Abstract
Background
Ischaemic preconditioning (IPC) of the right liver graft in the donor has not been studied in adult-to-adult living related liver transplantation (LRLT).
Objective
To assess the IPC effect of the graft on ischaemia reperfusion injury in the recipient and compare recipient and donor outcomes with and without preconditioned grafts.
Patients and methods
Alternate patients were transplanted with right lobe grafts that were (n =22; Group Precond) or were not (n =22; Group Control) subjected to IPC in the living donor. Liver ischaemia–reperfusion injury, liver/kidney function, morbidity/mortality rates and outcomes were compared. Univariate and multivariate analyses were performed to identify factors predictive of the aspartate aminotransferase (AST) peak and minimum prothrombin time.
Results
Both groups had similar length of hospital stay, morbidity/mortality, primary non-function and acute rejection rates. Post-operative AST (P =0.8) and alanine aminotransferase (ALT) peaks (P =0.6) were similar in both groups (307 ± 189 and 437 ± 302 vs. 290 ± 146 and 496 ± 343, respectively). In univariate analysis, only pre-operative AST and warm ischemia time (WIT) were significantly associated with post-operative AST peak (in recipients). In multivariate analysis, the graft/recipient weight ratio (P =0.003) and pre-operative bilirubin concentration (P =0.004) were significantly predictive of minimum prothrombin time post-transplantation.
Conclusions
Graft IPC in the living related donor is not associated with any benefit for the recipient or the donor and its clinical value remains uncertain.
Keywords: LRLT, graft ischemic preconditioning
Introduction
Living related right lobe liver transplantation (LRRLLT) was a breakthrough in the field of adult liver transplantation1 (for a review see2,3). This technique increased the availability of grafts at a time when there was a critical shortage of cadaveric organs resulting in a very high death rate among patients on waiting lists.4 It may also improve the prognosis of certain indications.5,6 Use of the right liver lobe has several advantages over use of the left lobe for adult-to-adult liver transplantation: better anatomic position for the vascular reconstruction, lower risk of hepatic venous outflow block and increased hepatic mass. To ensure donor safety, an adequate liver mass must be left to avoid post-operative hepatic dysfunction. Several strategies have been designed to optimize the function of the liver graft in the recipient and the remaining liver in the donor. These include stringent selection of the donor (with liver biopsy in many centres),2,3,7 preparation of the recipient, avoidance of vascular clamping during procurement, the choice of the preservation solution and the shortest ischaemia time possible.
Several recent animal studies have shown that ischaemic preconditioning (IPC) (especially brief exposure to warm ischemia) provides robust protection against ischemia–reperfusion injury during subsequent long periods of ischaemia. This phenomenon was first described for the heart.8 It has since been described in many experimental models (for review see9,10) and for several tissues and organs, including the liver.11–17 It has been shown to be protective in three clinical studies on liver resection.18–20 It has also been shown that a short period of warm ischaemia protects different organs including the heart, intestine, lung and kidney21,22 against subsequent cold ischaemia–reperfusion injury. Several studies in experimental models23–25 and humans26,27 suggest that IPC is suitable for use during liver transplantation. The aim of the present study was to evaluate the effects of IPC of the right lobe liver graft in the living donor on ischaemia–reperfusion injury in the recipient. We compared the rates of ischaemia–reperfusion injury, early graft function, mortality, morbidity and patient survival in the two groups of recipients. We also assessed the impact of graft IPC on donor outcome.
Patients and methods
Objectives of the study
The primary end point of the study was the graft tolerance to ischaemia reperfusion as assessed by the post-operative peak AST concentration in the recipient.18–20 Secondary end points included the analysis of the potential clinical relevance of IPC on early graft function, mortality, morbidity and patient survival. We also assessed the impact of graft IPC on donor outcome.
Study population and experimental design
This single centre prospective study was conducted during a 4-year period and included 44 consecutive cases of adult-to-adult LRRLLT. Alternate patients were assigned to the two study groups. Twenty-two patients received a graft that had been preconditioned in the donor (Group Precond): 10 min of right pedicle clamping including clamping of all arteries feeding the right liver, the right portal branch(es) and the main bile duct, followed by 10 min of reperfusion. Twenty-two patients received a graft that had not been preconditioned (Group Control). LRRLLT performed during the study period for fulminant hepatic failure (four cases) was the only exclusion criteria from the study. The protocol of preconditioning was identical to the one used in the two clinical studies reported so far.18–20 The sample size calculation was based on the results of the 15 first cases of LRRLLT performed at our centre, by the same surgeon (D.A.) and without any vascular clamping. In these 15 cases the mean peak aspartate aminotransferase (AST) concentration was 274 ± 126 IU/L (standard error =27.6 IU/L). The sample size needed to detect a significant difference for the peak AST concentration was calculated as 22 cases on each group, using a two-sided test (α =0.05, β =0.1). The protocol of the study was approved by the investigation and review board of our centre. Informed consent was obtained from all donors and recipients.
Surgical procedures
Right liver graft harvesting
The same procurement technique was used throughout the study. This technique has been reported in detail previously.7 No vascular clamping (except during the preconditioning stage, when applied) was performed during the harvesting procedure. The middle hepatic vein and/or the inferior hepatic vein were included in the graft as indicated.28,29 The graft was perfused ex situ with 1 L of cold University of Wisconsin (UW) solution. All donor hepatectomies were performed by the same surgeon (D.A.).
Transplantation technique
In brief, the native liver was totally removed with caval preservation.30 A temporary porta-caval shunt was performed.31 The right lobe was then implanted. A microscope was used for arterial and biliary anastomosis in all cases. All graft implantations were performed by the same surgeon (D.C.). Cold ischaemia time (CIT) was defined as the time between devascularization in the donor and portal reperfusion in the recipient. Warm ischaemia time (WIT) was defined as the time between portal unclamping and arterial revascularization. A liver biopsy was taken before closing the abdomen. This biopsy was available for 19/22 (86%) and 20/22 (91%) of Group Control and Group Precond recipients, respectively (P > 0.9). None of the graft was steatotic, as macrovacuolar steatosis >20% of hepatocytes was never observed. Ischaemia–reperfusion injury was classified as moderate to severe (vs. absent) when at least 10% of hepatocytes were necrotic, mainly in the centre of the lobule or disseminated throughout.32
Post-operative management
Donors
In addition to the usual post-operative care received after right hepatectomy, we measured the indocyanine green clearance rate at 15 min (ICG RT15′) and used CT imaging to measure the volume33–35 of the remaining left liver 8 and 30 days after donation.
Recipients
Transplanted patients received a standard immunosuppressive regimen including FK-506 and methylprednisolone. Early outcome was assessed by1 measuring ischaemia–reperfusion liver injury, as estimated by the peak AST concentration2 liver function tests, including determination of the minimum prothombin time and the peak bilirubin concentration within 10 days of transplantation3 measuring primary non-function, defined as immediate absence of graft function leading to retransplantation or death4. For the later group, the following technical complications were recorded: hemoperitoneum needing reoperation, and arterial, portal, outflow and biliary complications. Histologically proven acute rejection was recorded, provided it occurred within 6 weeks of transplantation and needed increased immunosuppression.36 Post-operative mortality was defined as death occurring during the initial hospitalization period after transplantation or within 60 days of transplantation.
Data analysis
All quantitative data are expressed as mean ± standard deviation. A P-value < 0.05 was considered statistically significant. Factors potentially predictive of the peak AST concentration in the recipient within 10 days of transplantation were assessed by univariate and multivariate analysis. The donor data included: age, pre-operative liver function tests, duration of operation, intra-operative blood loss, volume of autotransfusion and graft preconditioning; and the recipient data included: age, gender, pre-operative liver function tests, graft to recipient body weight ratio, cold ischaemia time, warm ischemia time and transfusion volume.
The same assessment was performed for the minimum prothrombin time. For continuous variables, differences between the groups were evaluated using the Student's t-test. For discrete variables, data were expressed as counts and percentages and were analysed using Fisher's exact test. Survival rates were calculated using the Kaplan–Meier method, and groups were compared with the log rank test. All statistical analyses were performed using SAS software (SAS Institute, Inc., Cary, NC, USA).
Biologists, intensive care specialists and pathologists were not informed whether the graft had been subjected to ischaemic preconditioning in the donor.
Results
Pre- and per-operative data (Tables 1 and 2)
Table 1.
Pre-, per- and post-operative characteristics of right lobe donors
| Characteristics | Donor of (R) liver without preconditionning n =22 cases | Donor of (R) liver with preconditionning n =22 cases | P-value |
|---|---|---|---|
| Male/female (ratio) | 6/16 | 8/14 | 0.5 |
| Age (years) | 37.8 ± 13.1 | 41.4 ± 15.4 | 0.4 |
| Left liver to body weight ratio (%) | 0.76 ± 0.15 | 0.83 ± 0.18 | 0.2 |
| Duration of operation (minutes) | 319 ± 107 | 274 ± 38 | 0.08 |
| Number of blood units autotransfused | 0.5 ± 0.8 | 0.5 ± 0.9 | 0.9 |
| Peak AST (IU/L) | 189.0 ± 70.5 | 231.5 ± 130.5 | 0.2 |
| Peak ALT (IU/L) | 193.1 ± 81.1 | 236.9 ± 135.2 | 0.2 |
| Minimum prothrombin time (% of normal) | 38.7 ± 7.9 | 36.0 ± 7.5 | 0.3 |
| Peak bilirubin (µmol/L) | 51.1 ± 22.3 | 68.8 ± 39.5 | 0.08 |
| Morbidity rate n cases (%) | 11 (50%) | 15 (68%) | 0.7 |
| Duration of hospital stay (days) | 13.4 ± 6.4 | 14.1 ± 4.1 | 0.6 |
| ICG RT 15′ | |||
| Day 8 | 12.7 ± 4.2 | 12.1 ± 6.7 | 0.8 |
| Day 30 | 11.3 ± 4.0 | 11.7 ± 6.5 | 0.9 |
| Left liver to body weight ratio (%) | |||
| Day 8 | 1.34 ± 0.29 | 1.34 ± 0.31 | >0.9 |
| Day 30 | 1.67 ± 0.25 | 1.50 ± 0.31 | 0.1 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Table 2.
Pre-, per- and post-operative characteristics of recipients
| Characteristics | Recipient of (R) liver without pre-conditionning n =22 cases | Recipient of (R) liver with pre-conditionning n =22 cases | P-value |
|---|---|---|---|
| Male/female (ratio) | 17/5 | 18/4 | >0.9 |
| Age (years) | 46.5 ± 13.6 | 47.6 ± 11.5 | 0.8 |
| No. transplanted for | 0.5 | ||
| Cirrhosis | 6 | 9 | |
| Cancer | 13 | 8 | |
| Other | 3 | 5 | |
| Graft to body weight ratio (%) | 1.14 ± 0.39 | 1.18 ± 0.31 | 0.7 |
| Cold ischaemia time (minutes) | 87 ± 31 | 92 ± 28 | 0.6 |
| Warm ischaemia time (minutes) | 68 ± 27 | 63 ± 27 | 0.6 |
| Number of blood units transfused | 4.5 ± 4.6 | 3.7 ± 3.5 | 0.9 |
| Peak AST (IU/L) | 290 ± 146 | 307 ± 189 | 0.4 |
| Peak ALT (IU/L) | 496 ± 343 | 437 ± 302 | 0.8 |
| Minimum prothrombin time (% of normal) | 36.4 ± 13.3 | 37.7 ± 8.5 | 0.7 |
| Bilirubin day 7 (µmol/L) | 107 ± 97 | 132 ± 104 | 0.4 |
| Duration of stay (days) | |||
| Overall | 41.3 ± 21.3 | 35.7 ± 8.7 | 0.3 |
| In intensive care unit | 10.4 ± 5.4 | 12.0 ± 6.6 | 0.4 |
| Morbidity n (%) | 7 (31.8) | 8 (36.4) | 0.9 |
| In-hospital mortality n (%) | 2 (9) | 1 (4.5) | 0.5 |
| Actuarial survival rate at 1 year (%) | |||
| Patient | 86.1 | 95.5 | 0.08 |
| Graft | 88.9 | 80.2 | 0.6 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
The two donor groups were similar (Table 1) in terms of age (P =0.4), gender ratio (P =0.5), pre-operative liver function tests including the ICG RT15′ (P≥ 0.2 in all cases), intra-operative data including duration of operation (P =0.08) and volume of auto-transfused blood (P =0.9). The remaining left liver to body weight ratio was similar in the two groups (P =0.2).
The two recipient groups were similar (Table 2) in terms of age (P =0.8), gender ratio (P > 0.9), indication for transplantation (P =0.5), liver function tests before transplantation (P > 0.2 in all cases) and intra-operative data including graft-to-body weight ratio (P =0.7), cold ischaemia time (P =0.6), warm ischaemia time (P =0.6) and number of blood units transfused (P =0.9).
Donor outcome (Table 1)
The peak AST concentration was similar in the two groups of donors (189.0 ± 70.5 vs. 231.5 ± 130.5 IU/L – group without vs. with graft preconditioning, respectively, P =0.2). Liver function tests were similar at all time points, including ICG RT 15′ at day 8 (P =0.8) and day 30 (P =0.9), and volume of remaining left liver at day 8 (P =0.7) and day 30 (P =0.6).
At least one complication occurred in 11 (morbidity rate =50%) and 15 (morbidity rate =68%) donors in the control and preconditioned group, respectively (P =0.7). None of these complications were life-threatening. The duration of hospitalization was similar in the two groups (P =0.6). All donors were alive and well at 24 ± 15 months after donation.
Tolerance to ischaemia–reperfusion in recipients (Table 2, Fig. 1)
Figure 1.
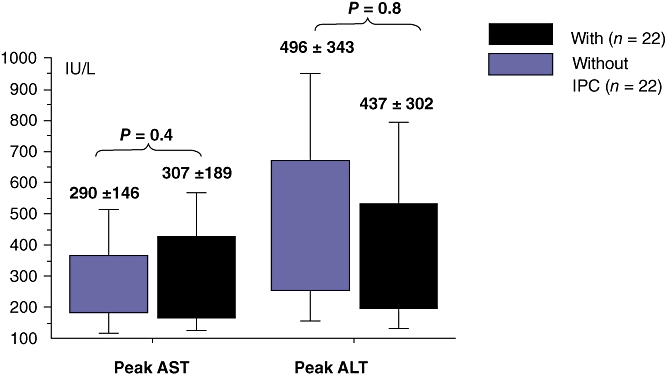
Ischaemia–reperfusion injury in the recipient after living related right lobe liver transplantation (LRRLLT)
We found no significant difference in serum concentrations of AST and ALT between the two groups at any time point. Patients in Group Precond had a similar peak AST concentration (307 ± 189 vs. 290 ± 146 IU/L, P =0.4) and a similar peak ALT concentration (437 ± 302 vs. 496 ± 343 IU/L, P =0.8) as patients in Group control (Fig. 1).
Factors associated with the maximum AST concentration within 10 days of transplantation
Factors potentially predictive of the maximum AST concentration in the recipient were assessed using univariate and multivariate analysis. The donor data included: age, pre-operative liver function tests, duration of operation, intra-operative blood loss, volume of autotransfusion and graft preconditioning; and the recipient data included: age, gender, pre-operative liver function tests, graft-to-recipient body weight ratio, CIT, WIT and transfusion volume.
Univariate analysis showed that only the pre-operative AST concentration in the recipient and the warm ischaemia time were associated with peak post-operative AST concentration (P =0.04 and P =0.03, respectively). Using multiple variable analysis none of the later factors remained independently significant.
Graft function and factors associated with the minimal prothrombin time
We found no significant difference in prothrombin time and bilirubin concentration between the two groups of recipients at any time point (Table 2, Fig. 2). Univariate analysis showed that the following factors were associated with the minimal prothrombin time: presence of cirrhosis (P =0.01), the combination of female donor to male recipient (P =0.04), graft to recipient body weight ratio (P =0.02), number of blood units transfused in the recipient (P =0.02), pre-operative bilirubin concentration in the recipient (P =0.002), and recipient preoperative prothrombin time (P =0.006). None of the other factors tested were significant, including preconditioning of the graft, CIT, and WIT (data not shown). In the multivariate analysis, graft-to-recipient body weight ratio (P =0.003) and recipient pre-operative bilirubin concentration (P =0.004) remained independently associated with the minimal prothrombin time.
Figure 2.
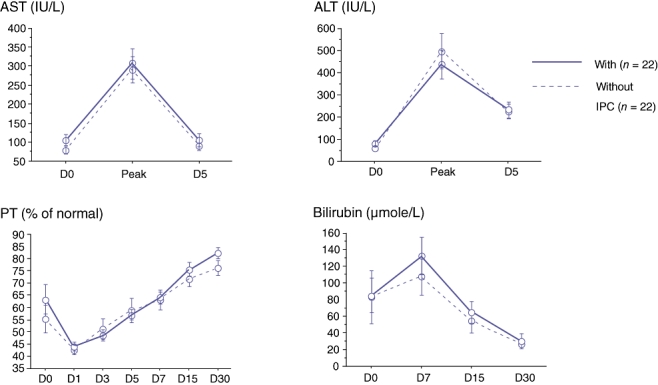
Post-living related right lobe liver transplantation (LRRLLT) liver function tests with versus without graft preconditioning. Comparision of groups was never statistically significant at any time point. AST, aspartate aminotransferase; ALT, alanine aminotransferase; PT, prothrombin time
Analysis of post-reperfusion biopsies
Analysis of post-reperfusion biopsies revealed no steatosis in any of the grafts. Liver biopsies revealed ischaemia–reperfusion injury in 2/20 (10%) and 4/19 (21%) of Group Precond and Group control patients, respectively (P =0.4).
Recipient outcome (Table 2)
Two patients in Group control and one patient in Group Precond died within 60 days of transplantation (P =0.5). Primary non function occurred in one case in Group control and none in Group Precond (P =0.9). Technical morbidity occurred in 7/22 (31.8%) and 8/22 (36.4%) patients in Group control and Group Precond, respectively (P =0.9). The incidences of biliary complications (6/22 in Group control and 7/22 in Group precond, P =0.9), arterial thrombosis (3/22 and 0/22, P =0.2) and hemoperitoneum requiring reoperation (3/22, and 0/22, P =0.2) were similar in the two groups. We observed no cases of portal vein thrombosis or outflow occlusion. The incidence of acute rejection needing increased immunosuppression was the same in Group control and Group Precond (4/22, and 1/22, P =0.3).
The durations of stay in intensive care and in hospital were similar in the two groups (P =0.4 and P =0.3, respectively).
Retransplantation was performed in three cases in Group control (due to arterial thrombosis n =2 and primary non-function n =1) and none in Group Precond (P =0.2). One patient from Group control who was on the waiting list for elective retransplantation for ischaemic cholangitis dropped out because of recurrent cancer.
Long-term survival
The mean follow-up period was 20.6 ± 14.4 months. Four patients in Group control and none in Group Precond (P =0.1) died. These four deaths occurred 3, 14, 21 and 24 months after transplantation and were as a result of cancer recurrence (3 cases) and intracerebral haemorrhage (1 case). No difference in 1-year survival was found between the groups (86.1% and 95.5% for Group control and Group Precond, respectively, P =0.08, logrank). Actuarial graft survival rate was 88.9% and 80.2% at 1 year for Group control and Group Precond, respectively (P =0.6).
Discussion
This single centre prospective study involving 44 consecutive cases of elective adult-to-adult living related right lobe liver transplantation is one of the few studies37,38 to evaluate the potential benefit of ischaemic preconditioning of the graft in this model.
Ischaemic preconditioning had no effect on graft tolerance to ischaemia–reperfusion injury, as shown by the similar peak AST concentrations in the two groups (Fig. 1, Table 2).
Two human studies have evaluated the preconditioning-like effect of reversible cardiac arrest in the cadaveric liver donor on the post-transplantation outcome.26,27 Totsuka et al.26 showed that livers from human donors who were resuscitated after cardiopulmonary arrest had similar survival and function to those from other donors. In addition, these authors showed that the post-transplantation serum concentrations of transaminases were lower in patients who received organs from donors with prior cardiopulmonary arrest compared with those without. Conversely, Wilson et al.27 reported that reversible cardiac arrest prior to graft procurement did not trigger any preconditioning benefit in liver transplantation. These two studies, together with the large body of experimental evidence showing that ischaemic preconditioning protects against cold ischaemia–reperfusion injury, were the impetus for our study. The discrepancy between our disappointing results and previous results in favour of ischaemic preconditioning may be as a result of several factors. First, the occurrence of ischaemic preconditioning differs between different tissues within a given species and within the same tissue in different species.8,39–47 Second, the many mechanisms beyond the scope of our study of ischaemic preconditioning9,10,48 might be involved differently in different models. Third, biases in our study might mask any effect of ischaemic preconditioning. These biases include small graft size, the preconditioning protocol and the choice of study design. One of the biases could be the short cold ischaemia time (below 3 h in all our cases; median =82 min). This potential bias, which remains to be proven, is however counterbalanced by the absence of an effect of preconditioning on ischaemia reperfusion subsequent to the warm ischaemia between portal and arterial revascularization (always >30 min, the cut-off value of clinical studies evaluating the value of IPC in warm ischemia during liver resection).18,19 Pharmacological preconditioning protocols that do not include a warm ischaemia step (with portal triad clamping) might prevent this negative effect.49–51
Ischaemic preconditioning did not improve graft function, as shown by the similar prothrombin time and bilirubin concentration in the two groups at all time points of the study (Fig. 2). This is in accordance with the results of the two human studies that evaluated the preconditioning-like effect of reversible cardiac arrest in the cadaveric donor.26,27 In these studies, post-transplantation prothrombin times and bilirubin concentrations were similar regardless of whether the donor had sustained temporary cardiac arrest prior to procurement or not. None of the animal studies on ischaemic preconditioning in cold ischaemia measured the coagulation factors and bilirubin levels after ischaemic preconditioning of the liver. However, Adam et al.52 showed that preconditioning of the liver graft was associated with altered liver function compared with a control group in a rat model. In a randomized study, we showed that 10 min of ischaemic preconditioning of the whole liver in the cadaveric donor improved graft tolerance to ischaemia–reperfusion. However, this protective effect tended to alter graft function.53 Conversely, Koneru et al.54 showed in a randomized study that 5 min of ischaemic preconditioning of the whole liver in the deceased donor did not trigger any benefit in the recipient in terms of ischaemia–reperfusion injury and graft function.
The preconditioning protocol had neither a positive nor a negative effect on the outcome of liver transplantation. The clamping of the graft pedicule was not associated with an increased risk of portal or arterial thrombosis in the recipient. Interestingly, when comparing the two groups, only patients in the Group control appeared to require retransplantation. Besides the fact that this difference was statistically non-significant and purely attributable to technical problems (2 patients) or graft primary non-function (1 patient), we could not confidently confirm that the clamping of the graft pedicule is not associated with an increased risk of portal or arterial thrombosis in the recipient as our study was underpowered to demonstrate a difference in this criterion.
Likewise, ischaemic preconditioning of the graft had no effect on donor outcome. Ischaemic preconditioning had no effect on the post-operative concentration of transaminases or on liver function tests i.e. prothombin time and bilirubin concentration. However, it is worth noting that the absence in our study of any effect of ischaemic preconditioning of the graft can also be interpreted as results of an intervention which is already compensated by advantages such as shorter transection time in LDLT donors, or healthy donors who have no underlying liver disease such as steatosis.
In addition, the results of this study differ from those reported by Arai et al. in a rat model.23 In the study by Arai et al. ischaemic preconditioning of one half of an individual rat liver decreased non-parenchymal cell killing, used as an index of sinusoidal endothelial cell killing, not only on the preconditioned side but also on the contralateral side. This potential effect, termed heterologous preconditioning, which has also been observed in the heart model,55 was not confirmed in the donors of our study.
Lastly, we also found that ischaemic preconditioning not only did not have any clinical impact on the recipients' outcome, but also that ischaemia–reperfusion injury was similar in the post-reperfusion biopsies of the patients belonging to the control and preconditioning group. However, our study did not provide any clinical information about the changes at a molecular level (such as over expression of IL1-Ra, Bcl-2 and NO), thought to be responsible for neutralizing the pro-inflammatory responses of interleukin-1 (IL-1) and tumour necrosis factor-alpha (TNF-α) to ischaemia reperfusion.56 Concerning the later changes (molecular) induced by ischaemia–reperfusion injury, we feel that newer prospective randomized studies in the same settings should be conducted to better evaluate their effects.
Conclusion
Ischaemic preconditioning of right hepatic lobe liver grafts in living donors does not seem to protect against ischaemia–reperfusion injury in the recipient. Ischaemic preconditioning of the graft did not have deleterious effects on graft function, or on morbidity or mortality rates. Ten minutes of ischaemic preconditioning had no consequence on the post-operative outcome of the donors. As donor safety and immediate and sufficient graft function are the primary goals of LRLT, the use of ischaemic preconditioning (ten minutes of warm ischemia) is not appropriate for living-related right liver transplantation.
Conflicts of interest
None declared.
References
- 1.Yamaoka Y, Washida M, Honda K, Tanaka K, Mori K, Shimahara Y, et al. Liver transplantation using a right lobe graft from a living related donor. Transplantation. 1994;57:1127–1130. [PubMed] [Google Scholar]
- 2.Todo S, Furukawa H, Jin MB, et al. Living donor liver transplantation in adults: outcome in Japan. Liver Transpl. 2000;6:S66–S72. doi: 10.1053/jlts.2000.19009. [DOI] [PubMed] [Google Scholar]
- 3.Brown RS, Jr, Russo MW, Lai M, Shiffman ML, Richardson MC, Everhart JE, et al. A survey of liver transplantation from living adult donors in the United States. N Engl J Med. 2003;348:818–825. doi: 10.1056/NEJMsa021345. [DOI] [PubMed] [Google Scholar]
- 4.Everhart JE, Lombardero M, Detre KM, et al. Increased waiting time for liver transplantation results in higher mortality. Transplantation. 1997;64:1300–1306. doi: 10.1097/00007890-199711150-00012. [DOI] [PubMed] [Google Scholar]
- 5.Sarasin FP, Majno PE, Llovet JM, et al. Living donor liver transplantation for early hepatocellular carcinoma: a life-expectancy and cost-effectiveness perspective. Hepatology. 2001;33:1073–1079. doi: 10.1053/jhep.2001.23311. [DOI] [PubMed] [Google Scholar]
- 6.Cheng SJ, Pratt DS, Freeman RB, Jr, et al. Living-donor versus cadaveric liver transplantation for non-resectable small hepatocellular carcinoma and compensated cirrhosis: a decision analysis. Transplantation. 2001;72:861–868. doi: 10.1097/00007890-200109150-00021. [DOI] [PubMed] [Google Scholar]
- 7.Marcos A. Right lobe living donor liver transplantation: a review. Liver Transpl. 2000;6:3–20. doi: 10.1002/lt.500060117. [DOI] [PubMed] [Google Scholar]
- 8.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 9.Carini R, Albano E. Recent insights on the mechanisms of liver preconditioning. Gastroenterology. 2003;125:1480–1491. doi: 10.1016/j.gastro.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 11.Pang CY, Yang RZ, Zhong A, et al. Acute ischemia preconditioning protects against skeletal muscle infarction in the pig. Cardiovasc Res. 1995;29:782–788. [PubMed] [Google Scholar]
- 12.Ishida T, Yarimizu K, Gute DC, Korthuis RJ. Mechanisms of ischemic preconditioning. Shock. 1997;8:86–94. doi: 10.1097/00024382-199708000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Bonventre JV. Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens. 2002;11:43–48. doi: 10.1097/00041552-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22:1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- 15.Du ZY, Hicks M, Winlaw D, et al. Ischemic preconditioning enhances donor lung preservation in the rat. J Heart Lung Transplant. 1996;15:1258–1267. [PubMed] [Google Scholar]
- 16.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic-ischemia reperfusion injury. Am J Surg. 2001;181:160–166. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 17.Selzner N, Rüdiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–936. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 18.Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155–162. doi: 10.1097/00000658-200008000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clavien PA, Selzner M, Rüdiger HA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–852. doi: 10.1097/01.sla.0000098620.27623.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nuzzo G, Giugliante F, Vellone M, et al. Pedicle clamping with ischemic preconditioning in liver resection. Liver Transpl. 2004;10:S53–S57. doi: 10.1002/lt.20045. [DOI] [PubMed] [Google Scholar]
- 21.Cleveland JC, Raeburn C, Harken AH. Clinical applications of ischemic preconditioning: from head to toe. Surgery. 2001;129:664–667. doi: 10.1067/msy.2001.111192. [DOI] [PubMed] [Google Scholar]
- 22.Raeburn CD, Cleveland JC, Zimmerman MA, Harken AH. Organ preconditioning. Arch Surg. 2001;136:1263–1266. doi: 10.1001/archsurg.136.11.1263. [DOI] [PubMed] [Google Scholar]
- 23.Arai M, Thurman RG, Lemasters JJ. Ischemic preconditioning of rat livers against cold storage-reperfusion injury: role of non parenchymal cells and the phenomenon of heterologous preconditioning. Liver Transpl. 2001;7:292–299. doi: 10.1053/jlts.2001.23080. [DOI] [PubMed] [Google Scholar]
- 24.Lloris-Carsis JM, Cejalvo D, Toledo-Pereyra LH, et al. Preconditioning: effect upon lesion modulation in warm liver ischemia. Transplant Proc. 1993;25:3303–3304. [PubMed] [Google Scholar]
- 25.Kume M, Yamamoto Y, Saad S, et al. Ischemic preconditioning of the liver in rats: implications of heat shock protein induction to increase tolerance of ischemia-reperfusion injury. J Lab Clin Med. 1996;128:251–258. doi: 10.1016/s0022-2143(96)90026-8. [DOI] [PubMed] [Google Scholar]
- 26.Totsuka E, Fung JJ, Urakami A, et al. Influence of donor cardiopulmonary arrest in human liver transplantation: possible role of ischemic preconditioning. Hepatology. 2000;31:577–580. doi: 10.1002/hep.510310305. [DOI] [PubMed] [Google Scholar]
- 27.Wilson DJ, Fisher A, Das K, et al. Donors with cardiac arrest: improved organ recovery but no preconditioning benefit in liver allografts. Transplantation. 2003;75:1683–1687. doi: 10.1097/01.TP.0000064542.63798.6B. [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Park K, Hwang S, et al. Congestion of right liver graft in living donor liver transplantation. Transplantation. 2001;71:812–814. doi: 10.1097/00007890-200103270-00021. [DOI] [PubMed] [Google Scholar]
- 29.Sugawara Y, Makuuchi M, Sano K, et al. Vein reconstruction in modified right liver graft for living donor liver transplantation. Ann Surg. 2003;237:180–185. doi: 10.1097/01.SLA.0000048444.40498.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calne RY, William R. Transplantation in man. I Observations on technique and organization in five cases. Br Med J. 1968;4:535–540. doi: 10.1136/bmj.4.5630.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belghiti J, Panis Y, Sauvanet A, et al. A new technique of side to side caval anastomosis during orthotopic hepatic transplantation without inferior vena caval occlusion. Surg Gynecol Obstet. 1992;175:271–272. [PubMed] [Google Scholar]
- 32.Adam R, Reynes M, Johann M, et al. The outcome of steatotic grafts in liver transplantation. Transplant Proc. 1991;23:1538–1540. [PubMed] [Google Scholar]
- 33.Heymsfield SB, Fulenwider T, Nordlinger B, Barlow R, Sones P, Kutner M. Accurate measurement of liver, kidney, and spleen volume mass by computerized axial tomography. Ann Intern Med. 1979;90:185–187. doi: 10.7326/0003-4819-90-2-185. [DOI] [PubMed] [Google Scholar]
- 34.Henderson JM, Heymsfield SB, Horowitz J, Kutner MH. Measurement of liver and spleen volume by computed tomography. Radiology. 1981;141:525–527. doi: 10.1148/radiology.141.2.6974875. [DOI] [PubMed] [Google Scholar]
- 35.Van Thiel DH, Hagler NG, Schade RR, et al. In vivo hepatic volume determination using sonography and computed tomography. Gastroenterology. 1985;88:1812–1817. doi: 10.1016/0016-5085(85)90005-8. [DOI] [PubMed] [Google Scholar]
- 36.Demetris A, Batts K, Dhillon A, Wight D, Williams J, Yamabe H. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 37.Miller CH, Masetti M, Cautero N, et al. Intermittent inflow occlusion in living liver donors: impact on safety and remnant function. Liver Transpl. 2004;10:244–247. doi: 10.1002/lt.20071. [DOI] [PubMed] [Google Scholar]
- 38.Testa G, Angelova V, Laricchia-Robbio L, et al. Unilateral ischemic preconditioning and heterologous preconditioning in living donor liver transplantation. Clin Transplant. 2009;24:334–340. doi: 10.1111/j.1399-0012.2009.01075.x. [DOI] [PubMed] [Google Scholar]
- 39.Park KM, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MARK kinase activation by remote ischemic pretreatment. J Biol Chem. 2001;276:1870–1876. doi: 10.1074/jbc.M007518200. [DOI] [PubMed] [Google Scholar]
- 40.Turman MA, Bates CM. Susceptibility of human proximal tubular cells to hypoxia: effect of hypoxic preconditioning and comparison to glomerular cells. Ren Fail. 1997;19:47–60. doi: 10.3109/08860229709026259. [DOI] [PubMed] [Google Scholar]
- 41.Berhends M, Walz MK, Kribben A, et al. No protection of the porcine kidney by ischaemic preconditioning. Exp Physiol. 2000;85:819–827. [PubMed] [Google Scholar]
- 42.Jefayri MK, Grace PA, Mathie RT. Attenuation of reperfusion injury by renal ischaemic preconditioning: the role of nitric oxide. BJU Int. 2000;85:1007–1013. doi: 10.1046/j.1464-410x.2000.00678.x. [DOI] [PubMed] [Google Scholar]
- 43.Arend LJ, Thompson CI, Spielman WS. Dypiramidol decreases glomerular filtration in the sodium-depleted dog. Evidence for mediation by intrarenal adenosine. Circ Res. 1985;56:242–251. doi: 10.1161/01.res.56.2.242. [DOI] [PubMed] [Google Scholar]
- 44.Kosieradzki M, Ametani M, Southard JH, Mangino MJ. Is ischemic preconditioning of the kidney clinically relevant? Surgery. 2003;133:81–90. doi: 10.1067/msy.2003.93. [DOI] [PubMed] [Google Scholar]
- 45.Cochrane J, Williams BT, Banerjee A, et al. Ischemic preconditioning attenuates functional, metabolic, and morphologic injury from ischemic renal failure in the rat. Ren Fail. 1999;21:135–145. doi: 10.3109/08860229909066978. [DOI] [PubMed] [Google Scholar]
- 46.Islam CF, Mathie RT, Dinneen MD, et al. Ischaemia-reperfusion injury in the rat kidney: the effect of preconditioning. Br J Urol. 1997;79:842–847. doi: 10.1046/j.1464-410x.1997.00209.x. [DOI] [PubMed] [Google Scholar]
- 47.Pagliaro P, Gattullo D, Rastaldo R, Losano G. Ischemic preconditioning: from the first to the second window of protection. Life Sci. 2001;69:1–15. doi: 10.1016/s0024-3205(01)01113-4. [DOI] [PubMed] [Google Scholar]
- 48.Sakon M, Ariyoshi H, Umeshita K, Monden M. Ischemia-reperfusion injury of the liver with special reference to calcium-dependent mechanisms. Surg Today. 2002;32:1–12. doi: 10.1007/s595-002-8105-8. [DOI] [PubMed] [Google Scholar]
- 49.Mokuno Y, Berthiaume F, Tompkins RG, et al. Technique for expanding the donor liver pool: heat shock preconditioning in a rat fatty liver model. Liver Transpl. 2004;10:264–272. doi: 10.1002/lt.20014. [DOI] [PubMed] [Google Scholar]
- 50.Glanemann M, Strenziok R, Kuntze R, et al. Ischemic preconditioning and methylprednisolone both equally reduce hepatic ischemia/reperfusion injury. Surgery. 2004;135:203–214. doi: 10.1016/j.surg.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Klein M, Geoghegan J, Wangemann R, et al. Preconditioning of donor livers with prostaglandin I2 before retrieval decreases hepatocellular ischemia-reperfusion injury. Transplantation. 1999;67:1128–1132. doi: 10.1097/00007890-199904270-00007. [DOI] [PubMed] [Google Scholar]
- 52.Adam R, Arnault I, Bao YM, Salvucci M, Sebagh M, Bismuth H. Effect of ischemic preconditioning on hepatic tolerance to cold ischemia in the rat. Transpl Int. 1998;11:S168–S170. doi: 10.1007/s001470050453. [DOI] [PubMed] [Google Scholar]
- 53.Azoulay D, Del Gaudio M, Andreani P, Ichai P, Sebag M, Adam R, et al. Effects of ten minutes of ischemic preconditioning of the cadaveric liver on the graft's preservation and function: the Ying and the Yang. Ann Surg. 2005;242:133–139. doi: 10.1097/01.sla.0000167848.96692.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koneru B, Fisher A, He Y, et al. Ischemic preconditioning in deceased donor liver trnsplantation: a prospective randomized clinical trial of safety and efficacy. Liver Transpl. 2005;11:196–202. doi: 10.1002/lt.20315. [DOI] [PubMed] [Google Scholar]
- 55.Przyklenk K, Bauer B, Ovize M, et al. Regional ‘ischemic preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 56.Barrier A, Olaya N, Chappini F, et al. Ischemic preconditioning modulates the expression of several genes, leading to the overproduction of IL-1Ra, iNOS, and Bcl-2 in a human model of liver ischemia-reperfusion. FASEB J. 2005;19:1617. doi: 10.1096/fj.04-3445com. [DOI] [PubMed] [Google Scholar]


