Abstract
Colorectal resection was traditionally associated with significant morbidity and prolonged stay in hospital. Laparoscopic colorectal resection was first described in 1991 as a minimally invasive form of colorectal surgery. It was later on assessed by multiple randomized controlled trials and meta-analysis and was found to be associated with a faster recovery, lower complication rates and a shorter stay in hospital compared with open resection. To assess the effect of enhanced recovery after surgery (ERAS) program on postoperative length of stay after elective colorectal resections, a literature review was conducted, supplemented by the results of 111 ERAS colorectal resections at regional NWS Hospital using a protocol based on the Fast Track approach described by Kehlet in 1999. ERAS has been shown to improve postoperative recovery, reduce length of stay and enhance early return to normal function when compared with traditional colorectal surgical protocols. The role of laparoscopic surgery in colorectal resections within a fast-track (ERAS) program is controversial. The current evidence suggests that within such a program, there is no difference between laparoscopic and open colorectal surgery in terms of postoperative recovery rates or length of hospital stay.
Keywords: Enhanced recovery after surgery, Colorectal surgery, Laparoscopy
INTRODUCTION
Conventional open resection (COR) has been reported to be associated with overall morbidity rates of 23% to 30% and an average hospital stay of 10 d (7 to 12 d)[1-3]. Laparoscopic colorectal resection (CLR) was introduced in 1991 as a proposed less invasive alternative to COR[4,5]. In a published series of 20 sigmoid resections, the authors achieved their aim of a five-day hospital stay in 70% of the cases. Subsequent meta-analysis of randomized trials (RCTs) and of non-randomized comparative studies as well as a Cochrane review showed that CLR was associated with faster recovery, lower complication rates and a shorter stay in hospital compared with COR[2,3,6].
The concept of fast-track (enhanced recovery after surgery, ERAS) was introduced to colorectal surgical practice by Kehlet in 1999 to improve postoperative recovery rates and reduce the length of hospital stay[7]. In a series of 16 open sigmoid colectomies, the authors achieved their aim of a two-day hospital stay in about 60% of the cases.
MAIN ASPECTS OF FAST TRACK COLORECTAL SURGERY
The main aspects of ERAS programs include preoperative patient education, no routine bowel preparation, minimal peri-operative starvation, carbohydrate and protein loading, tailored anesthesia and postoperative analgesia, maintaining high oxygen concentration and normothermia, avoiding peri-operative fluid overload and early postoperative mobilization[8]. Their implementation in a surgical unit requires a team approach involving the surgical, anesthetic, nursing and other staff including physiotherapists, dieticians and stoma therapists. ERAS protocols address almost all aspects of patient management before, during and after admission.
Bowel preparation
One of the main elements of ERAS programs is avoiding routine mechanical bowel preparation (MBP). For over a century, preoperative MBP has been the standard care in colorectal surgery. Although different agents were used for bowel cleansing, the rationale behind MBP includes the evacuation of stool to allow visualization of the luminal surfaces and to reduce fecal flora thereby reducing infections and anastomotic leakage after colorectal surgery. This was challenged as early as 1972 by Hughes who claimed that patients undergoing MBP had similar outcomes to those who did not[9]. In a recently published systematic review of 13 RCTs (4777 resections), the authors found no evidence to suggest that MBP reduced the rate of anastomotic leakage[10]. In patients undergoing low anterior resections, anastomotic leakage occurred in 10% of the MBP group, compared with 6.6% of the no preparation group. For other colorectal resections, anastomotic leakage occurred in 2.9% of the MBP group, compared to 2.5% of the no preparation group. Although the differences were not statistically significant, the results strongly suggested that there was no advantage in routine MBP. In fact there may be a disadvantage in adopting an approach of routine MBP in colorectal resections as a microbiological study found that MBP did not influence the median bacteria colony count in colonic mucosa[11]. A more recent RCT involving 244 participants added to the evidence that colorectal surgery can be performed safely without MBP[12]. MBP is not harmless as it can cause severe dehydration and electrolyte disturbance that may complicate the peri-operative course. The avoidance of MBP is, therefore, one of the central themes of most enhanced recovery or fast-track protocols.
Pre-operative starvation
Another important aspect of traditional colorectal surgery changed by the ERAS approach is the length of pre- and post-operative starvation. The aim of the traditional fasting before surgery is to ensure an empty stomach at the time of anesthetic induction to reduce the risk of aspiration. To avoid confusion, patients are instructed to avoid eating and drinking from midnight the night before surgery and no distinction is made between solid and fluid intake. This strict rule has been questioned as it was shown that drinking clear fluids up to two hours prior to surgery did not increase gastric fluid volume or acidity[13]. A systematic review of 22 trials showed no significant evidence to suggest that shortened preoperative fluid fast increases the risk of regurgitation or aspiration although the majority of trials used gastric fluid volume and acidity as indirect measures of patient safety14. Surgery induces catabolic response characterized by insulin resistance, release of stress hormones (glucagon, cortisol, and catecholamines), and negative nitrogen balance15. Several animal studies have shown that fed animals respond well to hemorrhage or endotoxemia compared to fasted animals[14,15]. Transferring these findings transferred to the clinical setting, patients were tried on oral carbohydrate loading prior to surgery in an attempt to attenuate postoperative insulin resistance. In a randomized controlled study by Kaska and colleagues[16], 221 patients were randomized to fasting, intravenous glucose, or oral carbohydrate fluid. While there was no difference found in the length of hospital stay or complications rate, patients who had preoperative oral carbohydrate had physiological insulin levels postoperatively. This suggests that insulin resistance was the lowest in this group.
Post-operative starvation
Postoperative starvation until flatus is passed per rectum has been a routine surgical practice for fear of anastomotic leakage and postoperative ileus. It is known that malnutrition is prevalent among patients with gastrointestinal cancer[17]. It is also known that the physiological stress of surgery increases the metabolic rate. If postoperative patients are not provided with adequate nutritional support, excessive muscle proteolysis occurs. Protein catabolism with negative nitrogen balance and insulin resistance are the main consequences of prolonged starvation following surgery. In addition, malnutrition is associated with increased intestinal permeability and impaired gut barrier function. A systematic review by Lewis et al[18] evaluated early commencement of post-operative enteral nutrition in 13 RCTs including 1173 patients. Although statistical significance was not reached, there was a trend in favor of early enteral feeding in reducing anastomotic dehiscence, intra-abdominal abscess and wound infection at the expense of a somewhat increased incidence of vomiting.
Routine nasogastric decompression
Routine nasogastric decompression is usually used in conjunction with postoperative fasting. The purpose of prophylactic gastric decompression is to prevent nausea and vomiting, reduce distension, and achieve an earlier return to bowel function. In a Cochrane review of 37 studies investigating the use of prophylactic nasogastric decompression in 5711 patients[19], the authors reported that patients who did not have a nasogastric tube inserted had an earlier return of bowel function. There was no significant difference between the two groups in terms of anastomotic leak rates. Hospital length of stay was shorter when tubes were not routinely used.
Routine prophylactic drainage
Routine prophylactic drainage of colorectal anastomoses has been used to evacuate peri-anastomotic fluid collection. This was thought to reduce the risk of anastomotic dehiscence and allow for early detection and management of anastomotic leakage. A systematic review of 6 RCTs involving 1140 patients randomized to prophylactic drainage or no drainage found no significant differences in the rates of clinical or radiological anastomotic dehiscence, wound infection, or extra abdominal complications between the two groups[20]. Even in rectal or anal anastomoses in which the rate of anastomotic dehiscence is higher than other colorectal resections, routine use of pelvic drainage has not been shown to reduce anastomotic leakage rates[21,22].
Defunctioning ileostomy
Diverting fecal material away from anastomosis site has been thought to reduce the risk of anastomotic leakage in colorectal surgery. However, a Cochrane review of six RCTs showed the use of defunctioning stoma was only useful for resections of very low rectal tumors[23]. A defunctioning ileostomy was found to be associated with a reduced risk of reoperation due to an anastomotic leak for the very low anastomoses (within 5 cm of the anal verge). This was also in agreement with the findings of an earlier systematic review by Hüser and colleagues[24].
Fluid management
Perioperative fluid management continues to be a challenge as patients are often dehydrated due to pre-operative fasting or use of mechanical bowel preparation. Liberal use of intra-operative and post-operative intra-venous isotonic fluids increases cardiopulmonary morbidity, delays return of gastrointestinal function and prolongs hospital stay[25]. Restrictive intra and postoperative fluid resuscitation is found to be associated with fewer complications, earlier return of gastrointestinal function, and shorter hospital stay[26,27].
Postoperative analgesia
A multimodal analgesic approach is an essential component of any ERAS program. Epidural analgesia can be a valuable adjunct to general anesthesia for major abdominal surgeries and has been reported to reduce the risks of postoperative pulmonary complications, nausea and vomiting compared to opiates patient controlled analgesia[28]. The use of epidural local anesthetics in patients undergoing abdominal surgery has also been shown to reduce the incidence of gastrointestinal ileus compared to traditional analgesia or opiate epidural analgesia with comparable analgesic effects[29]. The authors suggested that nociceptive receptors and sympathetic nerves supplying the laparotomy wound inhibit the gastrointestinal tract and that blocking those receptors and nerves reduces the incidence of postoperative ileus. However, in our experience, a multimodal analgesic approach significantly improves postoperative recovery even without epidural analgesia.
Normothermia
Maintaining normothermia is also an important element of ERAS programs. Intra-operative hypothermia occurs in as many as 20% of surgical patients and is usually due to the cold environment of the operating theatre in addition to impaired thermoregulation associated with anaesthesia[30]. Peri-operative hypothermia has been shown to be associated with an increase risk of morbid cardiac events, bleeding and transfusion requirement as well as wound infection[31].
EVIDENCE FOR ERAS PROTOCOLS IN COLORECTAL RESECTIONS
ERAS protocols have been shown to be associated with faster recovery and a reduced length of stay in hospital compared with traditional colorectal resection[8]. A systemic review that included eleven studies (four RCTs, and seven controlled clinical trials) examined the evidence for ERAS protocols when compared with traditional care[32]. ERAS protocols were associated with 2.45 d shorter primary hospital stay, and 2.46 d shorter total hospital stay. Morbidity was lower in the ERAS group and there were no significant differences in readmission rates.
IMPLEMENTATION OF ERAS PROGRAMS
Despite the current evidence supporting the benefits of ERAS protocols, such protocols have not yet been widely adopted[33], probably due to the cost and resources required to train medical, nursing and allied health staff to commit and adhere to a strict program. Some aspects of ERAS protocols have been adopted into traditional care (such as earlier enteral feeding, early mobilization, and multimodal analgesia) without necessarily implementing a structured ERAS protocol.
A REGIONAL HOSPITAL’S EARLY EXPERIENCE WITH ERAS PROTOCOLS IN COLORECTAL SURGERY
A “Fast Track” colorectal cancer resection program was introduced as a structured protocol in July 2006 to Coffs Harbour Health Campus, a regional teaching hospital of the University of New South Wales.
This comprised: (1) Targeted pre-operative education by the colorectal clinical nurse consultant during an unhurried interview at the preadmission clinic with the provision of an information booklet focusing on “What to expect”; (2) An interview with the stoma nurse when indicated; (3) Nutritional assessment if required; (4) Minimal peri-operative starvation; (5) Preoperative carbohydrate and protein loading; (6) No routine MBP. Enema preparation if required; (7) Transverse or oblique incision if seen fit by the operating surgeon; (8) High oxygen concentrations; (9) Actively maintaining normothermia (space blankets, warmers and warm intravenous fluids); (10) Actively avoiding excessive intravenous hydration; (11) No routine use of nasogastric tubes; (12) No routine use of drains; (13) Multimodal Analgesia: (a) Epidural analgesia if seen fit by the anaesthetist; (b) Subcostal nerve block when possible; (c) Continuous wound infiltration with a local anaesthetic agent (wound soaker); (d) Regular oral non-narcotic analgesia; and (e) Minimal morphia only (by using patient activated applications); (14) Routine use of regular prokinetic agents; (15) Routine use of regular anti-emetic drugs; (16) Structured early postoperative mobilization program; (17) Early oral feeding (clear fluids on the evening of surgery, free fluid intake on day one and a soft diet on day two); and (18) Discharge on day 5 whenever possible.
Surgical outcomes
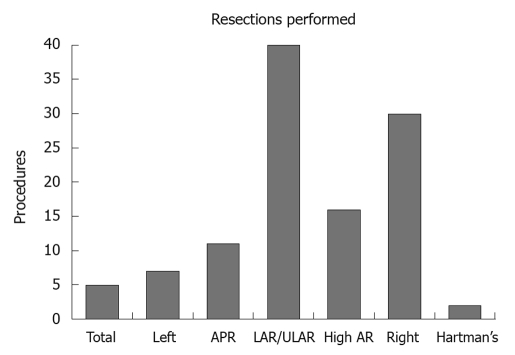
The outcomes of 111 colorectal resections by one surgeon using the ERAS protocol are presented (Figure 1). These comprised 40 low and ultralow anterior resections, 30 right hemicolectomies, 16 high anterior resections, 11 abdominoperineal resections, 7 left hemicolectomies, 5 total colectomies and 2 Hartman’s procedures. The relatively large number of left sided resections was the result of local referral patterns at the time. The median age was 67 years (28 to 88 years).
Figure 1.
Summary of 111 enhanced recovery after surgery colorectal resections by one surgeon at Coffs Harbour July 2006 to July 2010. APR: Abdominoperineal resection; AR: Anterior resection; LAR: Low anterior resection; ULAR: Ultralow anterior resection.
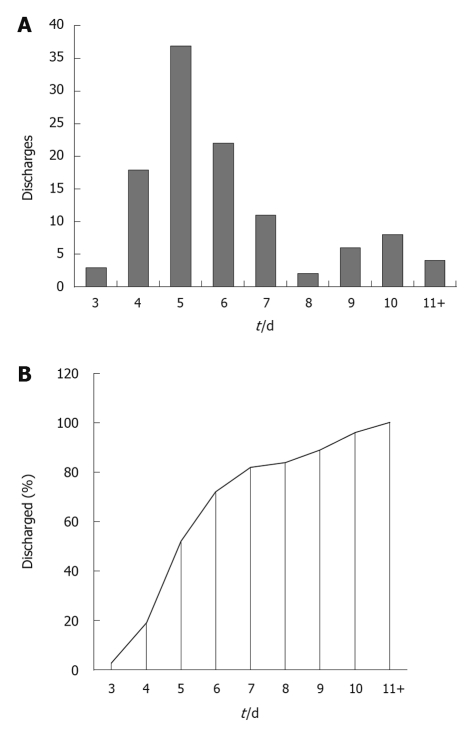
Sixteen patients (14.4%) had other simultaneous procedures. Nine (8.1%) had temporary stomas and 11 (9.9%) permanent. The great majority of the anastomoses were stapled. There were no deaths. Anastomotic leakage, wound and other complications occurred in 4.1%, 10.8% and 13.5% respectively. There were 3 (2.7%) unplanned returns to the operating theatre; all for anastomotic leaks. The median length of stay was 5 d (range: 3 to 21 d). There were 6 (5.4%) unplanned readmissions within a month of the procedure. The median length of stay for the 82 colorectal resections preceding the introduction of the ERAS protocol was 11 d (Figure 2). A patient survey showed high levels of satisfaction with preoperative education, pain management, minimal post-operative fatigue and the fast return to pre-operative mobility level.
Figure 2.
Number (A) and percentage (B) of patients discharged by postoperative day for 111 enhanced recovery after surgery colorectal resections at Coffs Harbour July 2006 to July 2010.
In 2009, a team from the Australian Safety and Efficacy Register of New Interventional Procedures - Surgical (ASERNIP-S) assessed the Coffs Harbour experience and that of others. They reported that fast-track surgery programs can result in beneficial outcomes for patients by reducing the length of hospital stay with no significant increase in readmission rates, that further work is required to assist in standardisation and implementation of protocols and that additional research is required to show how optimised approaches (Fast-track or ERAS programs) would differ from conventional methods[34].
ROLE OF LAPAROSCOPIC SURGERY WITHIN ERAS PROGRAMS
As pointed out above, the introduction of laparoscopic surgery has improved outcomes for patients undergoing colorectal resection with a conventional approach. However, the role of laparoscopic colorectal resection within a fast-track program is controversial. Most trials using the ERAS approach have so far failed to show an advantage in adopting the laparoscopic compared with the open technique. Basse and colleagues randomized 60 patients to either laparoscopic or open surgery within an ERAS rehabilitation program[35] and reported no difference between the two groups in terms of time of return to functional recovery, morbidity, mortality, length of stay or number of readmissions. King and colleagues randomized 62 patients to receive laparoscopic or open surgery within an enhanced recovery program[36] and reported statistically significant differences between the two groups. The sample sizes were small in those two trials. A systematic review of the above two RCTs and three controlled clinical trials again failed to show a significant difference between laparoscopic and open surgery in the context of ERAS rehabilitation[37]. In a subsequent meta-analysis of 11 studies (4 RCTs and 7 controlled trials) including 1021 patients, the authors reported a clear benefit in adopting the ERAS approach with no evidence for an advantage in adopting the laparoscopic technique[32]. Laparoscopic colorectal resection has been shown to be associated with an increase in operating time (about 35%) and cost (at least 20%) as well as a steep learning curve compared with open resection[2,3,38].
CONCLUSION
The current evidence suggests that the implementation of an ERAS Program is associated with a faster recovery and a shorter length of hospital stay with no increase in complication rates at the expense of a possible small increase in readmission rates. Furthermore, with the implementation of such a program, the laparoscopic technique does not seem to show any advantage over the conventional open surgical approach. We currently aim to prospectively assess the results of laparoscopic versus open colorectal resections within an ERAS program. The LAFA trial[39] will examine laparoscopic and open colorectal surgery with or without fast-track rehabilitation and should shed more light on the issue.
Acknowledgments
The authors would like to acknowledge the assistance of Moya Anderson (Clinical Nurse Consultant), Tracey Moore (Data Manager), Dr. Ross WB, Dr. Smith M and others for their assistance in establishing the ERAS protocol at the Coffs Harbour Health Campus in 2006.
Footnotes
Peer reviewer: Calogero Iacono, MD, Professor, Department of Surgery, University Hospital “GB Rossi”, Verona 37134, Italy
S- Editor Wang JL L- Editor Hughes D E- Editor Lin YP
References
- 1.Bokey EL, Chapuis PH, Fung C, Hughes WJ, Koorey SG, Brewer D, Newland RC. Postoperative morbidity and mortality following resection of the colon and rectum for cancer. Dis Colon Rectum. 1995;38:480–486; discussion 486-487. doi: 10.1007/BF02148847. [DOI] [PubMed] [Google Scholar]
- 2.Abraham NS, Byrne CM, Young JM, Solomon MJ. Meta-analysis of non-randomized comparative studies of the short-term outcomes of laparoscopic resection for colorectal cancer. ANZ J Surg. 2007;77:508–516. doi: 10.1111/j.1445-2197.2007.04141.x. [DOI] [PubMed] [Google Scholar]
- 3.Abraham NS, Young JM, Solomon MJ. Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br J Surg. 2004;91:1111–1124. doi: 10.1002/bjs.4640. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy) Surg Laparosc Endosc. 1991;1:144–150. [PubMed] [Google Scholar]
- 5.Redwine DB, Sharpe DR. Laparoscopic segmental resection of the sigmoid colon for endometriosis. J Laparoendosc Surg. 1991;1:217–220. doi: 10.1089/lps.1991.1.217. [DOI] [PubMed] [Google Scholar]
- 6.Schwenk W, Haase O, Neudecker J, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005:CD003145. doi: 10.1002/14651858.CD003145.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kehlet H, Mogensen T. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg. 1999;86:227–230. doi: 10.1046/j.1365-2168.1999.01023.x. [DOI] [PubMed] [Google Scholar]
- 8.Wind J, Polle SW, Fung Kon Jin PH, Dejong CH, von Meyenfeldt MF, Ubbink DT, Gouma DJ, Bemelman WA. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93:800–809. doi: 10.1002/bjs.5384. [DOI] [PubMed] [Google Scholar]
- 9.Hughes ES. Asepsis in large-bowel surgery. Ann R Coll Surg Engl. 1972;51:347–356. [PMC free article] [PubMed] [Google Scholar]
- 10.Guenaga KK, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2009:CD001544. doi: 10.1002/14651858.CD001544.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Jung B, Matthiessen P, Smedh K, Nilsson E, Ransjö U, Påhlman L. Mechanical bowel preparation does not affect the intramucosal bacterial colony count. Int J Colorectal Dis. 2010;25:439–442. doi: 10.1007/s00384-009-0863-3. [DOI] [PubMed] [Google Scholar]
- 12.Scabini S, Rimini E, Romairone E, Scordamaglia R, Damiani G, Pertile D, Ferrando V. Colon and rectal surgery for cancer without mechanical bowel preparation: one-center randomized prospective trial. World J Surg Oncol. 2010;8:35. doi: 10.1186/1477-7819-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Ljungqvist O, Søreide E. Preoperative fasting. Br J Surg. 2003;90:400–406. doi: 10.1002/bjs.4066. [DOI] [PubMed] [Google Scholar]
- 14.Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003:CD004423. doi: 10.1002/14651858.CD004423. [DOI] [PubMed] [Google Scholar]
- 15.Nygren J, Thorell A, Ljungqvist O. Preoperative oral carbohydrate nutrition: an update. Curr Opin Clin Nutr Metab Care. 2001;4:255–259. doi: 10.1097/00075197-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Kaska M, Grosmanová T, Havel E, Hyspler R, Petrová Z, Brtko M, Bares P, Bares D, Schusterová B, Pyszková L, et al. The impact and safety of preoperative oral or intravenous carbohydrate administration versus fasting in colorectal surgery--a randomized controlled trial. Wien Klin Wochenschr. 2010;122:23–30. doi: 10.1007/s00508-009-1291-7. [DOI] [PubMed] [Google Scholar]
- 17.Garth AK, Newsome CM, Simmance N, Crowe TC. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J Hum Nutr Diet. 2010;23:393–401. doi: 10.1111/j.1365-277X.2010.01058.x. [DOI] [PubMed] [Google Scholar]
- 18.Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J Gastrointest Surg. 2009;13:569–575. doi: 10.1007/s11605-008-0592-x. [DOI] [PubMed] [Google Scholar]
- 19.Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev. 2007:CD004929. doi: 10.1002/14651858.CD004929.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jesus EC, Karliczek A, Matos D, Castro AA, Atallah AN. Prophylactic anastomotic drainage for colorectal surgery. Cochrane Database Syst Rev. 2004:CD002100. doi: 10.1002/14651858.CD002100.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merad F, Hay JM, Fingerhut A, Yahchouchi E, Laborde Y, Pélissier E, Msika S, Flamant Y. Is prophylactic pelvic drainage useful after elective rectal or anal anastomosis? A multicenter controlled randomized trial. French Association for Surgical Research. Surgery. 1999;125:529–535. [PubMed] [Google Scholar]
- 22.Yeh CY, Changchien CR, Wang JY, Chen JS, Chen HH, Chiang JM, Tang R. Pelvic drainage and other risk factors for leakage after elective anterior resection in rectal cancer patients: a prospective study of 978 patients. Ann Surg. 2005;241:9–13. doi: 10.1097/01.sla.0000150067.99651.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montedori A, Cirocchi R, Farinella E, Sciannameo F, Abraha I. Covering ileo- or colostomy in anterior resection for rectal carcinoma. Cochrane Database Syst Rev. 2010:CD006878. doi: 10.1002/14651858.CD006878.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Hüser N, Michalski CW, Erkan M, Schuster T, Rosenberg R, Kleeff J, Friess H. Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg. 2008;248:52–60. doi: 10.1097/SLA.0b013e318176bf65. [DOI] [PubMed] [Google Scholar]
- 25.Lobo DN, Bostock KA, Neal KR, Perkins AC, Rowlands BJ, Allison SP. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 2002;359:1812–1818. doi: 10.1016/S0140-6736(02)08711-1. [DOI] [PubMed] [Google Scholar]
- 26.Nisanevich V, Felsenstein I, Almogy G, Weissman C, Einav S, Matot I. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology. 2005;103:25–32. doi: 10.1097/00000542-200507000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Holte K, Kehlet H. Fluid therapy and surgical outcomes in elective surgery: a need for reassessment in fast-track surgery. J Am Coll Surg. 2006;202:971–989. doi: 10.1016/j.jamcollsurg.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 28.White PF, Kehlet H, Neal JM, Schricker T, Carr DB, Carli F. The role of the anesthesiologist in fast-track surgery: from multimodal analgesia to perioperative medical care. Anesth Analg. 2007;104:1380–1396, table of contents. doi: 10.1213/01.ane.0000263034.96885.e1. [DOI] [PubMed] [Google Scholar]
- 29.Jørgensen H, Wetterslev J, Møiniche S, Dahl JB. Epidural local anaesthetics versus opioid-based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database Syst Rev. 2000:CD001893. doi: 10.1002/14651858.CD001893. [DOI] [PubMed] [Google Scholar]
- 30.Qadan M, Gardner SA, Vitale DS, Lominadze D, Joshua IG, Polk HC Jr. Hypothermia and surgery: immunologic mechanisms for current practice. Ann Surg. 2009;250:134–140. doi: 10.1097/SLA.0b013e3181ad85f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Díaz M, Becker DE. Thermoregulation: physiological and clinical considerations during sedation and general anesthesia. Anesth Prog. 2010;57:25–32; quiz 33-34. doi: 10.2344/0003-3006-57.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gouvas N, Tan E, Windsor A, Xynos E, Tekkis PP. Fast-track vs standard care in colorectal surgery: a meta-analysis update. Int J Colorectal Dis. 2009;24:1119–1131. doi: 10.1007/s00384-009-0703-5. [DOI] [PubMed] [Google Scholar]
- 33.Lassen K, Hannemann P, Ljungqvist O, Fearon K, Dejong CH, von Meyenfeldt MF, Hausel J, Nygren J, Andersen J, Revhaug A. Patterns in current perioperative practice: survey of colorectal surgeons in five northern European countries. BMJ. 2005;330:1420–1421. doi: 10.1136/bmj.38478.568067.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strum L, Cameron AL. Fast-track surgery and enhanced recovery after surgery (ERAS) programs. ASERNIP-S Report No. 74. Adelaide, South Australia: ASERNIP-S, March; 2009. [Google Scholar]
- 35.Basse L, Jakobsen DH, Bardram L, Billesbølle P, Lund C, Mogensen T, Rosenberg J, Kehlet H. Functional recovery after open versus laparoscopic colonic resection: a randomized, blinded study. Ann Surg. 2005;241:416–423. doi: 10.1097/01.sla.0000154149.85506.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King PM, Blazeby JM, Ewings P, Franks PJ, Longman RJ, Kendrick AH, Kipling RM, Kennedy RH. Randomized clinical trial comparing laparoscopic and open surgery for colorectal cancer within an enhanced recovery programme. Br J Surg. 2006;93:300–308. doi: 10.1002/bjs.5216. [DOI] [PubMed] [Google Scholar]
- 37.Vlug MS, Wind J, van der Zaag E, Ubbink DT, Cense HA, Bemelman WA. Systematic review of laparoscopic vs open colonic surgery within an enhanced recovery programme. Colorectal Dis. 2009;11:335–343. doi: 10.1111/j.1463-1318.2008.01679.x. [DOI] [PubMed] [Google Scholar]
- 38.Bokey EL, Moore JW, Chapuis PH, Newland RC. Morbidity and mortality following laparoscopic-assisted right hemicolectomy for cancer. Dis Colon Rectum. 1996;39:S24–S28. doi: 10.1007/BF02053802. [DOI] [PubMed] [Google Scholar]
- 39.Wind J, Hofland J, Preckel B, Hollmann MW, Bossuyt PM, Gouma DJ, van Berge Henegouwen MI, Fuhring JW, Dejong CH, van Dam RM, et al. Perioperative strategy in colonic surgery; LAparoscopy and/or FAst track multimodal management versus standard care (LAFA trial) BMC Surg. 2006;6:16. doi: 10.1186/1471-2482-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]