Abstract
Background
Adjuvant treatment for pancreatic adenocarcinoma has been shown to improve survival. An increasingly recognized ‘subtype’ of pancreatic adenocarcinoma is invasive intraductal papillary mucinous neoplasm (IPMN). It is unclear whether adjuvant treatment for invasive IPMN improves survival. This study aimed to determine the impact of adjuvant treatment in invasive IPMN.
Methods
We conducted a retrospective analysis of merged clinical databases including 412 patients undergoing resection for IPMN at two academic institutions between 1989 and 2006.
Results
Of 412 patients with IPMN who underwent pancreatectomy, 98 had invasive carcinoma. Median survival in invasive IPMN was 32 months. Adjuvant treatment did not affect median survival in node-positive or node-negative invasive IPMN. Biopsy-proven recurrence of invasive IPMN occurred in 45 patients (46%). The median disease-free interval from resection to recurrence was 27 months. Treatment of recurrences with chemotherapy or radiation therapy was not associated with a difference in survival; however, a subgroup of patients with recurrence in the remnant pancreas who underwent re-resection appeared to have more favourable outcomes.
Conclusions
An invasive component measuring >2 cm and lymph node involvement are associated with poorer prognosis. Adjuvant therapy in invasive IPMN appears to confer no survival benefit. In selected patients with recurrence of invasive IPMN in the remnant pancreas, re-resection should be considered.
Keywords: intraductal papillary mucinous neoplasm, adjuvant treatment, invasive
Introduction
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas has become a more commonly recognized neoplasm with overt invasion or progression into invasive carcinoma. Prior to the mid-1990s, IPMN had been reported under various names and had often been misinterpreted as mucinous cystic neoplasm, an entity now understood to be clearly distinct from IPMN.1 Intraductal papillary mucinous neoplasm was defined in 1996 by the World Health Organization (WHO)2 as a mucin-producing cystic lesion with pancreatic ductal communication. These lesions are classified radiologically and pathologically by the type of pancreatic ductal involvement. Main duct IPMN may involve the main duct exclusively (main-type IPMN) or the main duct and side branch ducts (mixed-type IPMN). Intraductal papillary mucinous neoplasms with exclusive involvement of branch ducts are classified as branch-type IPMN. The disease has variable malignant potential, but main duct IPMN carries a significantly greater risk of malignancy than IPMN with exclusively branch duct involvement according to natural history studies and surgical series.3 Malignant potential is graded pathologically by the degree of dysplasia or invasion (low grade, high grade, invasive); invasive IPMN confers a clearly distinct poor prognosis.
A number of investigators have suggested that patients with invasive IPMN may have better survival than patients with pancreatic ductal adenocarcinoma (PDAC). Complicating this comparison, invasive IPMN presents more commonly at earlier stages (stages I and II). Recently, investigators have examined matched comparisons of these groups to address this question.4 Because adjuvant treatment for PDAC has been shown to improve survival, it is often recommended for patients with invasive IPMN, particularly in cases with poor prognostic factors (e.g. positive lymph nodes and/or positive surgical margins).5 Nonetheless, it is unclear whether adjuvant treatment for invasive IPMN improves survival. Moreover, no studies to date have demonstrated a significant impact of adjuvant chemotherapy or chemoradiation in patients who have undergone resection for invasive IPMN. We utilized two, large, prospectively maintained databases from high-volume academic institutions with the aim of describing the characteristics of patients with invasive IPMN, determining predictors of survival in patients with invasive IPMN, and determining the impact of adjuvant therapy on survival in invasive IPMN. Although this is a retrospective study and therefore involves the potential pitfalls of selection bias, our hypothesis was that adjuvant treatment, with either chemotherapy or combination chemoradiation, would improve survival in patients with invasive IPMN.
Materials and methods
Patient selection
From 1 January 1989 to 31 December 2006, 412 patients underwent pancreatic resection for IPMN at Indiana University Hospital (Indianapolis, Indiana, n =181) and Mayo Clinic (Rochester, Minnesota, n =231) (Fig. 1). Starting in 1989, patients were accrued and entered prospectively into a clinical database approved by both the Indiana University Institutional Review Board and the Mayo Clinic Institutional Review Board. Patients were staged by history and physical examination, serum laboratory studies, chest radiography, dual-phase computed tomography and magnetic resonance imaging. Endoscopic ultrasonography with fine-needle aspiration biopsy and/or main duct brushing by endoscopic retrograde cholangiopancreatography were used commonly to establish preoperative cytopathological diagnoses. All resected IPMN were re-reviewed systematically by pathologists experienced in the classification of IPMN.6 Based on preoperative radiographic imaging, IPMN were classified into three morphological subtypes comprising main, mixed and branch duct variants. Based on surgical pathology, IPMN were graded histologically as adenoma (n =140), borderline neoplasm (n =124), carcinoma in situ (CIS) (n =50) or invasive carcinoma (invasive IPMN group, n =98) according to the WHO classification of IPMN.7 In the most recent classification, the adenoma and borderline lesions are grouped as low grade and CIS is considered high grade. These categories were defined collectively as non-invasive IPMNs (non-invasive IPMN group, n =314).
Figure 1.

Selection of patients with invasive intraductal papillary mucinous neoplasm (IPMN)
Surgery
For patients with invasive IPMN, resection consisted of pancreatoduodenectomy (PD) in 62 patients (63%) and distal pancreatectomy (DP) in 19 patients (20%). Seventeen patients (17%) with multifocal IPMN or extensive main duct IPMN underwent total pancreatectomy (TP). In the Mayo Clinic patients, extended lymph node dissection was commonly performed with the aim of eradicating residual cancer cells. Intraoperative frozen section examination of the pancreatic surgical margins was performed routinely. In the patients with positive margins (invasive IPMN or CIS), additional resection was continued until an R0 resection was obtained whenever possible. Inability to obtain a cancer-free margin was denoted in the analysis. Tumour–node–metastasis (TNM) staging was defined according to the sixth edition of the American Joint Committee on Cancer (AJCC).
Adjuvant treatment
None of the patients in this study underwent neoadjuvant treatment. Adjuvant treatment was employed most often in patients with positive lymph nodes (stage IIB and above) and/or positive surgical margins. According to performance status, adjuvant treatment was typically delivered 4–8 weeks postoperatively. The regimen of adjuvant treatment (chemotherapy, chemoradiation) was selected according to the institution and the suggestions of the oncologist. Agents including 5-fluorouracil (5-FU) and/or gemcitabine were used for either adjuvant chemotherapy or chemoradiation. For gemcitabine chemotherapy, the typical regimen involved 6 months of weekly intravenous infusion at 1000 mg/m2. The 5-FU chemotherapy regimen varied widely during the period of the study. A typical chemoradiation regimen involved 45 Gy administered over 5 weeks and either 5-FU or gemcitabine administered during the first and last weeks of radiotherapy. Patients who did not complete the planned adjuvant therapy were analysed on an intent-to-treat basis with their respective intended treatment group.
Endpoints
Variables evaluated in this study included age, sex, symptoms/conditions (pain, jaundice, weight loss, acute pancreatitis, chronic pancreatitis), and preoperative serum CA 19-9 level. Preoperative biopsies were graded as non-malignant or malignant (high-grade atypia or adenocarcinoma). Pathological examination determined tumour size (defined as maximum diameter), size of invasive component, histological differentiation (good, moderate or poor), margin of resection (positive or negative), node status (positive or negative), number of examined nodes, metastases, and perineural and perivascular invasion. Margins assessed included the pancreatic neck, bile duct, uncinate/retroperitoneal and duodenal in patients undergoing PD. Recurrences were classified as locoregional, distant metastasis or carcinomatosis. Postoperative outcomes were also assessed for duration of hospital stay, morbidity and mortality.
Statistical analysis
Data analyses were carried out with GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA) and stata 10.0 (StataCorp LP, College Station, TX, USA). Survival time was measured from the date of operation until death or last follow-up (the censoring date was 1 December 2008). Statistical associations between categorical factors were assessed using Fisher's exact test. The association of categorical factors with survival (univariate analysis) was assessed using the Kaplan–Meier method and was tested using the log-rank test. Multivariate analyses were undertaken via Cox proportional hazards regression and involved all preoperative factors, which were significantly associated with survival as determined by the univariable analysis. A stepwise model selection was used with probability of entry into the model set at 0.10 and the probability of a factor remaining in the model set at 0.20. Statistical significance within the Cox model was tested using the Wald test. Statistical significance was set at P < 0.05.
Results
Of 412 patients who underwent resection for IPMN, 98 (24%) had a pathological diagnosis of invasive IPMN.
Characteristics of patients with invasive IPMN are summarized in Table 1. The mean age of patients with invasive IPMN was 70 years. Male patients predominated. Overall, 90% of invasive IPMN patients presented with symptoms or associated conditions. Most commonly, patients presented with abdominal pain (56%), but approximately one-third presented with jaundice and/or weight loss. The majority (89%) of patients had pathological main duct involvement (main- and mixed-types) IPMN. Main duct dilation, however, was >1 cm in only 15% of patients. Of the patients with branch-type IPMN (11%), approximately half were multifocal (6%) vs. unifocal (5%). A little over half (53%) of patients had documented mural nodules on cross-sectional imaging or endoscopy prior to operation.
Table 1.
Characteristics of patients with invasive intraductal papillary mucinous neoplasm (IPMN)
| Invasive IPMN | |
|---|---|
| Patients, n | 98 |
| Mean age, years | 70 |
| Sex ratio, male : female | 1 : 5 |
| Symptoms/conditions, n | 87 (90%) |
| Abdominal pain, n | 54 (56%) |
| Jaundice, n | 33 (34%) |
| Weight loss, n | 32 (33%) |
| Pancreatitis, n | 23 (23%) |
| Operation | |
| Pancreaticoduodenectomy, n | 62 (62%) |
| Distal pancreatectomy, n | 19 (20%) |
| Total pancreatectomy, n | 17 (18%) |
| Duration of stay, days | 14.5 |
| Morbidity, n | 43 (44%) |
| Mortality, n | 3 (3%) |
| IPMN type | |
| Main, n | 41 (42%) |
| Mixed, n | 47 (47%) |
| Branch, n | 10 (11%) |
| Mean size, cm (range) | 4 (0.9–10) |
| AJCC stage | |
| I, n | 36 |
| IIA, n | 16 |
| IIB, n | 45 |
| III, n | 0 |
| IV, n | 1 |
| Margin +, n | 9 (9%) |
| Perineural invasion +, n | 47 (48%) |
| Perivascular invasion +, n | 18 (18%) |
AJCC, American Joint Committee on Cancer
Intraductal papillary mucinous neoplasms were located more commonly in the head than in the tail of the pancreas (62% vs. 20%), but 18% of patients required total pancreatectomy to achieve negative margins of resection. In addition, 10% of patients also required superior mesenteric and/or portal venous resection to achieve negative surgical margins. Patient duration of stay was 14.5 days, but varied considerably over the series. Patient morbidity and 30-day or in-hospital mortality rates were 44% and 3%, respectively.
The overall mean size of invasive IPMN was 4 cm. Mean sizes in main, mixed and branch types were 6 cm, 3 cm and 4 cm, respectively. The mean size of the actual invasive component was 3 cm. Mean sizes of invasive components in main, mixed and branch types were similar, at 4 cm, 3 cm and 3 cm, respectively. All patients were at disease stages I or II, except for one patient, who was at stage IV. This patient had a negative intraoperative liver biopsy, which, on permanent section, was read as adenocarcinoma.
A total of 91% of patients underwent R0 resection, leaving 9% with a positive margin of invasive IPMN (R1). Lymph nodes were positive in 46 (40%) patients, with 48% of the carcinomas showing perineural invasion and 18% showing perivascular invasion.
The mean follow-up in this combined series was 32 months (range 12–180 months). Table 2 summarizes the univariate analysis of preoperative factors (based on clinical examination and radiological findings) influencing survival in the 98 patients with invasive IPMN. Advanced age suggested a trend toward decreased survival (P =0.1), but jaundice, elevated serum CA 19-9, larger IPMN, and the presence of mural nodules demonstrated no trend toward decreased survival. Paradoxically, reported weight loss was associated with increased survival (48 months vs. 24 months) (P =0.04).
Table 2.
Univariate analysis of preoperative factors (clinical examination, radiological staging) influencing survival in patients with invasive intraductal papillary mucinous neoplasm (IPMN)
| Median survival, months (IQR) | P-valuea | |
|---|---|---|
| Age | ||
| <75 years | 35 (15–82) | 0.10 |
| ≥75 years | 28 (7–44) | |
| Sex | ||
| Female | 38 (11–79) | 0.77 |
| Male | 28 (13–80) | |
| Abdominal pain | ||
| Yes | 35 (13–127) | 0.16 |
| No | 21 (11–63) | |
| Jaundice | ||
| Yes | 23 (13–119) | 0.48 |
| No | 35 (11–80) | |
| Weight loss | ||
| Yes | 48 (11–127) | 0.04 |
| No | 24 (13–46) | |
| CA 19-9 serum levels | ||
| ≤37 IU/ml | 46 (8–80) | 0.64 |
| >37 IU/ml | 14 (11–37) | |
| IPMN size | ||
| <3 cm | 44 (15–80) | 0.20 |
| ≥3 cm | 21 (11–82) | |
| IPMN type | 0.21b | |
| Main duct | 33 (12–80) | 0.12 |
| Branch duct | 37 (21–119) | 0.08 |
| Mixed (main duct) | 30 (15–not defined) | 0.08 |
| Mixed (branch duct) | 13 (5–38) | |
| Mural nodule | ||
| Yes | 22 (11–79) | 0.22 |
| No | 38 (13–82) | |
Values in bold are significant at P < 0.05
Overall P-value. Subsequent P-values reflect the comparison between each of main, branch and mixed (primarily main duct) carcinomas with mixed (primarily branch duct) carcinomas
IQR, interquartile range
Pathological findings
Larger size of invasive component, positive lymph node status, positive margin status, poor tumour differentiation, and the presence of perineural or perivascular invasion were all factors which correlated on univariate analysis with poorer survival (Table 3). Overall median survival and 5-year survival rates in patients with invasive IPMN undergoing resection were 32 months and 30%, respectively.
Table 3.
Univariate analysis of pathological findings leading to poorer survival in patients with invasive intraductal papillary mucinous neoplasm (IPMN)
| Pathological findings | Median survival, months (IQR) | P-valuea |
|---|---|---|
| Invasive component | 0.01 | |
| <2 cm | 79 (30–not defined) | |
| ≥2 cm | 22 (10–41) | |
| Pathology determined IPMN type | 0.54b | |
| Main duct | 33 (15–80) | 0.12 |
| Side branch | 37 (10–not defined) | 0.56 |
| Mixed (main branch) | 22 (11–63) | 0.34 |
| Mixed (side branch) | 11 (2–not defined) | |
| Lymph nodes | <0.001 | |
| Involved | 15 (10–33) | |
| Not involved | 41 (21–127) | |
| Margins with invasive IPMN | 0.09 | |
| Involved | 15 (11–38) | |
| Not involved | 32 (12–82) | |
| Perineural invasion | 0.02 | |
| Yes | 15 (10–41) | |
| No | 41 (23–80) | |
| Perivascular invasion | <0.001 | |
| Yes | 11 (7–17) | |
| No | 41 (15–119) | |
| Poorly/dedifferentiated | <0.001 | |
| Yes | 15 (10–35) | |
| No | 44 (22–119) | |
| Adjuvant therapy | ||
| Yes | 23 (11–48) | 0.13 |
| No | 38 (12–119) | |
Values in bold are significant at P < 0.05
Overall P-value. Subsequent P-values reflect the comparison between each of main, branch and mixed (primarily main duct) carcinomas with mixed (primarily branch duct) carcinomas
IQR, interquartile range
Impact of adjuvant treatment
The characteristics of patients receiving adjuvant vs. no adjuvant therapy are demonstrated in Table 4. Notably, the adjuvant group had a larger mean size of neoplasm (4 cm vs. 3 cm) and larger mean size of invasive component (3.5 cm vs. 2.5 cm) (P =0.01), greater rates of positive lymph nodes (65% vs. 26%) (P =0.001), and a greater incidence of involved margins (19% vs. 3%) (P =0.02) than the group not receiving adjuvant therapy. No difference in perioperative morbidity or mortality was noted between these groups. Adjuvant therapy was achieved in 37 patients (37%) and consisted of chemotherapy based on 5-FU in two cases, gemcitabine-based chemotherapy in five and chemoradiation in 30 patients (four underwent gemcitabine-based chemotherapy and 26 underwent 5-FU-based chemotherapy) (Fig. 1). Sixty-one of 98 patients in the invasive IPMN group did not undergo any adjuvant treatment. Of these 61 patients, 14 were at disease stage IIB or higher and/or had positive surgical margins. Overall, the 37 patients who received adjuvant treatment had decreased 5-year overall survival (22% vs. 36%; P =0.002) and showed a trend toward decreased median survival (23 months vs. 38 months; 95% confidence interval [CI] 0.36–1.03) compared with the 61 patients who did not receive adjuvant treatment (Table 4) (Fig. 2). Patients with stage IIA or lower disease and a negative operative margin who received (n =5) or did not receive (n =47) adjuvant therapy demonstrated no difference in median survival (63 months vs. 48 months; P =0.98) (Fig. 3A). Patients with stage IIB or higher disease and/or positive surgical margins who received (n =37) or did not receive (n =14) adjuvant treatment had similar median survival (17 months vs. 22 months; P =0.67), which suggests that the overall poorer survival with adjuvant treatment may reflect a selection bias (Fig. 3B).
Table 4.
Pathological and perioperative characteristics of patients who did (n =37) or did not (n =61) receive adjuvant treatment
| Adjuvant treatment | No adjuvant treatment | P-valuea | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| n =37 | n =61 | ||
| Median invasive component size, cm (95% CI) | 3.5 (2.3–5.0) | 2.5 (1.1–4.0) | 0.01 |
| Lymph node involvement, % | 65 | 25 | <0.001 |
| Positive margin, % | 19 | 3 | 0.02 |
| Tumour differentiation | |||
| Poor/dedifferentiated, n | 20 (54%) | 25 (42%) | NS |
| Moderate | 14 (38%) | 31 (52%) | NS |
| High, n | 3 (9%) | 4 (8%) | NS |
| Multifocal IPMNs, n (%) | 3 (5%) | 3 (8%) | NS |
| Morbidity, n (%) | 16 (43%) | 27 (44%) | NS |
| 5-year overall survival (95% CI) | 22% (9–38) | 36% (22–50) | 0.002 |
Values in bold are significant at P < 0.05
95% CI, 95% confidence interval; NS, not significant
Figure 2.

The effect of adjuvant therapy on overall survival in all stages of invasive intraductal papillary mucinous neoplasm
Figure 3.

The effect of adjuvant chemotherapy or chemoradiation on overall survival of patients with invasive intraductal papillary mucinous neoplasm in (A) stage IIA or lower disease and negative operative margin (n =52) and (B) stage IIB and higher disease and positive operative margin (n =46)
Recurrence
Forty-five patients (46%) developed a biopsy-proven recurrence of invasive IPMN. Of these recurrences, nine (20%) were carcinomatosis, 13 (29%) recurred locally and 18 (40%) recurred as metastases, predominately to the liver (13 patients). The median post-surgery, recurrence-free survival of patients was 27 months (Fig. 4). Of the 45 patients who suffered recurrence, 19 had received chemotherapy, including two who received concomitant external beam radiation after recurrence had been identified. Three patients with recurrent invasive cancer in the pancreatic gland (at 11, 12 and 60 months after resection) were treated with re-resection; two remained alive at 74 months and 120 months follow-up, but one died of disease 37 months after the original operation. Thirteen patients received no further therapy for recurrence and 10 received care after the diagnosis of recurrence outwith the primary study institutions and thus information on their treatment is lacking (Fig. 5). No difference in median survival (from the date of initial surgery) was found between the 19 patients in whom recurrences were treated by chemotherapy or chemoradiation (13 months) and the 13 patients who did not receive any further treatment (21 months) (P =0.15). The few patients who were able to undergo re-resection, however, experienced an apparent survival advantage (P < 0.02).
Figure 4.

Recurrence-free survival with and without adjuvant therapy following pancreatectomy for invasive intraductal papillary mucinous neoplasm
Figure 5.
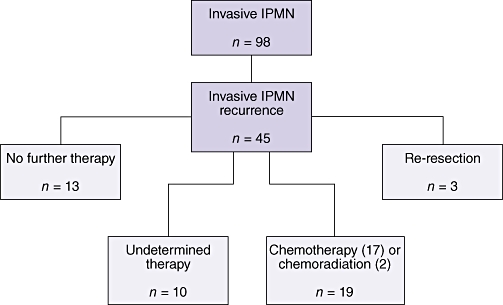
Treatment of patients who experienced recurrence of invasive intraductal papillary mucinous neoplasm (IPMN)
Multivariate analysis
Given the above results, we attempted to construct a global model of risk factors which, simultaneously, account for shorter survival associated with invasive IPMN. In this analysis, we considered all preoperative factors (age, weight loss and IPMN type) and histopathological factors (maximal size of invasive IPMN component, presence or absence of positive lymph nodes, final surgical margin status, carcinoma grading [poorly differentiated/dedifferentiated vs. well or moderately differentiated], presence or absence of perineural and perivascular invasion) associated with increased survival on univariate analysis. A stepwise Cox proportional hazards model was carried out as described in the statistical methods. Of the factors considered, weight loss, an invasive component >2 cm, and the presence of positive lymph nodes were related to shorter survival (P < 0.05) Table 5.
Table 5.
Multivariate analysis of risk factors for mortality caused by invasive intraductal papillary mucinous neoplasm
| Risk factor | Hazard ratio | 95% CI | P-valuea,b | |
|---|---|---|---|---|
| Age (<75 years vs. >75 years) | 0.61 | 0.33 | 1.11 | 0.11 |
| Weight loss | 0.34 | 0.17 | 0.69 | 0.003 |
| Differentiation (poor/dedifferentiated vs. good/moderate) | 1.62 | 0.82 | 3.19 | 0.17 |
| Size of invasive tumour (>2 cm vs. <2 cm) | 2.26 | 1.08 | 4.72 | 0.03 |
| Lymph node involvement | 2.34 | 1.25 | 4.38 | 0.01 |
| Perivascular involvement | 1.67 | 0.81 | 3.44 | 0.17 |
Wald test. The P-value is adjusted for the presence of all other factors in the model
Values in bold are significant at P < 0.05
95% CI, 95% confidence interval
Discussion
The current approach to adjuvant therapy for invasive IPMN is not standardized, predominately because data concerning efficacy in IPMN are lacking. Oncologists may be reluctant to administer adjuvant treatment to patients with invasive IPMN because there is little evidence to suggest that this approach is helpful. More commonly, others extrapolate their adjuvant treatment approach from the data available for the more common PDAC. As our experience with IPMN expands, it becomes evident that the natural history and behaviour of invasive IPMN may be distinct from that of PDAC. It is therefore uncertain whether the simple extension of our findings in PDAC is warranted. This large, retrospective, combined-institution study sought to determine the characteristics of patients with invasive IPMN, the predictors of poor prognosis, and the role of adjuvant therapy in patients with invasive IPMN.
These findings suggest that invasive IPMN occurs typically in elderly, symptomatic, male patients. These individuals most commonly present with abdominal pain and often with weight loss and jaundice. Abdominal pain is not a typical presenting symptom in PDAC. Invasive IPMN predominantly (89%) involves the main duct (main and mixed types), although the majority of these (85%) occur with main duct dilation of <1 cm, suggesting that a simple size cut-off alone may be inadequate in predicting main duct involvement or the potential for invasive malignancy.
Our study corroborates existing data in the literature regarding invasive IPMN related to lymph node status,8 margin status9 and perineural or perivascular invasion as factors influencing survival.10 However, on examining multivariate analysis of these factors, only lymph node involvement reached the level of statistical significance. The relationship between the size of IPMN and malignancy is less clear.11,12 Alternatively, the size of the invasive component in patients with invasive IPMN is an important factor in predicting survival outcome in this combined series.13 Serum CA 19-9 level was not found to be a prognostic factor as it is thought to be in PDAC.14 Paradoxically, weight loss actually appeared on multivariate analysis to confer a survival advantage in this group, which is at odds with findings for all IPMN as this factor is invariably correlated with the presence of invasive disease. One potential explanation for this observation may be that the weight loss present in these patients is related to steatorrhea, nausea, anorexia or pancreatitis associated with the mucin production of the IPMN, whereas malignant weight loss is more typical of the more common ductal adenocarcinoma. These symptoms may lead patients to seek medical care earlier, thus instigating a lead time bias that results in prolonged survival in this group.
Existing literature supports the finding that invasive IPMN has a poor prognosis, although it is significantly better than that in PDAC, with an overall 5-year survival of 30–42%.4,15,16 This runs counter to findings from some earlier studies in this regard.17 Given these differences in behaviour, the question remains whether patients with invasive IPMN, as in PDAC, may derive benefit from adjuvant or neoadjuvant treatment. Neoadjuvant approaches were not employed in this study, partly because of the inability to accurately differentiate between high-grade dysplasia (carcinoma in situ) and invasive IPMN preoperatively. Invasive IPMN was diagnosed correctly in only 68% of patients who underwent preoperative biopsies, and 20% of patients with non-invasive IPMN were mistakenly considered to have invasive IPMN.
An important aspect of examining the postoperative treatment of invasive IPMN is the role of adjuvant chemotherapy or radiation. It is well known that pancreatic surgery is characterized by substantial postoperative morbidity rates approaching 50%.18 In the case of PDAC, nearly 25% of patients who undergo PD do not receive adjuvant treatment as a result of complicated postoperative courses.19 Similarly, in the present series, 14 (27%) patients (with stage IIB or higher status or positive operative margins) were not able to finish or receive adjuvant treatment.
With regard to all invasive IPMN, a notable decrease in 5-year overall survival was noted in those patients who received any adjuvant therapy. Clearly, differences in disease stage contribute to the detrimental effect on survival seen in the adjuvant group. When further divided into advanced (stage IIB or higher disease or positive margins) or early (stage IIA or lower disease and negative margins) groups, no benefit was seen in patients receiving adjuvant therapies in either median or overall survival.
Gemcitabine-based chemotherapy alone was utilized in only 14% of patients receiving adjuvant treatment. Interestingly, postoperative chemoradiation was more common in this study than single-agent chemotherapy. Chemoradiation has not proven to be more effective than chemotherapy alone in the adjuvant setting for PDAC.20 In the setting of a positive postoperative margin, chemoradiation may be reasonable as in PDAC, but the number of R1 resections in our series precluded an adequately powered analysis of radiation specifically for positive margin status in the setting of invasive IPMN. A number of patients received 5-FU-based chemotherapy in our study. Although this regimen is no longer commonly employed for PDAC, it is notable that a recent European series suggested that 5-FU-based chemotherapy in PDAC remains effective and should not necessarily be abandoned.21
Recurrence is an important endpoint after resection for invasive IPMN. Just under half the patients in this series experienced an invasive recurrence. Additionally, the presence or absence of adjuvant chemotherapy or chemoradiation appeared to have no clear effect on recurrence-free survival. Analysis of the pattern of recurrence in patients with invasive IPMN showed that most patients with recurrence had distant recurrence in the form of either carcinomatosis or remote organ metastases (60% of recurrences). This tumour biology is consistent with the patterns of recurrence demonstrated previously for PDAC.22 Interestingly, no patient in this series was noted to develop recurrence of low or high grade IPMN in the remnant gland, although 13 developed invasive recurrence locally.
Given that nearly half of these patients will experience recurrence in either a locoregional or distant fashion, the approach to these recurrences is critical. Although this analysis referred to only a small subgroup, we observed that salvage treatment (chemotherapy or chemoradiation) failed to improve survival in patients with invasive recurrences compared with patients who underwent no further therapy. Interestingly, the few patients (n =3, 7%) with recurrence who underwent re-resection appeared to demonstrate increased survival relative to patients who underwent chemotherapy, chemoradiation or no additional therapy, although clearly these patients were amenable to re-operation as a result of favourable tumour and patient characteristics.
Importantly, our study involved several substantial confounding issues and limitations. Firstly, the small number of patients with advanced disease who did not receive planned adjuvant treatment (n =14) precludes us from drawing strong statistical conclusions about those who did receive adjuvant treatment. Secondly, patients received a number of different adjuvant treatment protocols. This heterogeneous approach affects the statistical power and confidence of conclusions in any one adjuvant treatment subgroup. Much of the variability in the adjuvant approach is accounted for by the long period encompassed in our series (1989–2007). Additionally, there are apparent differences in those patients who were selected for adjuvant therapy. As Table 4 shows, significant differences in the size of the invasive component, positive margins, and the presence of lymph node metastases are noted in the adjuvant therapy group. These factors would clearly mitigate potential benefits present in this group and these data must be interpreted with this in mind. Importantly, no differences in morbidity or tumour histology were seen between the two treatment groups.
Given the above limitations, it is important to note that any retrospective analysis is not definitive with regard to whether to employ or abandon adjuvant therapy. Rather, these findings call into question the benefit added by the addition of these potentially morbid treatments. Based on these findings, we feel that therapy should be reserved for fit patients and that tumour histology, stage (particularly node-positive and/or margin-positive disease) and patient factors should be considered by the medical oncologist and surgeon in combination, and that these patients should be surveyed closely for evidence of recurrence and response to therapy.
Conclusions
Factors associated with decreased survival in invasive IPMN included an invasive component measuring >2 cm and lymph node involvement. Preoperative weight loss appeared to be inversely related to survival in this group. Although main duct involvement is commonly correlated with invasive cancer, a minority of main duct IPMN cases had a pancreatic duct >1 cm in diameter. The addition of adjuvant treatment demonstrated no clear benefit in patients with invasive IPMN in either early- or later-stage disease; however, those patients given adjuvant treatment tended to have more aggressive pathology. Finally, re-operation for recurrence was associated with substantial longterm survival in three patients with invasive recurrence in the remnant pancreas in this series, and should be considered for patients with local recurrence amenable to re-resection.
Conflicts of interest
None declared.
References
- 1.Ohashi K, Murakami Y, Maruyama M, Takokochi T, Ohta H, Ohashi I. Four cases of ‘mucin-producing’ cancer of the pancreas on specific findings of the papilla of Vater. Prog Dig Endosc. 1982;20:348–351. [Google Scholar]
- 2.Kloeppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. Histological typing of tumours of the exocrine pancreas. In: Kloeppel G, editor. World Health Organization International Histological Classification of Tumours. 2nd edn. Berlin: Springer; 1996. pp. 1–60. [Google Scholar]
- 3.Waters JA, Schmidt CM. Intraductal papillary mucinous neoplasm – when to resect? Adv Surg. 2008;42:87–108. doi: 10.1016/j.yasu.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Poultsides GA, Reddy S, Cameron JL, Hruban RH, Pawlik TM, Ahuja N, et al. Histopathologic basis for the favourable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251:470–476. doi: 10.1097/SLA.0b013e3181cf8a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traverso LW. Pancreatic cancer: surgery alone is not sufficient. Surg Endosc. 2006;20(Suppl 2):446–449. doi: 10.1007/s00464-006-0052-1. [DOI] [PubMed] [Google Scholar]
- 6.Murakami Y, Uemura K, Ohge H, Hayashidani Y, Sudo T, Sueda T. Intraductal papillary-mucinous neoplasms and mucinous cystic neoplasms of the pancreas differentiated by ovarian-type stroma. Surgery. 2006;140:448–453. doi: 10.1016/j.surg.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Longnecker DS, Adler G, Hruban RH, et al. Intraductal papillary mucinous neoplasms of the pancreas. In: Hamilton SR, Aaltonen LA, editors. World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000. pp. 237–241. [Google Scholar]
- 8.House MG, Gönen M, Jarnagin WR, D'Angelica M, DeMatteo RP, Fong Y, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549–1555. doi: 10.1007/s11605-007-0243-7. [DOI] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Talamonti MS, Sener SF, Bilimoria MM, Stewart AK, Winchester DP, et al. Effect of hospital volume on margin status after pancreaticoduodenectomy for cancer. J Am Coll Surg. 2008;207:510–519. doi: 10.1016/j.jamcollsurg.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 10.Van Roest MH, Gouw AS, Peeters PM, Porte RJ, Slooff MJ, Fidler V, et al. Results of pancreaticoduodenectomy in patients with periampullary adenocarcinoma: perineural growth more important prognostic factor than tumour localization. Ann Surg. 2008;248:97–103. doi: 10.1097/SLA.0b013e31817b6609. [DOI] [PubMed] [Google Scholar]
- 11.Walsh RM, Vogt DP, Henderson JM, Hirose K, Mason T, Bencsath K, et al. Management of suspected pancreatic cystic neoplasms based on cyst size. Surgery. 2008;144:677–684. doi: 10.1016/j.surg.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246:644–651. doi: 10.1097/SLA.0b013e318155a9e5. [DOI] [PubMed] [Google Scholar]
- 13.D'Angelica M, Brennan MF, Suriawinata AA, Klimstra D, Conlon KC. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–408. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nara S, Shimada K, Kosuge T, Kanai Y, Hiraoka N. Minimally invasive intraductal papillary-mucinous carcinoma of the pancreas: clinicopathologic study of 104 intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2008;32:243–255. doi: 10.1097/PAS.0b013e3181484f1e. [DOI] [PubMed] [Google Scholar]
- 15.Wada K, Kozarek RA, Traverso LW. Outcomes following resection of invasive and non-invasive intraductal papillary mucinous neoplasms of the pancreas. Am J Surg. 2005;189:632–636. doi: 10.1016/j.amjsurg.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, et al. Longterm survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adsay NV, Conlon KC, Zee SY, Brennan MF, Klimstra DS. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62–77. doi: 10.1002/cncr.10203. [DOI] [PubMed] [Google Scholar]
- 18.Muscari F, Suc B, Kirzin S, Hay JM, Fourtanier G, Fingerhut A, et al. Risk factors for mortality and intra-abdominal complications after pancreatoduodenectomy: multivariate analysis in 300 patients. French Association for Surgical Research. Surgery. 2006;139:591–598. doi: 10.1016/j.surg.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Aloia TE, Lee JE, Vauthey JN, Abdalla EK, Wolff RA, Varadhachary GR, et al. Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg. 2007;204:347–355. doi: 10.1016/j.jamcollsurg.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 21.Neoptolemos JP, Stocken DD, Tudur Smith C, Bassi C, Ghaneh P, Owen E, et al. Büchler MW Adjuvant 5-fluorouracil and folinic acid vs. observation for pancreatic cancer: composite data from the ESPAC-1 and -3(v1) trials. Br J Cancer. 2009;100:246–250. doi: 10.1038/sj.bjc.6604838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van den Broeck A, Sergeant G, Ectors N, Van Steenbergen W, Aerts R, Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35:600–604. doi: 10.1016/j.ejso.2008.12.006. [DOI] [PubMed] [Google Scholar]


