokines with similar characteristics as LPS.
Th1 differentiation induced through LPS was unaffected by WSX-1 signaling. Although the percentage of CD4+ Vβ3+ T cells producing IFN-γ was lower in WSX-1−/− mice following immunization (Fig. 3B), the absolute number of specific T cells producing IFN-γ was statistically similar (Fig. 3C). In comparison, WSX-1 is required for Th1 differentiation in response to Listeria monocytogenes and Leishmania major (21, 36), possibly through the induction of T-bet and IL-12Rβ2 on T cells (37, 38). Since WSX-1−/− mice left untreated or given SEA alone had decreased numbers of splenic Th1 cells (data not shown), LPS stimulation in vivo bypasses the requirement for IL-27 in Th1 differentiation and may confer a powerful survival signal that is not seen with infections. Although further studies are necessary to identify the IL-27-independent Th1 factors, possible candidates include IL-12 (Fig. 1B), IL-18 (39) or other products downstream from the TLR4–TRIF pathway (40).
CD8+ Vβ3+ T cells did not produce IL-17 following SEA treatment in the presence or absence of WSX-1 (data not shown). CD8 T cells are a source of IL-17 in humans and mouse models of infection and contact hypersensitivity (41–46). Although antigen stimulation in vivo is not sufficient to generate IL-17-producing CD8 T cells, factors that down-regulate T-bet and Eomesodermin within this population may be involved (47). We found that WSX-1 blocked the accumulation of IFN-γ+ CD8 T cells in the LP but not spleen, suggesting a negative influence for IL-27 on intestinal CD8 T cell responses (Fig. 4; CD4-negative Vβ3+). In contrast, other models have found IL-27 to potentiate IFN-γ production and anti-tumor activity from CD8 T cells (48–51). Thus, the effects of IL-27 on specific cell populations are highly context dependent and warrant investigations on how different PAMPs or routes of immunization influence immunity. For example, intranasal immunization with SEA preferentially recruited CD8 T cells to lung, while the spleen and mesenteric lymph nodes harbored greater levels of CD4 T cells (52). The possibility for IL-27 to influence T-cell migration remains an important consideration for translational research.
The genes encoding individual IL-12 subunits differentially impacted Th polarization. Whereas p35 blocked Th17 commitment, p40 was required for the accumulation of Th17 cells in both spleen and LP (Fig. 1). This suggests that the balance of IL-12 family members determines Th polarization rather than absolute levels. In addition to forming IL-12, p40 can pair with p19 to form IL-23, and p40 also forms homodimers [reviewed in ref. (53)]. In our system, the phenotype of p40−/− mice was very similar to that of p19−/− mice following immunization [Fig. 1 and ref. (19)], suggesting that IL-23 rather than p40 homodimers supports the accumulation of intestinal Th17 cells. Our data correspond with an earlier finding that p40, but not p35, is required for Th17 cell accumulation in vivo (4).
Since p35 and p40 were both required for Th1 cell accumulation in the LP (Fig. 1C), IL-12 appears to be important for intestinal T-cell survival and/or recruitment. Expression of the T-cell homing markers α4β7 and CXCR3 were slightly reduced in IL-12-deficient mice, corresponding with a slight increase in CD62L expression (data not shown). This suggests a positive role for IL-12 in extra-lymphoid T-cell migration, although its impact on homing markers was not striking. In contrast, T-cell expression of α4β7 was significantly increased in the spleen and LP of WSX-1−/− mice, indicating that IL-27 can negatively influence T-cell homing to the intestine (Fig. 5). This hypothesis is supported by earlier findings showing that migration of CD8 T cells to the gut is mediated by beta 7 integrins (54) and that α4β7 expression on CD4 T cells is associated with their rapid egress from lymphoid tissue (20). Interestingly, CXCR3 expression was unaffected by WSX-1 deficiency (Fig. 5C), corresponding with a normal Th1 cytokine response (Fig. 3C). Therefore, IL-27 may control multiple facets of Th17 responses including priming and intestinal migration (Figs 3 and 5). Currently, it is not understood if IL-27 regulates the longevity of Th17 responses.
The impact of IL-27 on Treg development and function is not well understood. Although IL-27 promotes Foxp3 expression through STAT1 activation (55), IL-27 does not increase the percentage of Tregs in cell culture (56–58). In vivo, small T-cell populations are found to co-express Foxp3 and RORγt, the signature transcription factors for Tregs and Th17 cells, respectively (59–61). As IL-27 signaling inhibits RORγt expression (62), it is predicted to tip the balance in favor of Treg development. In support, antigen-specific Foxp3+ CD4 T cells produced less IL-10 and more IL-17 following immunization in the absence of WSX-1 signaling (Fig. 3D). Future studies are necessary to address the effects of IL-27 on Treg function.
Recently, mice from Taconic Farms were shown to have more Th17 cells in the gut than Jackson mice due to the presence of segmented filamentous bacteria (63, 64). In our experiments, gene-deficient mice were always compared with WT from the same vendor, and the WSX-1−/− strain was obtained from Taconic Farms. The extent to which commensal organisms are driving genetic phenotypes is an interesting question. It is notable, however, that increased Th17 numbers were observed in the gut of IL-12p35−/− mice that were purchased from Jackson Laboratory (Fig. 1) and therefore should not contain segmented filamentous bacteria in their microbiome. Overall, this suggests that commensal-dependent and IL-12 family-dependent mechanisms contribute to the balance of Th subsets in gut mucosa.
The role of IL-27/WSX-1 signaling in inflammatory bowel diseases is context dependent. Although WSX-1−/− mice are protected from a chronic low-dose dextran sulfate sodium (DSS)-induced colitis (65), IL-27R−/− mice have worse disease during high-dose DSS treatment, which results in a rapid onset of colitis (30). In the high-dose model, pathology correlated with increased IL-17 levels (30). Chronic colitis caused by IL-10 deficiency was also found to be IL-27R dependent (66). Interestingly, EBI3−/− mice that cannot produce IL-27 were protected from an acute Th2-driven colitis model (67). We favor the hypothesis that IL-27 is therapeutic during Th17-mediated colitis but pathologic during Th1- or Th2-mediated colitis. The finding that IL-27 polymorphisms are associated with human inflammatory bowel diseases warrants further investigations on its regulatory versus inflammatory functions (68).
Funding
National Institutes of Health (R01-AI42858, R01-AI52108 to A.T.V. and T32-AI07080 partially supported J.P.M.).
Disclosures
The authors declare no conflicting financial interests.
References
- 1.Magram J, Connaughton SE, Warrier RR, et al. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 2.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278:1910. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 4.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 6.Pflanz S, Hibbert L, Mattson J, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 2004;172:2225. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 7.Batten M, Li J, Yi S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 2006;7:929. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 8.Stumhofer JS, Laurence A, Wilson EH, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006;7:937. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura T, Takeda A, Hamano S, et al. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J. Immunol. 2006;177:5377. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 10.Villarino A, Hibbert L, Lieberman L, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 11.Holscher C, Holscher A, Ruckerl D, et al. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J. Immunol. 2005;174:3534. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 12.Rosas LE, Satoskar AA, Roth KM, et al. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to Leishmania donovani infection but develop severe liver immunopathology. Am. J. Pathol. 2006;168:158. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Awasthi A, Carrier Y, Peron JP, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007;8:1380. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald DC, Zhang GX, El-Behi M, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 2007;8:1372. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 15.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007;8:1363. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 16.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto SI, Suzuki T, Nagai S, Yamashita T, Toyoda N, Matsushima K. Identification of genes specifically expressed in human activated and mature dendritic cells through serial analysis of gene expression. Blood. 2000;96:2206. [PubMed] [Google Scholar]
- 18.Schuetze N, Schoeneberger S, Mueller U, Freudenberg MA, Alber G, Straubinger RK. IL-12 family members: differential kinetics of their TLR4-mediated induction by Salmonella enteritidis and the impact of IL-10 in bone marrow-derived macrophages. Int. Immunol. 2005;17:649. doi: 10.1093/intimm/dxh247. [DOI] [PubMed] [Google Scholar]
- 19.McAleer JP, Liu B, Li Z, et al. Potent intestinal Th17 priming through peripheral lipopolysaccharide-based immunization. J. Leukoc. Biol. 2010;88:21. doi: 10.1189/jlb.0909631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cucak H, Yrlid U, Reizis B, Kalinke U, Johansson-Lindbom B. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity. 2009;31:491. doi: 10.1016/j.immuni.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida H, Hamano S, Senaldi G, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 22.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2007;2:2307. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
- 23.McAleer JP, Zammit DJ, Lefrancois L, Rossi RJ, Vella AT. The lipopolysaccharide adjuvant effect on T cells relies on nonoverlapping contributions from the MyD88 pathway and CD11c+ cells. J. Immunol. 2007;179:6524. doi: 10.4049/jimmunol.179.10.6524. [DOI] [PubMed] [Google Scholar]
- 24.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J. Exp. Med. 1979;150:580. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 26.Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995;2:261. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 27.Tokoyoda K, Zehentmeier S, Hegazy AN, et al. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30:721. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Lefrancois L, Masopust D. T cell immunity in lymphoid and non-lymphoid tissues. Curr. Opin. Immunol. 2002;14:503. doi: 10.1016/s0952-7915(02)00360-6. [DOI] [PubMed] [Google Scholar]
- 29.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol. Rev. 2008;226:57. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 30.Troy AE, Zaph C, Du Y, et al. IL-27 regulates homeostasis of the intestinal CD4+ effector T cell pool and limits intestinal inflammation in a murine model of colitis. J. Immunol. 2009;183:2037. doi: 10.4049/jimmunol.0802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker C, Wirtz S, Blessing M, et al. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J. Clin. Invest. 2003;112:693. doi: 10.1172/JCI17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Artis D, Villarino A, Silverman M, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J. Immunol. 2004;173:5626. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 33.Bancroft AJ, Humphreys NE, Worthington JJ, Yoshida H, Grencis RK. WSX-1: a key role in induction of chronic intestinal nematode infection. J. Immunol. 2004;172:7635. doi: 10.4049/jimmunol.172.12.7635. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt C, Giese T, Ludwig B, et al. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn's disease but not in ulcerative colitis. Inflamm. Bowel Dis. 2005;11:16. doi: 10.1097/00054725-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J. Immunol. 2007;179:6421. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q, Ghilardi N, Wang H, et al. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 37.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc. Natl Acad. Sci. USA. 2003;100:15047. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeda A, Hamano S, Yamanaka A, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J. Immunol. 2003;170:4886. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 39.Maxwell JR, Yadav R, Rossi RJ, et al. IL-18 bridges innate and adaptive immunity through IFN-gamma and the CD134 pathway. J. Immunol. 2006;177:234. doi: 10.4049/jimmunol.177.1.234. [DOI] [PubMed] [Google Scholar]
- 40.McAleer JP, Rossi RJ, Vella AT. Lipopolysaccharide potentiates effector T cell accumulation into nonlymphoid tissues through TRIF. J. Immunol. 2009;182:5322. doi: 10.4049/jimmunol.0803616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin HC, Benbernou N, Fekkar H, Esnault S, Guenounou M. Regulation of IL-17, IFN-gamma and IL-10 in human CD8(+) T cells by cyclic AMP-dependent signal transduction pathway. Cytokine. 1998;10:841. doi: 10.1006/cyto.1998.0375. [DOI] [PubMed] [Google Scholar]
- 42.Happel KI, Zheng M, Young E, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 2003;170:4432. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanden Eijnden S, Goriely S, De Wit D, Willems F, Goldman M. IL-23 up-regulates IL-10 and induces IL-17 synthesis by polyclonally activated naive T cells in human. Eur. J. Immunol. 2005;35:469. doi: 10.1002/eji.200425677. [DOI] [PubMed] [Google Scholar]
- 44.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J. Immunol. 2006;177:6852. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kryczek I, Wei S, Vatan L, et al. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J. Immunol. 2007;179:1423. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- 46.Hamada H, Garcia-Hernandez Mde L, Reome JB, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 2009;182:3469. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Intlekofer AM, Banerjee A, Takemoto N, et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hisada M, Kamiya S, Fujita K, et al. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- 49.Matsui M, Moriya O, Belladonna ML, et al. Adjuvant activities of novel cytokines, interleukin-23 (IL-23) and IL-27, for induction of hepatitis C virus-specific cytotoxic T lymphocytes in HLA-A*0201 transgenic mice. J. Virol. 2004;78:9093. doi: 10.1128/JVI.78.17.9093-9104.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salcedo R, Stauffer JK, Lincoln E, et al. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J. Immunol. 2004;173:7170. doi: 10.4049/jimmunol.173.12.7170. [DOI] [PubMed] [Google Scholar]
- 51.Mayer KD, Mohrs K, Reiley W, et al. Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J. Immunol. 2008;180:693. doi: 10.4049/jimmunol.180.2.693. [DOI] [PubMed] [Google Scholar]
- 52.Muralimohan G, Rossi RJ, Guernsey LA, Thrall RS, Vella AT. Inhalation of Staphylococcus aureus enterotoxin A induces IFN-gamma and CD8 T cell-dependent airway and interstitial lung pathology in mice. J. Immunol. 2008;181:3698. doi: 10.4049/jimmunol.181.5.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol. Rev. 2008;226:191. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lefrancois L, Parker CM, Olson S, et al. The role of beta7 integrins in CD8 T cell trafficking during an antiviral immune response. J. Exp. Med. 1999;189:1631. doi: 10.1084/jem.189.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ouaked N, Mantel PY, Bassin C, et al. Regulation of the foxp3 gene by the Th1 cytokines: the role of IL-27-induced STAT1. J. Immunol. 2009;182:1041. doi: 10.4049/jimmunol.182.2.1041. [DOI] [PubMed] [Google Scholar]
- 56.Neufert C, Becker C, Wirtz S, et al. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur. J. Immunol. 2007;37:1809. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 57.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J. Immunol. 2008;180:2752. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 58.Huber M, Steinwald V, Guralnik A, et al. IL-27 inhibits the development of regulatory T cells via STAT3. Int. Immunol. 2008;20:223. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 59.Lochner M, Peduto L, Cherrier M, et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J. Exp. Med. 2008;205:1381. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voo KS, Wang YH, Santori FR, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc. Natl Acad. Sci. USA. 2009;106:4793. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diveu C, McGeachy MJ, Boniface K, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J. Immunol. 2009;182:5748. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 63.Ivanov II, Frutos Rde L, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Honda K, Nakamura K, Matsui N, et al. T helper 1-inducing property of IL-27/WSX-1 signaling is required for the induction of experimental colitis. Inflamm. Bowel Dis. 2005;11:1044. doi: 10.1097/01.mib.0000191611.05466.1f. [DOI] [PubMed] [Google Scholar]
- 66.Villarino AV, Artis D, Bezbradica JS, et al. IL-27R deficiency delays the onset of colitis and protects from helminth-induced pathology in a model of chronic IBD. Int. Immunol. 2008;20:739. doi: 10.1093/intimm/dxn032. [DOI] [PubMed] [Google Scholar]
- 67.Nieuwenhuis EE, Neurath MF, Corazza N, et al. Disruption of T helper 2-immune responses in Epstein-Barr virus-induced gene 3-deficient mice. Proc. Natl Acad. Sci. USA. 2002;99:16951. doi: 10.1073/pnas.252648899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li CS, Zhang Q, Lee KJ, et al. Interleukin-27 polymorphisms are associated with inflammatory bowel diseases in a Korean population. J. Gastroenterol. Hepatol. 2009;24:1692. doi: 10.1111/j.1440-1746.2009.05901.x. [DOI] [PubMed] [Google Scholar]