Abstract
Gene expression profiling has been used previously with spinal cord homogenates and laser capture microdissected motor neurons to determine the mechanisms involved in neurodegeneration in amyotrophic lateral sclerosis. However, while cellular and animal model work has focused on superoxide dismutase 1-related amyotrophic lateral sclerosis, the transcriptional profile of human mutant superoxide dismutase 1 motor neurons has remained undiscovered. The aim of this study was to apply gene expression profiling to laser captured motor neurons from human superoxide dismutase 1-related amyotrophic lateral sclerosis and neurologically normal control cases, in order to determine those pathways dysregulated in human superoxide dismutase 1-related neurodegeneration and to establish potential pathways suitable for therapeutic intervention. Identified targets were then validated in cultured cell models using lentiviral vectors to manipulate the expression of key genes. Microarray analysis identified 1170 differentially expressed genes in spinal cord motor neurons from superoxide dismutase 1-related amyotrophic lateral sclerosis, compared with controls. These genes encoded for proteins in multiple functional categories, including those involved in cell survival and cell death. Further analysis determined that multiple genes involved in the phosphatidylinositol-3 kinase signalling cascade were differentially expressed in motor neurons that survived the disease process. Functional experiments in cultured cells and primary motor neurons demonstrate that manipulating this pathway by reducing the expression of a single upstream target, the negative phosphatidylinositol-3 kinase regulator phosphatase and tensin homology, promotes a marked pro-survival effect. Therefore, these data indicate that proteins in the phosphatidylinositol-3 kinase pathway could represent a target for therapeutic manipulation in motor neuron degeneration.
Keywords: amyotrophic lateral sclerosis, SOD1, PTEN, PI3K, AKT
Introduction
Mutations in Cu/Zn superoxide dismutase (SOD1) were first associated with familial amyotrophic lateral sclerosis (ALS) in 1993 (Rosen et al., 1993). Following this landmark discovery, research has focused on the pathological mechanisms of mutant SOD1 that result in motor neuronal cell death. Oxidative stress, mitochondrial dysfunction, excitotoxicity, protein aggregation, endoplasmic reticulum stress, impairment of axonal transport, dysregulation of neuronal-glial interactions and apoptosis have all been demonstrated to contribute to motor neuron injury in the presence of mutant SOD1 (Shaw, 2005). Cellular and animal models expressing mutant SOD1 have been used to investigate strategies for therapeutic intervention, some of which have been successful in the laboratory, but have not to date translated into effective therapies in the clinic (Beghi et al., 2007; Aggarwal and Cudkowicz, 2008). A greater understanding at the molecular level of human SOD1-related ALS and how this relates to other subtypes of familial ALS and to sporadic disease is essential for the design of rational therapeutic approaches to neuroprotection in ALS.
ALS is an adult onset disorder and although SOD1 is ubiquitously expressed, it is the motor neurons that become selectively injured in patients as they age. Factors contributing to this selective vulnerability may include the post-mitotic nature of motor neurons, their large size, the high level of mitochondrial activity and their relatively low calcium buffering capacity. Thus, although not yet defined, we hypothesize that there are protective mechanisms initiated in response to the mutant SOD1 protein that protect the motor neuron during the early stages of life. These mechanisms then begin to fail during ageing, giving rise to the neurodegenerative process after several decades. Understanding these protective responses will allow the development of strategies for their upregulation, with the potential to prolong the survival of motor neuron in the face of a genetic stress.
Microarray analysis has been used previously to obtain gene expression profiles from brain and spinal cord homogenates of sporadic ALS cases in order to elucidate the genes and pathways that are involved in the neurodegenerative process (Malaspina et al., 2001; Ishigaki et al., 2002; Dangond et al., 2004; Offen et al., 2009). In subsequent reports, laser capture microdissection has been used to isolate motor neurons from the complex cell environment of the brain and spinal cord, in order to enrich the sample for neuronal gene expression (Jiang et al., 2005; Wang et al., 2006). However, to date there are no reports of the transcriptional profiles from human motor neurons associated with SOD1-related ALS. The aim of this work was to generate gene expression profiles of motor neurons from SOD1-related ALS and compare them to the profiles of motor neurons from neurologically normal control cases, in order to determine the pathways implicated in human SOD1-related neurodegeneration and to establish potential pathways suitable for therapeutic intervention. Multiple functional categories were identified as containing differentially expressed transcripts in motor neurons expressing mutant SOD1. This report focuses on the differential expression of cell survival and cell death genes, implicating the phosphatidylinositol-3 kinase (PI3K) signalling cascade as an important survival mechanism in motor neurons that remain identifiable in human ALS post-mortem material. This is supported by functional studies in vitro, which demonstrate that knockdown of the key negative regulator of the PI3K pathway, phosphatase and tensin homologue (PTEN), supports the survival of cultured wild-type and G93A SOD1 motor neurons.
Materials and methods
Microarray analysis
Cervical spinal cord blocks from three subjects with SOD1-related ALS (p.E100G: n = 1, p.I113T: n = 2) and five neurologically normal control cases (CON1–5) were obtained from the Sheffield Brain Tissue Bank. A further two control samples were obtained from NeuroResource, Institute of Neurology, University College London (CON6–7) (Table 1). All samples were collected following informed consent and the work was approved by the South Yorkshire Ethics Committee. Both p.I113T cases contained the characteristic hyaline conglomerate inclusions associated with this SOD1 mutation (Ince et al., 1998). Spinal cord sections were prepared, approximately 500 motor neurons were isolated and RNA was extracted using methods described previously (Ferraiuolo et al., 2007, 2009). RNA quantity and quality was assessed on the Nanodrop spectrophotometer and Agilent Bioanalyser, respectively, to ensure all samples were of comparable and sufficient quality to proceed. RNA (20–25 ng) was linearly amplified using the Affymetrix Two Cycle cDNA synthesis protocol to produce biotin-labelled copy RNA. Copy RNA (15 μg) was fragmented for 15 min and hybridized to the Human Genome U133 Plus 2.0 GeneChips, according to Affymetrix protocols. GeneChips were washed and stained in the Fluidics System 400 before being scanned in the GeneChip 3000 Scanner. Data analysis was performed in ArrayAssist®, using the PLIER algorithm for normalization and t-tests to identify differentially expressed transcripts. (Further details are available in online Supplementary material). The online Database for Annotation, Visualization and Integrated Discovery (DAVID) (Dennis et al., 2003; Huang da et al., 2009) and NetAffx™ (Affymetrix) were used to assign Gene Ontology terms (Biological Process, Molecular Function or Cellular Component) to each of the differentially expressed transcripts.
Table 1.
Details of the three SOD1-related ALS cases and seven neurologically normal controls
| Sample | SOD1 status | Sex | Age (years) | PMI (h) | Disease duration (m)/cause of death |
|---|---|---|---|---|---|
| ALS1 | p.E100G | Female | 40 | 23 | 41 |
| ALS2 | p.I113T | Female | 60 | 27 | 13 |
| ALS3 | p.I113T | Female | 66 | 50 | 14 |
| CON1 | Normal | Female | 87 | 14 | Haemopericardium |
| CON2 | Normal | Male | 53 | 19 | Ischaemic heart disease |
| CON3 | Normal | Male | 63 | 19 | Ischaemic heart disease |
| CON4 | Normal | Male | 63 | 20 | Septic shock |
| CON5 | Normal | Female | 59 | 5 | Atypical pneumonia |
| CON6 | Normal | Male | 81 | 19 | Acute abdominal obstruction |
| CON7 | Normal | Female | 80 | 24 | Pulmonary embolism |
PMI = post-mortem interval.
Quantitative polymerase chain reaction
Total RNA was amplified using the QuantiTech® Whole Transcriptome kit (Qiagen). Quantitative polymerase chain reaction (qPCR) primers were either designed using Eurofins online primer design software (www.eurofinsdna.com) or were identified from previous publications (Supplementary Table 1) (Zhou et al., 2003). QPCR of the SOD1 cases and controls was performed using Brilliant® II SYBR® Green qPCR Master Mix (Stratagene) on the Stratagene 3000, as described previously (Kirby et al., 2005). Unpaired two-tailed t-tests (Graphpad Prism 5) were used to determine if the relative differences between the SOD1 mutant cases and controls were statistically significant.
Small interfering RNA design and viral production
A 19-nucleotide sequence targeting mouse PTEN (Supplementary material) (Ning et al., 2004) was subcloned in the pLVTHM genome vector (Addgene plasmid 12247) (Wiznerowicz and Trono, 2003) according to the manufacturer’s protocol. This approach allowed generation of a stem-loop-stem small hairpin RNA to effectively reduce PTEN expression levels (Brummelkamp et al., 2002). Wild-type and mutant C124S PTEN complementary DNA were also cloned between the BamHI/XhoI sites of a lentiviral vector genome. Self-inactivating lentiviral vectors stocks were prepared and viral titres were estimated using enzyme-linked immunosorbent assay (Supplementary material) (Deglon et al., 2000).
Lentiviral transduction of NSC34 cells
Untransfected NSC34 cells and mutant G93A SOD1 stably transfected NSC34 cells were cultured as previously described (Menzies et al., 2002). Cells were transduced either with lentiviral vectors carrying a PTEN knockdown small interfering RNA (multiplicity of infection = 50), a scrambled small interfering RNA (multiplicity of infection = 50) or with vectors expressing normal or mutant PTEN or green fluorescent protein. On post-transduction Day 5, cells were oxidatively stressed with 10 mM H2O2 for 2 h and cell survival measured using a propidium iodide assay (Supplementary material).
Lentiviral transduction of primary motor neuron cultures
Cultures of embryonic motor neurons were prepared as described previously (Jablonka et al., 2007) (Supplementary material). Motor neurons were transduced with lentiviral vectors expressing the normal or mutant PTEN or green fluorescent protein or with the PTEN small interfering RNA and scrambled small interfering RNA vectors (multiplicity of infection = 10), 24 h after plating. Motor neurons for western blotting were cultured for 7 days post-transduction at 37°C with 5% CO2. For survival assays, 1500 cells were plated without cover slips in four-well dishes coated with polyornithine and mouse laminin. Cells were observed under a phase-contrast microscope and the number of initially plated cells was determined after the cells were attached for 20 h after plating. After 7 days in culture, the numbers of surviving cells were counted.
Western blotting
Protein lysates from untransfected NSC34 cells and G93A SOD1 stably transfected NSC34 cells were collected on Day 7 post-transduction. For each sample, 10 μg of protein was loaded on to the gel. For western blotting of the primary motor neuron cultures, 200 000 cells were plated on 3.5 cm cell culture dishes coated with polyornithine and mouse laminin. Protein lysates were collected on Day 7 post-transduction (multiplicity of infection = 10 for lentiviral-scrambled small interfering RNA against PTEN-green fluorescent protein and lentiviral-small interfering RNA against PTEN-green fluorescent protein). For each sample, 2 µg of protein was loaded on to the gel. Primary antibodies used were anti-mouse GAPDH antibody (1:5000; Calbiochem), anti-rabbit PTEN (1:1000; Cell Signalling), Phospho-AKT Ser473 (1:1000; Cell Signalling), Phospho-Bad Ser155 (1:1000; Cell Signalling).
Results
Gene expression profiling of motor neurons
Gene expression profiles were generated from RNA extracted from motor neurons isolated by laser capture microdissection to ensure a motor neuron-enriched transcriptome was sampled. RNA was obtained from motor neurons located in the cervical spinal cord of three SOD1-related ALS cases (mtSOD1 motor neurons) (p.E100G: n = 1, p.I113T: n = 2) and seven neurologically normal control cases (control motor neurons) (Table 1). Expression profiles of the three mtSOD1 motor neurons gave an average of 22.1% of genes present in the samples, compared with 28.2% of genes present in the seven control motor neurons. Quality control parameters were comparable between the two groups. Comparative analysis in ArrayAssist® identified 1170 transcripts differentially expressed in SOD1-related motor neurons compared with control motor neurons, of which 524 were increased and 646 were decreased in mtSOD1 motor neurons compared with controls. (.cel files for each of the 10 GeneChips have been submitted to the Gene Expression Omnibus Repository, Accession GSE20589).
Functional categorization of the differentially expressed genes revealed that the largest changes in gene expression were in genes involved in transcription, signalling and metabolism (Table 2). The majority of genes involved in transcription showed decreased expression, which is in keeping with the transcription repression induced by mutant SOD1 described in previous reports (Kirby et al., 2005; Ferraiuolo et al., 2007). Functional categories affected reflect those mechanisms known to be involved in neurodegeneration, such as programmed cell death, the cytoskeleton and calcium binding. In contrast, very few RNA processing genes were differentially expressed, suggesting that motor neuron cell death in SOD1-related ALS is distinct from that proposed in TARDBP and FUS-related ALS. The complete list of differentially expressed genes, classified by functional category, is given in Supplementary Table 2. The Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathways, which contain a significant number of differentially expressed genes, as identified by the Database for Annotation, Visualization and Integrated Discovery (DAVID), are provided in Supplementary Tables 3 and 4.
Table 2.
Functional classification of differentially expressed transcripts in the presence of the mutant SOD1
| Functional category | Number of genes | Genes increased | Genes decreased |
|---|---|---|---|
| Transcription | 185 | 65 | 120 |
| Signalling | 139 | 73 | 66 |
| Metabolism | 103 | 44 | 59 |
| Transport | 96 | 47 | 49 |
| Modification | 63 | 26 | 37 |
| Nucleic acid binding | 52 | 27 | 25 |
| Cell development | 48 | 22 | 26 |
| Response | 44 | 24 | 20 |
| Cell adhesion | 39 | 17 | 22 |
| Programmed cell death | 34 | 14 | 20 |
| Cytoskeleton | 33 | 19 | 14 |
| Cell cycle | 28 | 12 | 16 |
| Translation | 24 | 9 | 15 |
| Reproduction | 19 | 7 | 12 |
| Oxidoreductase/ electron transport | 19 | 10 | 9 |
| Calcium binding | 16 | 9 | 7 |
| Protein binding | 13 | 7 | 6 |
| Development—nervous system | 12 | 4 | 8 |
| Cell differentiation/ proliferation | 12 | 6 | 6 |
| Extracellular | 7 | 3 | 4 |
| RNA processing | 4 | 4 | 0 |
| Lipid binding | 2 | 1 | 1 |
| Miscellaneous | 115 | 51 | 64 |
| Unknown | 63 | 24 | 39 |
In order to focus our downstream analysis of the data, we looked at the KEGG pathways identified as most significantly affected. In these highlighted pathways, it was evident that genes involved in cell development, differentiation and survival and in programmed cell death signalling appeared in several of the significant KEGG pathways. Since these genes, particularly the PI3K/PTEN pathway, represent obvious targets for therapeutic interventional strategies and a review of the literature provided evidence that these genes play an important role in the CNS as well as being dysregulated in the G93A SOD1 mouse model of SOD1-related ALS (Warita et al., 2001; Nagano et al., 2002), we chose to further investigate the role of these genes and their protein interactions and validate their differential expression. We propose that the increase in the PI3K survival pathway is one of the neuroprotective mechanisms that determines the late-onset of SOD1-related ALS.
Cell survival: the RHOA/PI3K/PTEN pathway
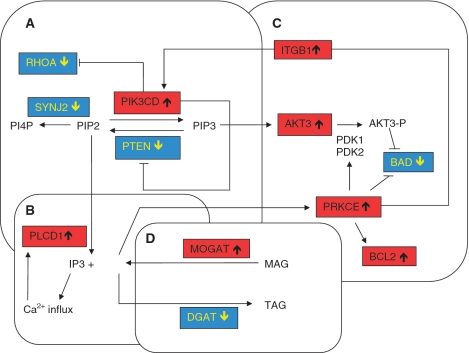
RHOA is a member of the Rho GTPase family, intracellular proteins that play an important role in neuronal cell survival and cell death by transducing extracellular signals to the cytoskeleton (Linseman and Loucks, 2008). Expression levels of RHOA are decreased 4.25-fold in the mtSOD1 motor neurons and this was confirmed by qPCR (P = 0.0017) (Supplementary Fig. 1A). The expression of RHOA is negatively regulated by the p110-delta catalytic subunit (p110Δ/PIK3CD) of phosphatidylinositol-3 kinase (PI3K) (Papakonstanti et al., 2007), which is highly expressed in the nervous system, including in spinal cord motor neuron (Eickholt et al., 2007). This subunit is increased (+3.95) in mtSOD1 motor neurons and this was confirmed by qPCR (P = 0.0005) (Supplementary Fig. 1B). PI3K is a heterodimeric enzyme that phosphorylates phosphatidylinositol-4,5-P2 (PI[4,5]P2) to phosphatidylinositol-3,4,5-P3 (PI[3,4,5]P3) (Fig. 1A). PI[3,4,5]P3 then recruits protein kinase B (AKT) to the membrane where AKT is activated by phosphorylation (see below). The PIK3CD subunit also negatively regulates PTEN (Papakonstanti et al., 2007), which shows decreased expression in mtSOD1 motor neurons (−2.07). This differential expression has been confirmed by qPCR (P = 0.0017) (Supplementary Fig. 1C). PTEN (phosphatase and tensin homologue) is a dual phosphatase, which negatively regulates the PI3K-AKT pathway by catalysing the conversion of PI[3,4,5]P3 to PI[4,5]P2. PI[4,5]P2 can then be catalysed to PI[4]P, by the action of inositol polyphosphate 5-phosphatases (IPP) and synaptojanin2, which is a ubiquitiously expressed inositol polyphosphate 5-phosphatase. Synaptojanin2 is decreased (−3.50) in mtSOD1 motor neurons. PI[4,5]P2 can also be converted by phospholipase C to inositol 1,4,5 triphosphate and diacylglycerol (Fig. 1B). Diacylglycerol activates protein kinase C epsilon (see below) (Basu and Sivaprasad, 2007), while inositol 1,4,5 triphosphate causes cytosolic Ca2+ influx. Thus, the decrease in PTEN favours the PI3K pathway and activation of AKT.
Figure 1.
Interacting pathways of cell survival present in the mtSOD1 motor neurons. (A) Increase in PIK3CD, which inhibits RHOA and PTEN, (B) the connection between PIP2, phospholipase CD1 (PLCphosp), diacylglycerol (DAG) and protein kinase C epsilon (PRKCEpr). (C) Increased protein kinase C epsilon activates AKT3 and increases BCL2 and inhibits Bcl2-antagonist of death expression (BAD) and (D) shows how the increase in monoacylglycerol O-transferase (MOGAT) and decrease in diacylglycerol O-acyltransferase (DGAT) ensure levels of diacylglycerol are available for protein kinase C epsilon activation. AKT3-P = phophorylated AKT3; IP3 = inositol 1,4,5 triphosphate; ITGB1be = beta-1-integrin; MAG = monoacylglycerol; PI4P = phosphatidylinositol-4 phosphate; PIK3CD = phosphatidylinositol-3 kinase catalytic subunit delta; PIP2 = phosphatidylinositol-4,5-bisphosphate; RHOA = ras homology gene family member A; SYNJ2 = synaptojanin 2; TAG = triacylglycerol.
Cell survival: the AKT3/protein kinase C epsilon pathway
AKT3 (also known as protein kinase B gamma) is primarily expressed in the brain and has been shown to be essential for normal brain development (Easton et al., 2005; Tschopp et al., 2005). Once activated by phosphorylation at Thr308 and Ser473 by phosphatidylinositol-3 kinase 1 and 2 (PDK1 and PDK2), respectively, AKT enzymes regulate cell survival, cell cycle and proliferation and glycogen and protein metabolism (Blume-Jensen and Hunter, 2001). AKT has been shown to inhibit the action of B cell lymphoma 2 (Bcl2)-antagonist of death through phosphorylation (Frebel and Wiese, 2006). In mtSOD1 motor neurons, AKT3 expression is increased (+3.24) and this has been confirmed by qPCR (P = 0.02) (Supplementary Fig. 1D), and while PDK1 and PDK2 are not differentially expressed, CD9, encoding a tetraspanin that has been shown to enhance the phosphorylation of AKT (Kotha et al., 2009), is increased (+2.83). In contrast, the level of Bcl2-antagonist of death gene expression is decreased (−2.59) (Fig. 1C).
AKT phosphorylation has been shown to be increased by overexpression of protein kinase C epsilon. AKT can also be indirectly regulated through protein kinase C epsilon causing an increase of beta-1-integrin at the cell surface which subsequently triggers the PI3K pathway (Basu and Sivaprasad, 2007) (Fig. 1C). In mtSOD1 motor neurons, expression of protein kinase C epsilon is increased (+5.22), as confirmed by qPCR (P = 0.004) (Supplementary Fig. 1E) as is beta-1-integrin (+2.76). Protein kinase C epsilon also mediates anti-apoptotic signalling by altering the expression of the BCL2 family of proteins (Basu and Sivaprasad, 2007) and this is demonstrated in the mtSOD1 motor neurons with increased expression of the anti-apoptotic BCL2 (+2.84), as confirmed by qPCR (P = 0.045) (Supplementary Fig. 1F) and decreased expression of the pro-apoptotic protein Bcl2-antagonist of death (−2.59). As mentioned previously, protein kinase C epsilon requires diacylglycerol for activation; monoacylglycerol O-transferase 1 (MOGAT1), which synthesizes diacylglycerol, is increased in mtSOD1 motor neurons (+2.10), while the downstream enzyme diacylglycerol O-acyltransferase (DGAT1), which synthesizes triacylglycerol, is decreased (−3.59). These changes are predicted to ensure a supply of diacylglycerol for protein kinase C epsilon and support for cell survival (Fig. 1D).
Cell death: the programmed cell death pathway
The differentially expressed genes PIK3CD, AKT3, protein kinase C epsilon and BCL2 provide evidence of increased expression of anti-apoptotic genes to protect the motor neurons from cell death. This is supported by additional anti-apoptotic BCL2-related genes that show increased expression including BCL2 associated athanogene 4 (BAG4 +2.26) and BCL11B (+2.45). The pro-apoptotic genes that show decreased expression, in addition to Bcl2-antagonist of death, include apoptotic peptidase activating factor (APAF1; −2.75), BCL2 associated transcription factor 1 (BCLAF1/BTF; −2.35), death inducer obliterator 1 (DIDO1; −2.83) and deoxyribonuclease 1 (DNASE1, −2.28).
There are also multiple genes decreased in the p53 apoptotic pathway. Although p53 (TP53) itself is not differentially expressed, p63 (TP63), a homologue of p53 that also induces apoptosis is decreased (−2.31). Tumour necrosis factor receptor associated factor 4 (TRAF4), activated by both p53 and p63, is decreased (−3.75), as are other p53-induced genes, leucine-rich repeats and death domain containing gene (LRDD; −4.80); phorbo-12-myristate-13-acetate-induced protein 1 (PMAIP1/NOXA; −4.89); TP53 inducible protein 3 (TP53I3; −3.02) and TP53 inducible protein 13 (TP53I13; −2.36). APAF1 (−2.75) is also a downstream target of p53. Inhibition of p53 can occur through the action of mouse double minute (MDM) proteins, and MDM4 is increased 2.18 in the mtSOD1 motor neurons.
In neuronal cells, members of the inhibitor of apoptosis protein family have previously been shown to be increased to prevent apoptosis (Frebel and Wiese, 2006). Survivin (BIRC5) interacts with X-linked inhibitor of apoptosis protein (XIAP/BIRC4) to inhibit the apoptosome, while X-linked inhibitor of apoptosis protein also directly interacts with caspase 3, 7 and 9 to inhibit apoptosis. Contrary to the anti-apoptotic expression changes described above, in mtSOD1 motor neurons, both survivin (BIRC5) and X-linked inhibitor of apoptosis protein (XIAP/BIRC4) are decreased (−2.98 and −2.43, respectively), while the pro-apoptotic septin 4, which binds X-linked inhibitor of apoptosis protein, is increased (+2.44). However, ring finger protein 34 (RNF34), which has a similar function to X-linked inhibitor of apoptosis protein and increases the expression of BCL2, is increased (+2.66).
In contrast to the anti-apoptotic response demonstrated above, there is also an increase in the pro-apoptotic apoptosis signal-regulating kinase 1/mitogen activated protein 3 kinase 5 (ASK1/MAP3K5 +2.59). ASK1 is activated by oxidative stress and once phosphorylated, initiates c-Jun N-terminal kinase (JNK) and p38 MAP kinase pathways (Matsuzawa and Ichijo, 2008). Sustained activation of these pathways by ASK1 is required for apoptosis. However, there are no apparent downstream effectors of ASK1, such as MAP2 kinases or JNK/p38, that show differential expression.
Effect of PTEN knockdown on survival in a motor neuronal cell line
The key regulators of the cell survival pathway that have been shown have altered expression in mtSOD1 motor neurons are PTEN (negative regulator) and PIK3CD (positive regulator). While there are several PI3K enzymes encoded in the human genome, there is only one functional PTEN protein. We therefore chose to investigate the specific effect of reducing the expression of PTEN, as a logical way to upregulate the PI3K signalling pathway.
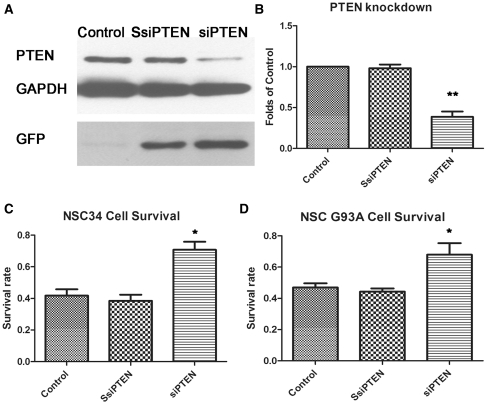
To demonstrate the effect of reduced expression of PTEN on motor neuron survival in functional assays, the mouse motor neuronal cell line, NSC34, was transduced with a lentivirus expressing a small interfering RNA against PTEN. The vector also encodes the green fluorescent protein reporter gene downstream from a elongation factor 1 alpha EF1a promoter. Cells transduced with small interfering RNA against PTEN showed reduced protein expression of PTEN, while the scrambled small interfering RNA had no effect on PTEN protein levels (Supplementary Fig. 2). Western blotting with an antibody to PTEN demonstrated that PTEN protein expression is knocked down by >50% using small interfering RNA against PTEN (Fig. 2A and B). Following transduction with the small interfering RNA and scrambled small interfering RNA against PTEN for 5 days, the NSC34 cells were stressed with 10 mM H2O2 for 2 h. Knockdown of PTEN by small interfering RNA was demonstrated to significantly increase the survival of the untransfected NSC34 cells from 41.7–70.7% and mutant G93A SOD1 expressing NSC34 cells from 47 to 68% in response to this oxidative insult (P < 0.05) (Fig. 2C and D).
Figure 2.
Results of transduction of NSC34 cells with small interfering RNA against PTEN (siPTEN) and scrambled small interfering RNA against PTEN (SsiPTEN). (A) Representative western blot illustrating the knockdown of PTEN expression in the presence of the small interfering RNA against PTEN. The control is untransfected NSC34 cells. (B) Graph showing the level of PTEN protein expression in control and in small interfering RNA against PTEN and scrambled small interfering RNA against PTEN transduced (green fluorescent protein positive) NSC34 cells relative to GAPDH (n = 4; **P < 0.01; values represent means ± SD). (C) Effect of PTEN knockdown on survival of untransfected NSC34 cells and (D) survival of G93A SOD1 stably transfected NSC34 cells, following treatment with 10 mM H2O2 for 2 h (n = 4; *P < 0.05; values represent means ± SD). GFP = green fluorescent protein.
Effect of PTEN knockdown in primary motor neurons
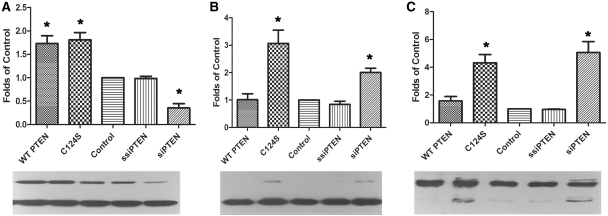
To investigate whether PTEN knockdown has a similar effect in post-mitotic neurons, purified motor neuronal cultures were transduced with small interfering RNA against PTEN or scrambled small interfering RNA against PTEN. In addition, the primary motor neurons were incubated with lentivector encoding either wild-type PTEN or a p.C124S dominant PTEN mutant (C124S), which has no phosphatase activity. Compared with untransduced cells, the small interfering RNA against PTEN treated motor neurons showed a reduction of 62% in PTEN protein expression, while those transduced with the wild-type or mutant PTEN showed an increase of 75% (Fig. 3A). Western blotting of the PI3K downstream targets showed an increase in phosphorylated AKT in the presence of small interfering RNA against PTEN and also in motor neurons incubated with the dominant mutant C124S PTEN (Fig. 3B). There was also a concomitant increase in the phosphorylation of Bcl2-antagonist of death in these two motor neuron preparations (Fig. 3C), demonstrating that reduced PTEN protein expression has a functional effect on downstream targets.
Figure 3.
Western blotting of primary motor neuronal cultures transfected with constructs over expressing wild-type (WT) or C124S mutant PTEN or transduced with small interfering RNA against PTEN (siPTEN) and scrambled small interfering RNA against PTEN (SsiPTEN). Using antibodies against (A) PTEN, (B) phosphorylated AKT and (C) phosphorylated Bcl2-antagonist of death, reduced PTEN expression (A) is shown to be associated with increased phosphorylation of AKT and Bcl2-antagonist of death (B, C). In each graph: lane 1 = wild-type PTEN; lane 2 = dominant negative mutant C124S PTEN; lane 3 = untransfected primary motor neurons; lane 4 = scrambled small interfering RNA against PTEN; and lane 5 = small interfering RNA against PTEN (n = 3; *P < 0.05; values represent means ± SD).
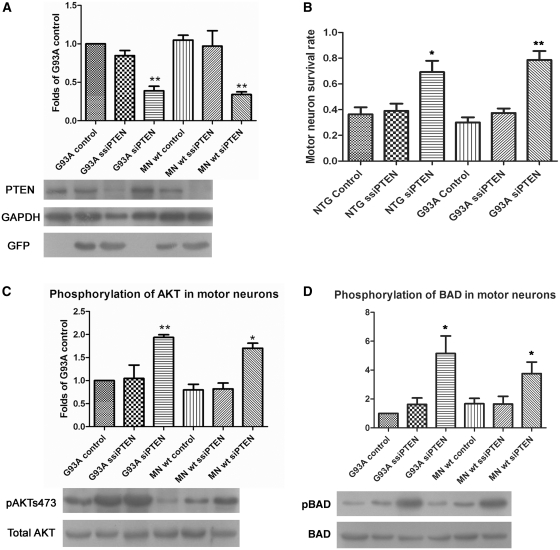
Additional experiments using primary motor neuron originating from the human G93A SOD1 transgenic mouse, confirmed the specificity of the small interfering RNA against PTEN to knockdown PTEN (Fig. 4A) and this correlated with an increase in survival of motor neuron in culture at Day 7 (Fig. 4B). Small interfering RNA against PTEN-mediated PTEN downregulation was also shown to be associated with an increase in phosphorylation of AKT (Fig. 4C) and increased phosphorylation levels of Bcl2-antagonist of death in primary motor neurons originating from G93A SOD1 mice (Fig. 4D). Thus, these small interfering RNA experiments demonstrate that the increased survival seen in cells following PTEN knockdown are mediated through increased phosphorylation and activation of AKT and increased phosphorylation promoting inhibition of Bcl2-antagonist of death.
Figure 4.
Results of transduction of primary motor neuronal cultures originating from G93A SOD1 mice and litter mates with small interfering RNA (siRNA) against PTEN and scrambled small interfering RNA against PTEN (ssiPTEN). Antibodies against (A) PTEN, (C) phosphorylated AKT and (D) phosphorylated Bcl2-antagonist of death (BAD) show the concomitant decrease in expression of PTEN and increase in phosphorylation of AKT and Bcl2-antagonist of death (n = 3; *P < 0.05, **P < 0.01; values represent means ± SD). (B) Survival of motor neuron in transduced primary motor neuronal cultures on Day 7 (n = 3; *P < 0.05, **P < 0.01; values represent means ± SD). MN = motor neurons; wt = wild-type.
Discussion
The aim of this investigation was to generate gene expression profiles from motor neurons of normal human and mutant SOD1-related ALS, to determine those pathways dysregulated in SOD1-related ALS neurodegeneration and to establish potential pathways for therapeutic intervention. We identified 1170 differentially expressed genes in mtSOD1 motor neuron, of which 524 were increased and 646 were decreased in the presence of the mutant SOD1 protein. While multiple functional categories of genes were altered in expression, we focused on the significant differential expression of numerous genes in signalling pathways promoting cell development, differentiation and survival, with a concomitant decrease in programmed cell death associated genes. We propose that these particular genes reflect those expressed in motor neurons that have survived the human ALS disease process, thereby highlighting mechanisms the motor neurons are using to protect against mutant SOD1-mediated neuronal cell death and that extension of life, through invasive ventilation, is likely to see the balance of the survival/cell death genes switch such that these motor neurons would also degenerate. Our hypothesis is supported by evidence from the SOD1 transgenic mouse, which showed that loss of PI3K and AKT immunoreactivity occurs in the majority of large spinal cord motor neurons, prior to disease onset (Nagano et al., 2002). However, injection of an adenoviral vector containing glial cell line-derived neurotrophic factor significantly preserved the number of phosphor-AKT positive motor neurons and this was associated with an increase in the total number of motor neurons (Manabe et al., 2002).
The role of PTEN and PI3K in neurodegeneration
The p110Δ subunit of PI3K negatively regulates both RHOA and PTEN expression (Papakonstanti et al., 2007). In the mtSOD1 motor neurons, a decreased level of RHOA is predicted to result in maintenance of the growth cone and neurite extension, as well as continued formation of actin/myosin cross bridges, thereby supporting axon integrity. Increased activation of RHOA in the CNS is associated with ischaemic injury or glutamate induced excitotoxicity, leading to apoptosis (Semenova et al., 2007). Thus, reduced RHOA expression in the mtSOD1 motor neuron is predicted to reflect cellular conditions promoting neuronal regeneration and survival in motor neuron isolated at the end-stage of disease. This conclusion is supported by the strategy taken by Gross and colleagues (2007) who used lentiviral vectors to reduce RHOA expression and promote regeneration of motor neuron in models of spinal cord ischaemia.
PTEN is a tumour suppressor gene implicated in both familial and sporadic cancers. As a key regulator of the conversion of PI[3,4,5]P3 to PI[4,5]P2, in cancer PTEN acts to protect against the downstream pro-proliferative, anti-apoptotic AKT-dependent pathways (Chalhoub and Baker, 2009). In contrast, in SOD1-related ALS, induction of anti-apoptotic pathways in the motor neuron is predicted to be neuroprotective. The decreased expression of PTEN and concomitant increase of PIK3CD, promotes generation of the signalling molecule PI[3,4,5]P3, which is in turn essential for activation of the downstream AKT signalling pro-survival pathways. The importance of the activation of this pathway in the human CNS is supported by in vitro models. In both the NSC34 motor neuronal cell line and in primary motor neurons, reduced PTEN protein expression is associated with an increase in survival, increased phosphorylated (and therefore activated) AKT and increased phosphorylated (and therefore inactive) Bcl2-antagonist of death. These findings are present in untransfected cells and in the presence of G93A mutant SOD1.
PI3K, AKT and protein kinase C epsilon as neuroprotective agents
The downstream effect of increased PI3K is increased activation of the AKT pathway. In the mtSOD1 motor neurons, AKT3 was increased, which in combination with the increase in protein kinase C epsilon, would lead to increased AKT3 activation and reduced levels of the pro-apoptotic Bcl2-antagonist of death, while protein kinase C epsilon concomitantly causes increased BCL2 and decreased Bcl2-antagonist of death expression (Basu and Sivaprasad, 2007). In addition, we have shown that phosphorylated Bcl2-antagonist of death, which is inactive, is also increased when PTEN is inhibited (and AKT is activated). It has been shown previously that overexpression of Bcl2 in the G93A SOD1 transgenic mouse model delayed onset and prolonged survival (Kostic et al., 1997) and the result was reproduced by adeno-associated viral injection of Bcl2 into the spinal cord (Azzouz et al., 2000). Thus, increased expression of BCL2, induced by protein kinase C epsilon, is neuroprotective in mtSOD1 motor neurons. In addition, AKT3 has been implicated previously as having a neuroprotective role against SOD1-mediated neurotoxicity (Kanekura et al., 2005).
Multiple reports have demonstrated that the neuroprotective effects of both vascular endothelial growth factor and angiogenin act via PI3K to induce AKT activation. Vascular endothelial growth factor-mediated cell survival via PI3K/AKT has been shown in vitro in the NSC34 cell line transfected with G93A SOD1 (Li et al., 2003), rat primary motor neurons transduced with adenoviral G93A SOD1 (Lunn et al., 2009) and in vivo in G93A SOD1 transgenic rats (Dewil et al., 2007). Similarly, in vitro and in vivo treatment of cultured motor neurons and G93A SOD1 mice, respectively, with angiogenin increased motor neuron survival and AKT signalling (Kieran et al., 2008). In addition, PI3K/AKT is also activated during vascular endothelial growth factor-mediated neuroprotection following glutamate excitotoxicity and serum withdrawal (Tolosa et al., 2008, 2009). Finally, activators of PI3K, including epigallocatechin gallate, have been shown to increase cell survival in cellular models transfected with mutant SOD1 (Koh et al., 2004, 2005) and treatment of the G93A SOD1 mice with epigallocatechin gallate significantly delayed the onset of disease, and extended life span (Xu et al., 2006). Thus, activation of the PI3K/AKT pathway by several different stimuli (angiogenin, vascular endothelial growth factor, epigallocatechin gallate) all result in neuroprotection and support our evidence for the role of this pathway as a mechanism that is protecting the surviving mtSOD1 motor neurons.
Comparison with microarray studies of human ALS
The results presented in this report provide a novel insight into the pathways responding to the presence of mtSOD1 in human motor neurons that survived the neurodegenerative process prior to death. Earlier gene expression profiling studies used RNA extracted from homogenates of anterior horn spinal cord (Malaspina et al., 2001; Ishigaki et al., 2002; Dangond et al., 2004; Lederer et al., 2007; Offen et al., 2009) and while these studies clearly demonstrated reactive gliosis, an inflammatory response and apoptotic cell death, the differential expression primarily reflected the increasing number of glial cells, rather than the loss of motor neurons. Thus, any increased gene expression in survival pathways of the remaining motor neurons would have been diluted by the multiple other non-neuronal cell types in the homogenates.
More recently, laser capture microdissection has been used to isolate lumbar spinal cord motor neurons from the surrounding CNS tissue from sporadic and control cases, to generate motor neuron-enriched gene expression profiles (Jiang et al., 2005). There was concordance in several functional categories of genes between those genes differentially expressed in sporadic ALS lumbar motor neurons and those differentially expressed in the SOD1-related ALS cervical spinal cord motor neurons. These categories include transcription-related genes, cell surface proteins, cell survival and cell death associated genes and neuroprotective neurotrophic factors, such as hepatocyte growth factor (Supplementary Tables 2 and 3) (Jiang et al., 2005).
Comparison with microarray studies of SOD1-related models
Microarray analysis of SOD1 models of ALS, including NSC34 cell lines transfected with mutant G93A SOD1 and G93A SOD1 transgenic mice have previously been reported by the authors of this report (Kirby et al., 2005; Ferraiuolo et al., 2007). In both of these reports, there was evidence of a decrease in transcription, with more genes decreased in both G93A SOD1 transfected NSC34 cells, 120d G93A SOD1 transgenic mice and in mtSOD1 motor neurons compared with controls. Although neither of the previous reports used genome-wide microarrays, key pathways were found to be consistently dysregulated in the presence of mtSOD1, including cell development, differentiation and survival, metabolism and transcription.
It is important to note that the cultured murine motor neuronal cell line, NSC34, does show a different transcriptional response to that of post-mitotic, aged human motor neuron-enriched samples isolated from spinal cord. Analysis of expression profiles from the immortalized NSC34 cell line demonstrated an unexpected decrease in programmed cell life genes, in response to the presence of human mutant SOD1, as well as a decrease in genes involved in antigen processing and presentation (Kirby et al., 2005). Such responses have not been detected in the mtSOD1 motor neurons isolated from the CNS or the G93A SOD1 transgenic mouse model, most likely due to the interaction with astrocytes and other glial cells in the CNS, which play a supporting role during the lifetime of the motor neuron. In addition, the SOD1 transgenic mouse model expresses mutant SOD1 protein at a level 10-fold greater that of endogenous murine SOD1, while in the human disease, the ratio of mutant to normal SOD1 is 1:1. Such an increased stress and the very rapid disease course in the murine model may account for some of the differences seen in the motor neuron responses from the mouse model and human cases. However, as discussed above, the SOD1 transgenic mice do show loss of PI3K and AKT immunoreactivity in the majority of large spinal cord motor neurons, prior to disease onset (Nagano et al., 2002).
We present here the first report of transcriptional profiling of motor neurons from SOD1-related ALS and establish a role for the RHOA/PI3K/PTEN and AKT3/protein kinase C epsilon pathways as a central mechanism involved in preventing neuronal cell death in the motor neurons that have survived the disease process in human SOD1-related ALS. While only a small number of SOD1-related ALS cases with a range of post-mortem intervals have been sampled, the pathways implicated have been supported by our functional data and evidence in the published literature. We have demonstrated that knockdown of PTEN, which increases PI3K signalling, promotes motor neuron survival in the presence of oxidative stress or mutant G93A SOD1, with concomitant increases in phosphorylated AKT and Bcl2-antagonist of death. While it is unknown if these pathways are altered as a direct consequence of the mutant SOD1 protein’s toxic gain of function or as a secondary defence, the data contribute further evidence of the potential for PI3K and AKT activators and PTEN inhibitors as therapeutic agents to slow the progression of motor neuron degeneration. Such agents could potentially support the intrinsic attempts of the motor neuron to survive the cell stress induced by the presence of mutant SOD1, a response that in human subjects is usually successful in preventing motor neuron degeneration for several decades.
Supplementary Material
Acknowledgements
We thank Hazel Holden and Matthew Wyles for technical assistance, NeuroResource, UCL for additional control material and Nicole Déglon for providing us with lentiviral vector plasmids and technical advice on viral production. We are extremely grateful to the patients with ALS and their families for the valuable gift to research of the donation of CNS tissue to the Sheffield Brain Tissue Bank.
Funding
Wellcome Trust programme award (to P.J.S.); Motor Neurone Disease Association (to J.K.).
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- ALS
amyotrophic lateral sclerosis
- BCL2
B cell lymphoma 2
- KEGG
Kyoto Encyclopaedia of Genes and Genomes
- qPCR
quantitative polymerase chain reaction
- PI3K
phosphatidylinositol-3 kinase
- PTEN
phosphatase and tensin homologue
- SOD 1
superoxide dismutase 1
References
- Aggarwal S, Cudkowicz M. ALS drug development: reflections from the past and a way forward. Neurotherapeutics. 2008;5:516–27. doi: 10.1016/j.nurt.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzouz M, Hottinger A, Paterna JC, Zurn AD, Aebischer P, Bueler H. Increased motoneuron survival and improved neuromuscular function in transgenic ALS mice after intraspinal injection of an adeno-associated virus encoding Bcl-2. Hum Mol Genet. 2000;9:803–11. doi: 10.1093/hmg/9.5.803. [DOI] [PubMed] [Google Scholar]
- Basu A, Sivaprasad U. Protein kinase Cepsilon makes the life and death decision. Cell Signall. 2007;19:1633–42. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghi E, Mennini T, Bendotti C, Bigini P, Logroscino G, Chio A, et al. The heterogeneity of amyotrophic lateral sclerosis: a possible explanation of treatment failure. Curr Med Chem. 2007;14:3185–200. doi: 10.2174/092986707782793862. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243–7. doi: 10.1016/s1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Ann Rev Pathol. 2009;4:127–50. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangond F, Hwang D, Camelo S, Pasinelli P, Frosch MP, Stephanopoulos G, et al. Molecular signature of late-stage human ALS revealed by expression profiling of postmortem spinal cord gray matter. Physiol Genomics. 2004;16:229–39. doi: 10.1152/physiolgenomics.00087.2001. [DOI] [PubMed] [Google Scholar]
- Deglon N, Tseng JL, Bensadoun JC, Zurn AD, Arsenijevic Y, Pereira de Almeida L, et al. Self-inactivating lentiviral vectors with enhanced transgene expression as potential gene transfer system in Parkinson’s disease. Human Gene Ther. 2000;11:179–90. doi: 10.1089/10430340050016256. [DOI] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- Dewil M, Lambrechts D, Sciot R, Shaw PJ, Ince PG, Robberecht W, et al. Vascular endothelial growth factor counteracts the loss of phospho-Akt preceding motor neurone degeneration in amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol. 2007;33:499–509. doi: 10.1111/j.1365-2990.2007.00850.x. [DOI] [PubMed] [Google Scholar]
- Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, et al. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–78. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickholt BJ, Ahmed AI, Davies M, Papakonstanti EA, Pearce W, Starkey ML, et al. Control of axonal growth and regeneration of sensory neurons by the p110delta PI 3-kinase. PLoS ONE. 2007;2:e869. doi: 10.1371/journal.pone.0000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraiuolo L, De Bono JP, Heath PR, Holden H, Kasher P, Channon KM, et al. Transcriptional response of the neuromuscular system to exercise training and potential implications for ALS. J Neurochem. 2009;109:1714–24. doi: 10.1111/j.1471-4159.2009.06080.x. [DOI] [PubMed] [Google Scholar]
- Ferraiuolo L, Heath PR, Holden H, Kasher P, Kirby J, Shaw PJ. Microarray analysis of the cellular pathways involved in the adaptation to and progression of motor neuron injury in the SOD1 G93A mouse model of familial ALS. J Neurosci. 2007;27:9201–19. doi: 10.1523/JNEUROSCI.1470-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frebel K, Wiese S. Signalling molecules essential for neuronal survival and differentiation. Biochem Soc Trans. 2006;34:1287–90. doi: 10.1042/BST0341287. [DOI] [PubMed] [Google Scholar]
- Gross RE, Mei Q, Gutekunst CA, Torre E. The pivotal role of RhoA GTPase in the molecular signaling of axon growth inhibition after CNS injury and targeted therapeutic strategies. Cell Transplant. 2007;16:245–62. doi: 10.3727/000000007783464740. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Ince PG, Tomkins J, Slade JY, Thatcher NM, Shaw PJ. Amyotrophic lateral sclerosis associated with genetic abnormalities in the gene encoding Cu/Zn superoxide dismutase: molecular pathology of five new cases, and comparison with previous reports and 73 sporadic cases of ALS. J Neuropathol Exp Neurol. 1998;57:895–904. doi: 10.1097/00005072-199810000-00002. [DOI] [PubMed] [Google Scholar]
- Ishigaki S, Niwa J, Ando Y, Yoshihara T, Sawada K, Doyu M, et al. Differentially expressed genes in sporadic amyotrophic lateral sclerosis spinal cords–screening by molecular indexing and subsequent cDNA microarray analysis. FEBS Lett. 2002;531:354–8. doi: 10.1016/s0014-5793(02)03546-9. [DOI] [PubMed] [Google Scholar]
- Jablonka S, Beck M, Lechner BD, Mayer C, Sendtner M. Defective Ca2+ channel clustering in axon terminals disturbs excitability in motoneurons in spinal muscular atrophy. J Cell Biol. 2007;179:139–49. doi: 10.1083/jcb.200703187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YM, Yamamoto M, Kobayashi Y, Yoshihara T, Liang Y, Terao S, et al. Gene expression profile of spinal motor neurons in sporadic amyotrophic lateral sclerosis. Ann Neurol. 2005;57:236–51. doi: 10.1002/ana.20379. [DOI] [PubMed] [Google Scholar]
- Kanekura K, Hashimoto Y, Kita Y, Sasabe J, Aiso S, Nishimoto I, et al. A Rac1/phosphatidylinositol 3-kinase/Akt3 anti-apoptotic pathway, triggered by AlsinLF, the product of the ALS2 gene, antagonizes Cu/Zn-superoxide dismutase (SOD1) mutant-induced motoneuronal cell death. J Biol Chem. 2005;280:4532–43. doi: 10.1074/jbc.M410508200. [DOI] [PubMed] [Google Scholar]
- Kieran D, Sebastia J, Greenway MJ, King MA, Connaughton D, Concannon CG, et al. Control of motoneuron survival by angiogenin. J Neurosci. 2008;28:14056–61. doi: 10.1523/JNEUROSCI.3399-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby J, Halligan E, Baptista MJ, Allen S, Heath PR, Holden H, et al. Mutant SOD1 alters the motor neuronal transcriptome: implications for familial ALS. Brain. 2005;128:1686–706. doi: 10.1093/brain/awh503. [DOI] [PubMed] [Google Scholar]
- Koh SH, Kwon H, Kim KS, Kim J, Kim MH, Yu HJ, et al. Epigallocatechin gallate prevents oxidative-stress-induced death of mutant Cu/Zn-superoxide dismutase (G93A) motoneuron cells by alteration of cell survival and death signals. Toxicology. 2004;202:213–25. doi: 10.1016/j.tox.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Koh SH, Roh H, Lee SM, Kim HJ, Kim M, Lee KW, et al. Phosphatidylinositol 3-kinase activator reduces motor neuronal cell death induced by G93A or A4V mutant SOD1 gene. Toxicology. 2005;213:45–55. doi: 10.1016/j.tox.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Kostic V, Jackson-Lewis V, de Bilbao F, Dubois-Dauphin M, Przedborski S. Bcl-2: prolonging life in a transgenic mouse model of familial amyotrophic lateral sclerosis. Science. 1997;277:559–62. doi: 10.1126/science.277.5325.559. [DOI] [PubMed] [Google Scholar]
- Kotha J, Zhang C, Longhurst CM, Lu Y, Jacobs J, Cheng Y, et al. Functional relevance of tetraspanin CD9 in vascular smooth muscle cell injury phenotypes: a novel target for the prevention of neointimal hyperplasia. Atherosclerosis. 2009;203:377–86. doi: 10.1016/j.atherosclerosis.2008.07.036. [DOI] [PubMed] [Google Scholar]
- Lederer CW, Torrisi A, Pantelidou M, Santama N, Cavallaro S. Pathways and genes differentially expressed in the motor cortex of patients with sporadic amyotrophic lateral sclerosis. BMC Genomics. 2007;8:26. doi: 10.1186/1471-2164-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Xu W, Luo C, Gozal D, Liu R. vascular endothelial growth factor -induced activation of the PI3-K/Akt pathway reduces mutant SOD1-mediated motor neuron cell death. Brain Res Mol Brain Res. 2003;111:155–64. doi: 10.1016/s0169-328x(03)00025-1. [DOI] [PubMed] [Google Scholar]
- Linseman DA, Loucks FA. Diverse roles of Rho family GTPases in neuronal development, survival, and death. Front Biosci. 2008;13:657–76. doi: 10.2741/2710. [DOI] [PubMed] [Google Scholar]
- Lunn JS, Sakowski SA, Kim B, Rosenberg AA, Feldman EL. Vascular endothelial growth factor prevents G93A-SOD1-induced motor neuron degeneration. Develop Neurobiol. 2009;69:871–84. doi: 10.1002/dneu.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina A, Kaushik N, de Belleroche J. Differential expression of 14 genes in amyotrophic lateral sclerosis spinal cord detected using gridded cDNA arrays. J Neurochem. 2001;77:132–45. doi: 10.1046/j.1471-4159.2001.t01-1-00231.x. [DOI] [PubMed] [Google Scholar]
- Manabe Y, Nagano I, Gazi MS, Murakami T, Shiote M, Shoji M, et al. Adenovirus-mediated gene transfer of glial cell line-derived neurotrophic factor prevents motor neuron loss of transgenic model mice for amyotrophic lateral sclerosis. Apoptosis. 2002;7:329–34. doi: 10.1023/a:1016123413038. [DOI] [PubMed] [Google Scholar]
- Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta. 2008;1780:1325–36. doi: 10.1016/j.bbagen.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Menzies FM, Cookson MR, Taylor RW, Turnbull DM, Chrzanowska-Lightowlers ZM, Dong L, et al. Mitochondrial dysfunction in a cell culture model of familial amyotrophic lateral sclerosis. Brain. 2002;125:1522–33. doi: 10.1093/brain/awf167. [DOI] [PubMed] [Google Scholar]
- Nagano I, Murakami T, Manabe Y, Abe K. Early decrease of survival factors and DNA repair enzyme in spinal motor neurons of presymptomatic transgenic mice that express a mutant SOD1 gene. Life Sci. 2002;72:541–8. doi: 10.1016/s0024-3205(02)02249-x. [DOI] [PubMed] [Google Scholar]
- Ning K, Pei L, Liao M, Liu B, Zhang Y, Jiang W, et al. Dual neuroprotective signaling mediated by downregulating two distinct phosphatase activities of PTEN. J Neurosci. 2004;24:4052–60. doi: 10.1523/JNEUROSCI.5449-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offen D, Barhum Y, Melamed E, Embacher N, Schindler C, Ransmayr G. Spinal cord mRNA profile in patients with ALS: comparison with transgenic mice expressing the human SOD-1 mutant. J Mol Neurosci. 2009;38:85–93. doi: 10.1007/s12031-007-9004-z. [DOI] [PubMed] [Google Scholar]
- Papakonstanti EA, Ridley AJ, Vanhaesebroeck B. The p110delta isoform of PI 3-kinase negatively controls RhoA and PTEN. Embo J. 2007;26:3050–61. doi: 10.1038/sj.emboj.7601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Semenova MM, Maki-Hokkonen AM, Cao J, Komarovski V, Forsberg KM, Koistinaho M, et al. Rho mediates calcium-dependent activation of p38alpha and subsequent excitotoxic cell death. Nat Neurosci. 2007;10:436–43. doi: 10.1038/nn1869. [DOI] [PubMed] [Google Scholar]
- Shaw PJ. Molecular and cellular pathways of neurodegeneration in motor neurone disease. J Neurol Neurosurg Psychiatry. 2005;76:1046–57. doi: 10.1136/jnnp.2004.048652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa L, Mir M, Asensio VJ, Olmos G, Llado J. Vascular endothelial growth factor protects spinal cord motoneurons against glutamate-induced excitotoxicity via phosphatidylinositol 3-kinase. J Neurochem. 2008;105:1080–90. doi: 10.1111/j.1471-4159.2007.05206.x. [DOI] [PubMed] [Google Scholar]
- Tolosa L, Mir M, Olmos G, Llado J. Vascular endothelial growth factor protects motoneurons from serum deprivation-induced cell death through phosphatidylinositol 3-kinase-mediated p38 mitogen-activated protein kinase inhibition. Neuroscience. 2009;158:1348–55. doi: 10.1016/j.neuroscience.2008.10.060. [DOI] [PubMed] [Google Scholar]
- Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, et al. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–54. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- Wang XS, Simmons Z, Liu W, Boyer PJ, Connor JR. Differential expression of genes in amyotrophic lateral sclerosis revealed by profiling the post mortem cortex. Amyotroph Lateral Scler. 2006;7:201–10. doi: 10.1080/17482960600947689. [DOI] [PubMed] [Google Scholar]
- Warita H, Manabe Y, Murakami T, Shiro Y, Nagano I, Abe K. Early decrease of survival signal-related proteins in spinal motor neurons of presymptomatic transgenic mice with a mutant SOD1 gene. Apoptosis. 2001;6:345–52. doi: 10.1023/a:1011334018804. [DOI] [PubMed] [Google Scholar]
- Wiznerowicz M, Trono D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol. 2003;77:8957–61. doi: 10.1128/JVI.77.16.8957-8961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Chen S, Li X, Luo G, Li L, Le W. Neuroprotective effects of (-)-epigallocatechin-3-gallate in a transgenic mouse model of amyotrophic lateral sclerosis. Neurochem Res. 2006;31:1263–9. doi: 10.1007/s11064-006-9166-z. [DOI] [PubMed] [Google Scholar]
- Zhou YH, Tan F, Hess KR, Yung WK. The expression of PAX6, PTEN, vascular endothelial growth factor, and epidermal growth factor receptor in gliomas: relationship to tumor grade and survival. Clin Cancer Res. 2003;9:3369–75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.