Abstract
Background
Minimization of blood loss during pancreatoduodenectomy requires careful surgical technique and specific preventative measures. Therefore, red blood cell (RBC) transfusions and operative time are potential surgical quality indicators. The aim of the present study was to compare peri-operative RBC transfusion and operative time with 30-day morbidity/mortality after pancreatoduodenectomy.
Methods
All pancreatoduodenectomies (2005 to 2008) were identified using the American College of Surgeons-National Surgical Quality Improvement Program (ACS-NSQIP). RBC transfusions and operative time were correlated with 30-day morbidity/mortality.
Results
Pancreatoduodenectomy was completed in 4817 patients. RBC transfusions were given to 1559 (32%) patients (1–35 units). Overall morbidity and mortality rates were 37% and 3.0%, respectively. Overall 30-day morbidity increased in a stepwise manner with the number of RBC transfusions (R =0.69, P < 0.01). Although RBC transfusions and operative times were not statistically linked (P =0.87), longer operative times were linearly associated with increased 30-day morbidity (R =0.79, P < 0.001) and mortality (R =0.65, P < 0.01). Patients who were not transfused also displayed less morbidity (33%) and mortality (1.9%) (P < 0.05).
Discussion
Peri-operative RBC transfusion after pancreatoduodenectomy is linearly associated with 30-day morbidity. Longer operative time also correlates with increased morbidity and mortality. Therefore, blood transfusions and prolonged operative time should be considered quality indicators for pancreatoduodenectomy.
Keywords: pancreatoduodenectomy, whipple, NSQIP, transfusion
Introduction
Despite a history now approaching 100 years,1,2 pancreatoduodenectomy remains a formidable and lengthy procedure with substantial risks. While the mortality rate associated with this operation has been dramatically reduced from more than 20% in the 1970s to less than 3% in modern reports,3–5 peri-operative morbidity has remained relatively consistent at approximately 40%.6,7 A clear improvement in outcomes is also observed when pancreatoduodenectomies are performed in high volume centers by experienced pancreatic surgeons.8,9
Allogenic blood transfusions increase the risk of infection, acute lung injury and potentially cancer recurrence.10–15 While the precise mechanism of transfusion-induced immunosuppression is debated,16,17 significant operative blood loss that leads to a subsequent need for blood transfusion has both short- and long-term negative consequences. Because surgical blood loss is often considered a modifiable factor that may reflect surgical technique, rates of peri-operative blood transfusion have been advocated as robust quality indicators.17
Extended operative duration has also been identified to negatively impact outcomes after general surgical procedures.18–20 Both an increased rate of infectious complications (surgical site infections, sepsis, septic shock, pneumonia and urinary tract infections), as well as a longer duration of hospital stay correlate with operative time in patients undergoing both pancreatectomies and biliary procedures.21 Moreover, these risks appear to increase in a linear manner with lengthening operative times at 90-min intervals.21 Therefore, the aim of this analysis was to identify the associations between both peri-operative red blood cell (RBC) transfusion and operative time, with 30-day morbidity/mortality after pancreatoduodenectomy using the American College of Surgeons-National Surgical Quality Improvement Program (ACS-NSQIP).22
Methods
ACS-NSQIP
The ACS-NSQIP is a nationally validated, outcomes-based, risk-adjusted, peer-controlled programme for the measurement and enhancement of the quality of surgical care.23–26 One hundred and thirty-six pre-operative (patient characteristics), intra-operative (processes of care) and post-operative (adverse outcomes) variables were prospectively collected by trained, certified nurse reviewers in the ACS-NSQIP. Patients younger than 16 years of age were excluded. Quality is ensured by inter-rater reliability audits, as well as online decision support so that the level of disagreement is currently only 1.53% for all variables. Surgical clinical nurse reviewers also ensured the validity of their data by assessing physician documentation, and/or contacting patients directly. Outcomes are assessed at 30 days after the index operation (e.g. pancreatoduodenectomy), and highly standardized and validated definitions were employed. Because all ACS-NSQIP data undergoes a logistic regression analysis, observed-to-expected (O/E) ratios can be calculated for morbidity and mortality. While low ratios indicate superior quality of care, high ratios confirm inferior care. ACS-NSQIP uses a systematic random sampling process that employs an 8-day cycle, whereby the first 40 cases are used to fulfil the inclusion and exclusion criteria at each participating centre. This process therefore excludes an over-sampling of minor cases (i.e. cholecystectomy, inguinal hernia and breast procedures).
Participant use file
The ACS-NSQIP Participant Use File (PUF) was queried to find all patients who had undergone a pancreatoduodenectomy from 1 January 2005 to 31 December 2008. The pancreatoduodenectomy procedure codes (48150, 48152, 48153, 48154) were used to identify all patients for analysis. Patient demographics and comorbidities were summarized. Operative procedure duration was queried in 60-min intervals. Blood transfusions were quantified by the specific number of transfused RBC units within 72 h of the operative procedure. Morbidity and mortality observed within 30 days of a pancreatoduodenectomy were analysed. Morbidity included organ space infections, wound disruption, cerebrovascular accident or stroke, myocardial infarction, cardiac arrest, pulmonary embolism, ventilator dependence longer than 48 h, acute renal failure, bleeding complications, sepsis, septic shock, superficial surgical site infections, pneumonia, unplanned intubation, progressive renal insufficiency, urinary tract infection and deep vein thrombosis. The overall complication pool is composed of approximately two-thirds serious morbidity and one-third minor complications. Expected morbidity and mortality are available in the PUF based on logistic regressions of all procedures in the database. Observed/expected (O/E) ratios for morbidity and mortality were calculated for comparisons with regards to both RBC transfusion and operative duration.
Statistical analysis
Analysis was performed using Stata version 8.0 (Stata Corp, College Station, TX, USA). Normally or near-normally distributed variables were reported as means and non-normally distributed variables as medians. Means were compared using the Student's t-test and medians using the Mann-Whitney U-test. Differences in proportions among categorical data were assessed using Fischer's exact test. Correlations were determined with Pierson's coefficients. A P-value less than 0.05 was considered to represent statistical significance for all comparisons.
Results
Patient demographics and comorbidities
Over the 4-year study interval, 4817 patients who underwent a pancreatoduodenectomy were tracked using the ACS-NSQIP registry. Three additional patients were excluded from the analysis as a result of missing patient data. As seen in Table 1, the majority of patients were functionally independent prior to their procedure. Most patients also had 1 (29.2%), 2 (23.6%), 3 (14.3%), 4 (7.0%) or more (5.5%) comorbidities. Specific definitions for these comorbidities may be found at http://www.acsnsqip.org. A minority of patients received neoadjuvant chemoradiotherapy (3.6%) or used chronic steroids (2.0%). Moreover, 20% of patients had lost at least 10% of their pre-illness body weight prior to their pancreatoduodenectomy.
Table 1.
Patient demographics and comorbidities
| Demographic | |
|---|---|
| N | 4782 |
| Age, mean, y | 64.3 ± 4.3 |
| BMI, kg/m2 | 26.6 ± 5.8 |
| General (%) | |
| Diabetes | 22.7 |
| Smoking | 21.9 |
| Functionally independent (%) | |
| Pre-illness | 98.3 |
| Pre-surgery | 96.5 |
| Cardiac (%) | |
| HTN | 52.1 |
| CHF | 0.3 |
| MI | 0.4 |
| Respiratory (%) | |
| Vent dependent | 0.2 |
| Dyspnea | 9.0 |
| Pneumonia | 0.3 |
| COPD | 4.5 |
| Renal (%) | |
| Dialysis | 0.3 |
| Renal insufficiency | 0.3 |
| Ascites (%) | 0.9 |
| Bleeding disorder (%) | 2.5 |
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; HTN, hypertension; MI, myocardial infarction; Vent dependent, pre-operative ventilator dependent.
Blood transfusions
Thirty-two per cent (1559/4817) of patients received a RBC transfusion within 72 h of their pancreatoduodenectomy. A median of three units was utilized (range: 1–35) amongst patients who actually received a blood transfusion (Fig. 1). The most frequent number of units transfused was two followed by one.
Figure 1.
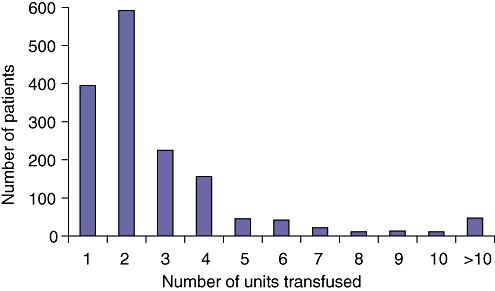
Number of red blood cell units for patients who received a transfusion
Operative duration
Figure 2 displays the distribution of operative time. The mean operative duration for a pancreatoduodenectomy was between 5 and 6 h. Fifty-nine per cent of the operations took between 4 and 7 h to perform.
Figure 2.
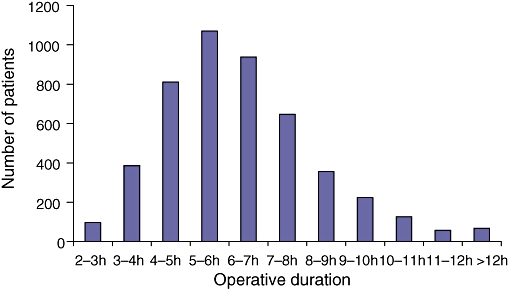
Distribution of operative duration
Outcomes – transfusion
Of the 4817 patients undergoing a pancreatoduodenectomy, overall morbidity was 37% (1775/4817). Overall mortality within 30 days was 3.0% (145/4817). The expected 30-day morbidity was 42% and the expected mortality was 3.3%. Thus, the morbidity O/E ratio was 0.89, and the mortality O/E ratio was 0.91. Overall 30-day morbidity increased in a stepwise manner with the number of RBC transfusions (R =0.69, P < 0.01), as seen in Fig. 3a. Overall mortality was not statistically associated with transfusion of blood (R =0.07, P =0.44). These relationships remained consistent in comparisons for observed/expected morbidity (R =0.82, P < 0.001) and mortality (R =0.03, P =0.59) ratios, respectively. Patients who were not transfused also displayed a lower morbidity (33%) and mortality (1.9%) than patients receiving RBC transfusions (morbidity =46%; mortality =5.3%) (P < 0.00001 for both comparisons), as seen in Fig. 3b.
Figure 3.
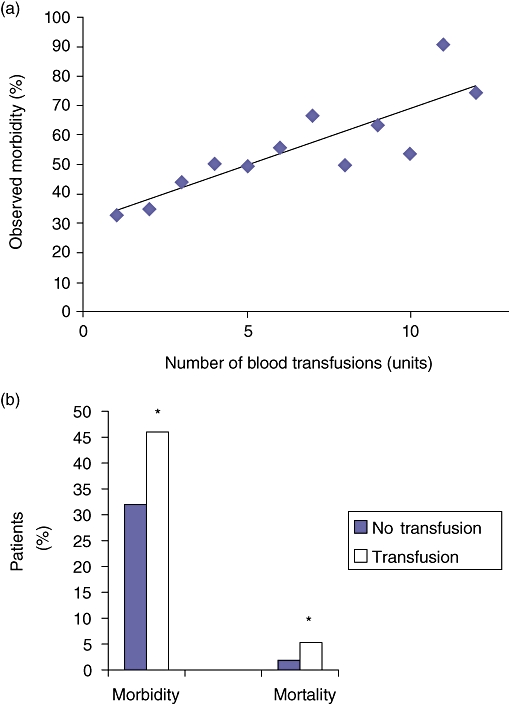
(a) Morbidity associated with blood transfusions (R =0.69, P < 0.01). (b Morbidity and mortality without and with blood transfusions. *P < 0.00001 (no transfusion vs. transfusion)
Outcomes – duration
Longer operative duration was linearly associated with both increased 30-day morbidity (R =0.79, P < 0.001) and mortality (R =0.65, P < 0.01) (Fig. 4a,b). Similar results were noted in comparisons for observed/expected morbidity (R =0.84, P < 0.0001) and mortality (R =0.74, P < 0.001). Although no statistically significant association was observed between RBC transfusion and operative duration (R =0.001, P =0.87), there was a significant increase in the data variation at the extremes of operative times (i.e. very brief, as well as extended duration cases).
Figure 4.
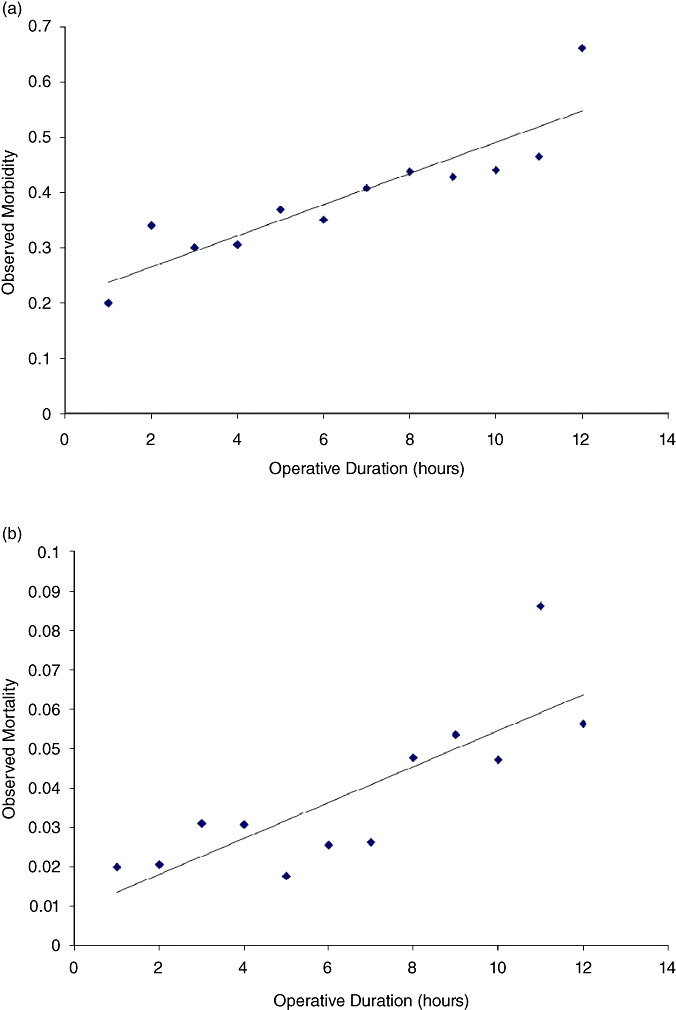
(a) Morbidity associated with operative duration (R =0.79, P < 0.001). (b Mortality associated with operative duration (R =0.65, P < 0.01)
Discussion
Analysis of the ACS-NSQIP for 4817 patients who underwent a pancreatoduodenectomy from 2005 to 2008 demonstrated that peri-operative RBC transfusion was associated with 30-day morbidity in a stepwise manner. Moreover, longer operative times also linearly correlated with increases in both 30-day morbidity and mortality. This analysis suggests that peri-operative blood transfusions and prolonged operative time should be considered quality indicators for performance of pancreatoduodenectomy.
Throughout the nearly 100-year history of the pancreatoduodenectomy procedure,1,2 numerous modifications have been reported. The goal of each update has been to decrease the morbidity and mortality rates that often accompany this complex procedure. Potential complications include, but are not limited to, post-operative sepsis, haemorrhage, delayed gastric emptying, as well as leakage from the pancreatico-enteric anastamosis. With modern improvements in critical care, nutritional support and percutaneous drainage techniques, peri-operative mortality has been dramatically reduced from 20% to less than 3%.3–5 Unfortunately, despite both technical and medical advancements, peri-operative morbidity rates have remained relatively consistent at approximately 40%.5–7
The NSQIP concept of prospectively collecting data on major operations and then providing risk adjusted 30-day morbidity and mortality outcomes for quality of care feedback originated in the Department of Veterans Affairs (National Veterans Affairs Surgical Risk Study).27–29 Subsequently, private sector health systems adopted NSQIP in an attempt to reduce morbidity, mortality and costs for participating hospitals.30 In 2004, the American College of Surgeons initiated a modified version of NSQIP which currently includes data from approximately 250 hospitals, and is being utilized for both quality of care assessment, as well as for surgical research.31,22 Approximately 60% of these hospitals are large academic medical centres. As a result, more than half of the pancreatoduodenectomies being performed annually in the United States are captured in this database.
Despite advances in surgical technique and peri-operative care, a blood transfusion is still required in a significant number of patients who undergo pancreatoduodenectomy. In this ACS-NSQIP analysis, 32% of patients received a RBC transfusion within 72 h of their operative procedure (median =3 units). The risks of allogenic blood transfusions are well documented and include an increased incidence of post-operative infection, acute lung injury and cancer recurrence.10–15 Unlike the potential transmission of infectious agents,32 these post-operative effects appear to be the direct result of transfusion-related immunosuppression.14–17 As a consequence of these data, as well as results from a multicentre randomized trial indicating that a transfusion trigger haemoglobin level of 7.0 g/dL is as safe as more liberal thresholds in nearly all patients,33‘bloodless’ surgery is being encouraged more frequently.17
In the context of hepato-pancreato-biliary (HPB) surgery, a near paucity of literature explores the relationship between RBC transfusion and outcomes. On univariate analysis, the Johns Hopkins group identified blood loss as a predictor of subsequent pancreatic fistula after pancreatoduodenectomy.34 Unfortunately, this relationship was not maintained in a multivariate analysis. In elective hepatic surgery, 10% to 33% of patients typically receive a blood transfusion.35–39 On retrospective review, these transfusions were shown to be associated with both an increase in peri-operative morbidity and mortality, as well as in the length of hospital stay.40 In a study attempting to predict the need for transfusion in elective liver resections, a median of two units were transfused when needed.41 Given the strong negative associations between RBC transfusion and outcomes in non-pancreatic surgery, it is not surprising that the ACS-NSQIP population displayed a stepwise increase in 30-day morbidity with increasing RBC transfusions after pancreatoduodenectomy. Although a statistical increase in 30-day mortality was not observed, we believe this observation is most likely the result of small sample sizes in patient subgroups who died after receiving varying numbers of RBC unit transfusions. This concept is strengthened by the observation that patients who received absolutely no blood had a significantly lower 30-day morbidity and mortality rate than those who were transfused with any volume of RBCs.
Clearly, pancreatoduodenectomies are technically challenging procedures. As a result, intra-operative blood loss and, therefore, subsequent RBC transfusions, are robust quality indicators because they are ‘specific and measurable elements of practice that can be used to assess quality of care’. 17,42 Moreover, this element of care is also modifiable because it is often surgeon or anaesthesiologist determined. As a result, it is one of the few modifiable factors that we have available for both short- and long-term outcomes.17 The need for a transfusion can also be altered by technical modifications such as minimizing blunt dissection, ensuring intra-operative haemostasis and maintaining normothermia. Non-technical approaches to reduce blood loss are also helpful and include pre-operative improvement of haemoglobin levels (iron and erythropoietin), minimizing redundant blood tests (pre- and post-operative), reducing central venous pressures for hepatic surgery, employing blood cell salvage devices and following evidence-based guidelines for lower transfusion triggers.17
After general surgical procedures, increased operative duration has been shown to predict infectious complications (surgical site infections, sepsis, septic shock, pneumonia and urinary tract infections).18–20 This association, as well as a longer duration of hospital stay, has also been reported in patients undergoing both pancreatectomies and biliary procedures.21 The specific operative time needed to produce an increase in subsequent morbidity is debated.18,20,21 Although no previous studies related to pancreatic resection were available for comparison, the ACS-NSQIP study cohort showed a strong linear correlation between operative duration and increased 30-day morbidity and mortality. While it could be argued that operative time is most commonly a direct result of patient anatomy and local disease progression, rather than surgical technique, it is interesting to note that there was no statistical association between operative duration and RBC transfusion. If patient variables were the primary determinant of operative time, one might also expect to see an increase in the rate of blood transfusion with lengthened operative duration.
This study has several limitations. First, it is retrospective and registry based; therefore the possibility of bias cannot be eliminated. Second, the ACS-NSQIP is not designed to track and evaluate pancreatoduodenectomy specific outcomes. As a result, relevant complications such as pancreatic fistula and exocrine or endocrine pancreatic dysfunction are not available for analysis. An HPB-specific NSQIP option is currently under development.43 Third, the small number of patients who actually died within 30 days of their pancreatoduodenectomy under-powered our statistical evaluation of the potential relationship between RBC transfusion and mortality. Fourth, we were unable to analyse the precise impact of additional operative components (i.e. diagnostic laparoscopy and/or concurrent vein resection) because of a lack of detail within the ACS-NSQIP registry. Fifth, although morbidity and mortality are powerful indicators of quality, additional metrics that may also be helpful in assessing quality (i.e. hospital and surgeon volume, cost and emergency status) are not yet available in ACS-NSQIP.44,45 Finally, although a RBC transfusion is considered a reasonable surrogate for peri-operative blood loss, it remains an indirect measure and may be potentially influenced by individual surgeon, anaesthesiologist, intensivist and trainee transfusion thresholds (i.e. defiance of current level-one data regarding indicated transfusion triggers33). However, peri-operative transfusion does reduce the known inaccuracies in surgeon-determined estimations of blood loss.46
In conclusion, 30-day morbidity is associated with both RBC transfusion and extended operative duration in patients undergoing a pancreatoduodenectomy. A similar stepwise relationship is present for 30-day mortality and operative duration. As a result, limiting blood loss and avoiding prolonged operative times should be goals for the pancreatic surgeon. These variables are robust quality indicators for pancreatoduodenectomy.
Conflicts of interest
None declared.
References
- 1.Kaush W. Das carcinoma der papilla und seine radikale entfernung. Beitr Klinishen Chirugie. 1912;78:29–33. [Google Scholar]
- 2.Whipple AO. The rationale of radical surgery for cancer of the pancreas and ampullary region. Ann Surg. 1941;114:612–615. doi: 10.1097/00000658-194111440-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crile G., Jr The advantage of bypass operations over radical pancreaticoduodenectomy in the treatment of pancreatic carcinoma. Surg Gynecol Obstet. 1970;130:1049–1053. [PubMed] [Google Scholar]
- 4.Herter FP, Cooperman AM, Ahlborn TN, Antinori C. Surgical experience with pancreatic and periampullary cancer. Ann Surg. 1982;195:274–281. doi: 10.1097/00000658-198203000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poon RTP, Fan ST. Decreasing the pancreatic leak rate after pancreaticoduodenectomy. Adv Surg. 2008;42:33–48. doi: 10.1016/j.yasu.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–260. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD, Kaufman HS, Coleman J. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430–435. doi: 10.1097/00000658-199305010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langer B. Role of volume outcome data in assuring quality in HPB surgery. HPB. 2007;9:330–334. doi: 10.1080/13651820701611234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sosa JA, Bowman HM, Gordon TA, Bass EB, Yeo CJ, Lillemoe KD, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998;228:429–438. doi: 10.1097/00000658-199809000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal N, Murphy JG, Cayten CG, Stahl WM. Blood transfusion increases the risk of infection after trauma. Arch Surg. 1993;128:171–177. doi: 10.1001/archsurg.1993.01420140048008. [DOI] [PubMed] [Google Scholar]
- 11.Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogenic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54:908–914. doi: 10.1097/01.TA.0000022460.21283.53. [DOI] [PubMed] [Google Scholar]
- 12.McAlister FA, Clark HD, Wells PS, Laupacis A. Perioperative allogenic blood transfusion does not cause adverse sequelae in patients with cancer: a meta-analysis of unconfounded studies. Br J Surg. 2001;85:171–178. doi: 10.1046/j.1365-2168.1998.00698.x. [DOI] [PubMed] [Google Scholar]
- 13.Busch OR, Hop WC, Marquet RL, Jeekel J. Blood transfusions and local tumor recurrence in colorectal cancer: evidence of a noncausal relationship. Ann Surg. 1994;220:791–797. doi: 10.1097/00000658-199412000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miki C, Hiro J, Ojima E, Inoue Y, Mohri Y, Kusunoki M. Perioperative allogenic blood transfusion, the related cytokine response and long-term survival after potentially curative resection of colorectal cancer. Clin Oncol (R Coll Radiol) 2006;18:60–66. doi: 10.1016/j.clon.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Crowe JP, Gordon NH, Fry DE, Shuck JM, Hubay CA. Breast cancer survival and perioperative blood transfusion. Surgery. 1989;106:836–841. [PubMed] [Google Scholar]
- 16.Nielson HJ. Detrimental effects of perioperative blood transfusion. Br J Surg. 1995;82:582–587. doi: 10.1002/bjs.1800820505. [DOI] [PubMed] [Google Scholar]
- 17.Dixon E, Datta I, Sutherland FR, Vauthey J. Blood loss in surgical oncology: neglected quality indicator? J Surg Oncol. 2009;99:508–512. doi: 10.1002/jso.21187. [DOI] [PubMed] [Google Scholar]
- 18.Pessaux P, Msika S, Atalla D, Hay JM, Flamant Y. Risk factors for postoperative infectious complications in noncolorectal abdominal surgery: a multivariate analysis based on a prospective multicenter study of 4718 patients. Arch Surg. 2003;138:314–324. doi: 10.1001/archsurg.138.3.314. [DOI] [PubMed] [Google Scholar]
- 19.Cruse PJ, Foord R. A five-year prospective study of 23 649 surgical wounds. Arch Surg. 1973;107:206–210. doi: 10.1001/archsurg.1973.01350200078018. [DOI] [PubMed] [Google Scholar]
- 20.Galandiuk S, Polk HC, Jagelman DG, Fazio VW. Re-emphasis of priorities in surgical antibiotic prophylaxis. Surg Gyencol Obstet. 1989;169:219–222. [PubMed] [Google Scholar]
- 21.Proctor LD, Davenport DL, Bernard AC, Zwischenberger JB. General surgical operative duration is associated with increased risk-adjusted infectious complication rates and length of hospital stay. J Am Coll Surg. 2010;210:60–65. doi: 10.1016/j.jamcollsurg.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 22.Birkmeyer JD, Shahian DM, Dimick JB, Finlayson SR, Flum DR, Ko CY, et al. Blueprint for a new American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2008;207:777–782. doi: 10.1016/j.jamcollsurg.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Daley J, Khuri SF, Henderson W, Hur K, Gibbs JO, Barbour G, et al. Risk adjustment of the postoperative morbidity rate for comparative assessment of the quality of surgical care. J Am Coll Surg. 1997;185:315–327. [PubMed] [Google Scholar]
- 24.Khuri SF. The NSQIP: a new frontier in surgery. Surgery. 2005;138:837–843. doi: 10.1016/j.surg.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Fink AS, Campbell DA, Mentzer RM, Henderson WG, Daley J, Bannister J, et al. The National Surgical Quality Improvement Program in non-Veterans Administration hospitals: initial demonstration of feasibility. Ann Surg. 2002;236:344–354. doi: 10.1097/00000658-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khuri SF, Henderson WG, Daley J, Jonasson O, Jones RS, Campbell DA, et al. The patient safety surgery study: background, study design, and patient populations. J Am Coll Surg. 2007;204:1089–1102. doi: 10.1016/j.jamcollsurg.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Khuri SF, Daley J, Henderson WG, Hur K, Demakis J, Aust JB, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comprehensive assessment of the quality of surgical care. J Am Coll Surg. 1995;180:519–531. [PubMed] [Google Scholar]
- 28.Khuri SF, Daley J, Henderson WG, Barbour G, Lowry P, Irvin G, et al. The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk adjusted, and peer-controlled programme for the measurement and enhancement of the quality of surgical care. Ann Surg. 1998;228:491–507. doi: 10.1097/00000658-199810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daley J, Khuri SF, Henderson WG, Hur K, Gibbs JO, Barbour G, et al. Risk adjustment of the postoperative mortality rate for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1997;185:328–340. [PubMed] [Google Scholar]
- 30.Henderson WG, Khuri SF, Mosca C, Fink AS, Hutter MM, Neumayer LA. Comparison of risk-adjusted 30-day postoperative mortality and morbidity in Department of Veterans Affairs hospitals and selected university medical centers: general surgical operations in men. J Am Coll Surg. 2007;204:1103–1114. doi: 10.1016/j.jamcollsurg.2007.02.068. [DOI] [PubMed] [Google Scholar]
- 31.Parikh PY, Pitt HA, Kilbane M, Howard TJ, Nakeeb A, Schmidt CM, et al. Pancreatic necrosectomy: North American mortality is much lower than expected. J Am Coll Surg. 2010;209:712–719. doi: 10.1016/j.jamcollsurg.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Goodnough LT. Risks of blood transfusion. Crit Care Med. 2003;31:S678–S686. doi: 10.1097/01.CCM.0000100124.50579.D9. [DOI] [PubMed] [Google Scholar]
- 33.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 34.Lin JW, Cameron JL, Yeo CJ, Riall TS, Lillemoe KD. Risk factors and outcomes in postpancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg. 2004;8:951–959. doi: 10.1016/j.gassur.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 35.Bui LL, Smith AJ, Bercovici M, Szalai JP, Hanna SS. Minimising blood loss and transfusion requirements in hepatic resection. HPB. 2002;4:5–10. doi: 10.1080/136518202753598672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mariette D, Smadja C, Naveau S, Borgonovo G, Vons C, Franco D. Preoperative predictors of blood transfusion in liver resection for tumor. Am J Surg. 1997;173:275–279. doi: 10.1016/S0002-9610(96)00400-X. [DOI] [PubMed] [Google Scholar]
- 37.Nagimo M, Kamiya J, Arai R, Nishio H. One hundred consecutive hepatobiliary resections for biliary malignancy: preoperative blood donation, blood loss, transfusion, and outcome. Surgery. 2005;137:148–155. doi: 10.1016/j.surg.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Pulitano C, Arru M, Bellio L, Rossini S, Ferla G, Aldrighetti L. A risk score for predicting perioperative blood transfusion in liver surgery. Br J Surg. 2007;94:860–865. doi: 10.1002/bjs.5731. [DOI] [PubMed] [Google Scholar]
- 39.Verma V, Schwartz RE. Factors influencing perioperative blood transfusions in patients with gastrointestinal cancer. J Surg Res. 2007;141:97–104. doi: 10.1016/j.jss.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 40.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869. doi: 10.1097/01.SLA.0000072371.95588.DA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cockbain AJ, Masudi T, Lodge PA, Toogood GJ, Prasad KR. Predictors of blood transfusion requirement in elective liver resection. HPB. 2010;12:50–55. doi: 10.1111/j.1477-2574.2009.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gagliardi AR, Fung MF, Langer B, Stern H, Brown AD. Development of ovarian cancer surgical quality indicators using a modified Delphi approach. Gynecol Oncol. 2005;97:446–456. doi: 10.1016/j.ygyno.2004.12.059. [DOI] [PubMed] [Google Scholar]
- 43.Pitt HA, Kilbane M, Strasberg SM, Pawlik TM, Dixon E, Zyromski NJ, et al. ACS-NSQIP has the potential to create an HPB-NSQIP option. HPB. 2009;11:405–413. doi: 10.1111/j.1477-2574.2009.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vollmer CM, Pratt W, Vanounou T, Maithel SK, Callery MP. Quality assessment in high-acuity surgery. Arch Surg. 2007;142:371–380. doi: 10.1001/archsurg.142.4.371. [DOI] [PubMed] [Google Scholar]
- 45.Howard TJ, Krug JE, Yu J, Zyromski NJ, Schmidt CM, Jacobson LE, et al. A margin-negative R0 resection accompolished with minimal postoperative complications is the surgeon's contribution to long-term survival in pancreatic cancer. J Gastrointest Surg. 2006;10:1338–1346. doi: 10.1016/j.gassur.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Sutherland FR, Mackenzie S, Bathe O, et al. Measuring blood loss during hepatectomy. HPB. in press. [Google Scholar]


