Abstract
Behavioural variant frontotemporal dementia is characterized by a change in comportment. It is associated with considerable functional decline over the course of the illness albeit with sometimes dramatic variability among patients. It is unknown whether any baseline features, or combination of features, could predict rate of functional decline in behavioural variant frontotemporal dementia. The aim of this study was to investigate the effects of different baseline clinical, neuropsychological, neuropsychiatric, genetic and anatomic predictors on the rate of functional decline as measured by the Clinical Dementia Rating Sum of Boxes scale. We identified 86 subjects with behavioural variant frontotemporal dementia that had multiple serial Clinical Dementia Rating Sum of Boxes assessments (mean 4, range 2–18). Atlas-based parcellation was used to generate volumes for specific regions of interest at baseline. Volumes were utilized to classify subjects into different anatomical subtypes using the advanced statistical technique of cluster analysis and were assessed as predictor variables. Composite scores were generated for the neuropsychological domains of executive, language, memory and visuospatial function. Behaviours from the brief questionnaire form of the Neuropsychiatric Inventory were assessed. Linear mixed-effects regression modelling was used to determine which baseline features predict rate of future functional decline. Rates of functional decline differed across the anatomical subtypes of behavioural variant frontotemporal dementia, with faster rates observed in the frontal dominant and frontotemporal subtypes. In addition, subjects with poorer performance on neuropsychological tests of executive, language and visuospatial function, less disinhibition, agitation/aggression and night-time behaviours at presentation, and smaller medial, lateral and orbital frontal lobe volumes showed faster rates of decline. In many instances, the effect of the predictor variables observed across all subjects was also preserved within anatomical subtypes. Furthermore, some of the predictor variables improved our prediction of rate of functional decline after anatomical subtype was taken into account. In particular, age at onset was a highly significant predictor but only after adjusting for subtype. We also found that although some predictor variables, for example gender, Mini-Mental State Examination score, and apathy/indifference, did not affect the rate of functional decline; these variables were associated with the actual Clinical Dementia Rating Sum of Boxes score estimated for any given time-point. These findings suggest that in behavioural variant frontotemporal dementia, rate of functional decline is driven by the combination of anatomical pattern of atrophy, age at onset, and neuropsychiatric characteristics of the subject at baseline.
Keywords: frontotemporal dementia, behaviour, functional decline, brain volumes, mixed effects models
Introduction
Behavioural variant frontotemporal dementia (FTD) is a clinical syndrome characterized by the insidious onset of behavioural and personality change accompanied by impairment in executive function (Neary et al., 1998; Josephs, 2008). It is the most common of the three clinical syndromes subsumed under the umbrella term FTD, which has a reported prevalence of 15 per 100 000 for subjects in the age range 45–64 years (Ratnavalli et al., 2002). Subjects are typically ‘young’ with disease onset before the age of 65 years and typically experience substantial functional decline over the course of their illness. In fact, the rate of functional decline in behavioural variant FTD is faster than the rate in typical Alzheimer’s disease (Rascovsky et al., 2005). It is not surprising therefore, that subjects with behavioural variant FTD perform poorly on tests of functional ability (Rosen et al., 2004), with performance progressively declining over time (Whitwell et al., 2008; Knopman et al., 2009). Unfortunately, the rate of functional decline can vary dramatically (Knopman et al., 2009) making prognosis for individual subjects difficult. It is unknown whether specific baseline characteristics, or combinations of characteristics, could predict functional decline in behavioural variant FTD.
Subjects with behavioural variant FTD can have variable clinical and neuropsychological profiles (Snowden et al., 2001; Rascovsky et al., 2002; Le Ber et al., 2006), and can be anatomically heterogeneous (Whitwell et al., 2009d). Using the analytical technique of cluster analysis, we recently demonstrated that behavioural variant FTD is associated with at least four different anatomical subtypes characterized by predominant frontal atrophy, frontotemporal atrophy, temporal atrophy and temporofrontoparietal atrophy (Whitwell et al., 2009d). We therefore aimed to determine whether baseline characteristics, including anatomical subtype, or a combination of characteristics, could predict functional decline in behavioural variant FTD measured using the Clinical Dementia Rating Sum of Boxes (CDR-SB) scale (Hughes et al., 1982; Fillenbaum et al., 1996). The CDR-SB ranges from 0–18 points (0 = no impairment, 18 = maximum impairment) and has been proven to be a valid instrument with good inter-rater reliability (Kendall’s correlation coefficient = 0.90) (Burke et al., 1988; Fillenbaum et al., 1996; Morris, 1997; Choi et al., 2003). Reliability has only been formally tested in Alzheimer’s disease, although the CDR-SB has been shown to be sensitive to functional impairment (Rosen et al., 2004) and correlates well with patterns and rates of cerebral atrophy in behavioural variant FTD (Seeley et al., 2008; Whitwell et al., 2008, 2009c), suggesting that it is a valid measure of disease progression in behavioural variant FTD. The sum of boxes measure was used in this analysis as it is a more detailed quantitative measure with a greater range than the CDR global score, is less influenced by the memory component, and is more pertinent to behavioural variant FTD.
An important motivating hypothesis for this study was that anatomical subtype would predict functional decline given that the anatomical subtypes have different genetic and varying pathological underpinnings (Whitwell et al., 2009d).
Materials and methods
Subject selection
We identified all subjects from the Mayo Clinic Alzheimer’s Disease Research Centre or Alzheimer’s Disease Patient Registry that had a clinical diagnosis of behavioural variant FTD (The Lund and Manchester Groups, 1994; Neary et al., 1998) with a documented onset year and at least two serial clinical assessments at the Mayo Clinic, Rochester, between 1994 and 2009. The Alzheimer’s Disease Research Centre and Alzheimer’s Disease Patient Registry are longitudinal prospective studies in which subjects undergo approximately annual clinical and neuropsychological assessments and MRI scans. All subjects had been evaluated within the Department of Neurology by a subspecialist in the Division of Behavioural Neurology. The CDR (Hughes et al., 1982), Mini-Mental State Examination (MMSE) (Folstein et al., 1975), and the brief questionnaire form of the Neuropsychiatric Inventory (NPI-Q) (Cummings, 1998; Kaufer et al., 2000) were administered independently. The CDR was completed by the evaluating physician, the MMSE by the neuropsychometrist, and the NPI-Q by the carer. The results of the NPI-Q were not used in the clinical diagnosis of the subjects and NPI-Q data was not available for five subjects seen before 1998. Age at onset was recorded for all subjects and was based on the symptom history provided by the carer. Disease onset was considered to be the year and month of the appearance of the first symptom(s) deemed to be specific to behavioural variant FTD (Neary et al., 1998) by the evaluating behavioural neurologist. A family history was recorded as positive if at least one first degree relative of the subject had a clinical or pathological diagnosis of FTD or amyotrophic lateral sclerosis, or a clinical diagnosis of young onset dementia (onset <65 years). Apolipoprotein E genotyping was performed as previously described (Josephs et al., 2004).
A total of 97 subjects were identified with a behavioural variant FTD diagnosis. Eleven subjects were subsequently excluded because they did not have a documented month and year of disease onset, did not have serial CDR assessments or had a baseline CDR-SB score of 18 and hence reached the limit of the measurement, allowing for no further measurable functional decline. Therefore, a total of 86 subjects were included in this study. Subject characteristics at baseline including: demographics, CDR global score, CDR-SB, MMSE, subscores of the NPI-Q and apolipoprotein E epsilon 4 (ε4) carrier status are shown in Table 1 and Fig. 1. This study was approved by the Mayo Clinic Institutional Review Board. All subjects provided written informed consent before participating in any research activity.
Table 1.
Subject characteristics at baseline
| Characteristic | n (%) | Median (IQR) | Range |
|---|---|---|---|
| Male gender | 38 (44%) | ||
| Apolipoprotein E ε4 carrier | 20 (24%) | ||
| Positive family history | 35 (42%) | ||
| Age at onset, year | 56 (50, 66) | 20–84 | |
| Age at baseline, year | 62 (55, 71) | 25–89 | |
| Disease duration at baseline, year | 4 (2, 6) | 0–24 | |
| Disease duration at last assessment, year | 8 (5, 11) | 2–25 | |
| Education, year | 15 (12, 17) | 5–20 | |
| MMSE | 25 (22, 27) | 1–30 | |
| CDR global score | |||
| 0 | 1 (1) | ||
| 0.5 | 35 (41) | ||
| 1 | 35 (41) | ||
| 2 | 12 (14) | ||
| 3 | 3 (3) | ||
| CDR sum of boxes | 4 (2, 7) | 0–17 | |
| Neuropsychiatric Inventory | |||
| Total | 8 (5, 13) | 0–23 | |
| Delusions | 0 (0, 0) | 0–3 | |
| Hallucinations | 0 (0, 0) | 0–2 | |
| Agitation/aggression | 1 (0, 2) | 0–3 | |
| Depression/dysphoria | 0 (0, 1) | 0–2 | |
| Anxiety | 0 (0, 2) | 0–3 | |
| Elation/euphoria | 0 (0, 0) | 0–3 | |
| Apathy/indifference | 2 (1, 2) | 0–3 | |
| Disinhibition | 1 (0, 2) | 0–3 | |
| Irritability | 1 (0, 2) | 0–3 | |
| Motor disturbance | 0 (0, 2) | 0–3 | |
| Night-time behaviours | 0 (0, 1) | 0–3 | |
| Appetite/eating | 1 (0, 2) | 0–3 | |
| Executive | |||
| Composite | 0.1 (−1.0, 1.1) | −2.0–2.8 | |
| Trails Aa | 50 (37, 74) | 20–300 | |
| Trails B | 300 (96, 300) | 41–300 | |
| Controlled Oral Word Association Test | 26 (16, 32) | 1–53 | |
| Language | |||
| Composite | 0.1 (−0.8, 0.8) | −2.9–2.6 | |
| Boston naming | 45 (35, 53) | 7–60 | |
| Category fluency | 21 (15, 30) | 2–51 | |
| Memory | |||
| Composite | 0.0 (−1.0, 1.0) | −2.0–2.5 | |
| Wechsler Memory Scale-Revised Logical Memory | 5 (1, 11) | 0–30 | |
| Wechsler Memory Scale-Revised Visual Reproduction | 6 (1, 14) | 0–38 | |
| Visuospatial | |||
| Composite | −0.1 (−0.7, 0.7) | −2.2–3.0 | |
| Picture completionb | 5 (4, 6) | 1–12 | |
| Block designb | 6 (5, 8) | 1–14 | |
| Anatomical subtype | |||
| Temporal dominant | 14 (18) | ||
| Temporofrontoparietal | 34 (43) | ||
| Frontotemporal | 13 (16) | ||
| Frontal dominant | 18 (23) |
aTrails A was not included in executive composite score.
bBased on scaled scores from either the revised or version III of the Wechsler Adult Intelligence Scale.
IQR = interquartile range.
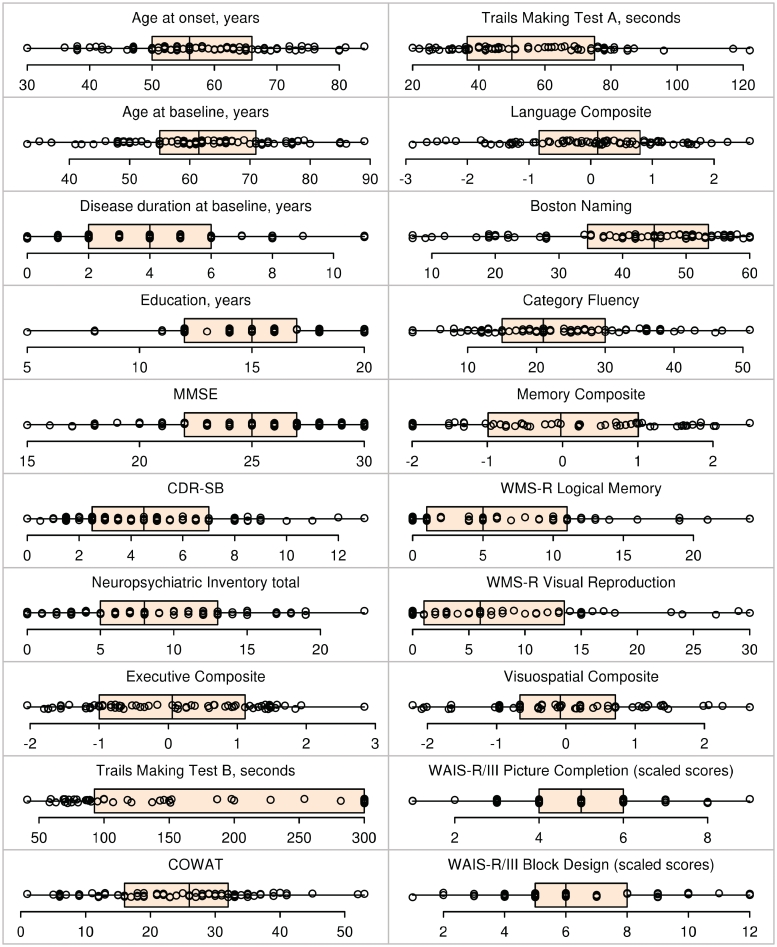
Figure 1.
Box-plots with superimposed data points showing baseline demographic, cognitive and neuropsychological test results for all 86 subjects. Boxes indicate the median and quartiles and have whiskers extending to the furthest data point within 1.5 interquartile ranges of the box. Individual data points have been randomly perturbed in the vertical direction to reduce overlap. COWAT = Controlled Oral Word Association Test; WAIS-R/lll = Wechsler Adult Intelligence Scale-Revised/version three; WMS-R = Wechsler Memory Scale-Revised.
Genetic analysis
All subjects with a positive family history that had available DNA were screened for mutations in the microtubule associated protein tau (MAPT) or progranulin (GRN) gene, as previously described (Hutton et al., 1998; Gass et al., 2006).
Neuropsychological assessments
Neuropsychological assessments performed within 6 months of the baseline clinical assessment were collected and analysed. Our neuropsychological battery included two neuropsychological tests to represent the domains of executive, language, memory and visuospatial function. Trail Making Test A was also completed but was not utilized for any analysis. The Trail Making Test B (War Department, 1944) and Controlled Oral Word Association Test (Benton and Hamsher, 1989) were used to test executive function. Subjects who could not complete Trail Making Test B were given a score of 300 s corresponding to the maximum. Boston Naming Test (Kaplan et al., 1983) and Category Fluency Test (sum of animals, vegetables and fruits) (Lucas et al., 1998) were used to test language function. The Wechsler Memory Scale-Revised Logical Memory delayed recall and Visual Reproduction delayed recall subtests (Rey, 1964) were used to test memory function. Scaled scores of the Picture Completion and Block Design subtests of the Wechsler Adult Intelligence Scale-Revised or Third Edition (Wechsler, 1981, 1997) were used to test visuospatial function. All tests were administered by experienced psychometrists and were supervised by clinical neuropsychologists. Baseline neuropsychological test scores are shown in Table 1.
Magnetic resonance imaging analysis
All MRI studies were performed with a standardized imaging protocol (Jack et al., 2008b). All images underwent preprocessing correction for gradient non-linearity (Jovicich et al., 2006) and intensity non-uniformity (Sled et al., 1998) as previously described (Jack et al., 2008a). A total of 79 subjects had a usable volumetric MRI. Four subjects did not have a volumetric MRI and in three subjects the MRI was unusable due to movement artefact.
An atlas-based parcellation technique was employed using SPM5 and the automated anatomic labelling atlas (Tzourio-Mazoyer et al., 2002) in order to generate grey matter volumes for different regions of interest across the brain of each subject. The processing steps have been previously described in detail (Whitwell et al., 2009d). Briefly, all subject MRIs and the automated anatomic labelling atlas (Tzourio-Mazoyer et al., 2002) were spatially normalized to a customized template (Ashburner and Friston, 2005). Then for each subject, the inverse transformation was applied to the custom-space atlas in order to warp the atlas to the patient’s native anatomical space. Each native-space MRI was segmented into grey matter, white matter and CSF. The grey matter probability maps for each subject were thresholded to create a binary mask and were multiplied by the native-space automated anatomic labelling atlas to generate a custom grey matter atlas for each patient, parcellated into different regions of interest.
The region of interest data were utilized for two separate analyses. First, to group subjects into the four anatomical subtypes of behavioural variant FTD (Whitwell et al., 2009d). For this analysis, we analysed the same 26 regions of interest that were originally used to perform the cluster analysis and, as before, all volumes were divided by the total grey matter volume for each subject (Whitwell et al., 2009d). The methods used to assign each subject to an anatomical subtype are described below in the ‘Statistical analysis’ section.
Second, we were interested in determining whether baseline region of interest volumes could help predict CDR-SB increase. For this analysis we chose a smaller, more manageable, number of regions of interest that represented regions that have previously been shown to be affected in behavioural variant FTD. We therefore calculated grey matter volume of the left and right hemispheres for the following eight mutually exclusive regions of interest: medial frontal [medial frontal gyrus (Brodmann area, BA 8 + 9 + 10) and anterior cingulate gyrus (BA 32 + 33)]; orbital frontal (BA 11 + 12); lateral frontal [inferior (BA 43 + 44 + 45 + 47), middle (BA 6 + 10 + 46) and superior frontal gyri (BA 8 + 9)]; medial temporal [hippocampus, amygdala, parahippocampal gyrus (BA 34 + 35 + 36), fusiform (BA 37)]; lateral temporal [inferior, middle and superior temporal gyri (BA 20 + 21, +22)]; temporal pole (BA 38), parietal [posterior cingulate gyrus (BA 23 + 31), inferior parietal (BA 39 + 40) and superior parietal lobe (BA 5+7)]; and caudate nucleus. Volumes for the left and right hemisphere were averaged for each region of interest. In addition, all region of interest volumes were divided by the total grey matter volume for each subject. By doing this, regional grey matter volumes were scaled to, or relative to, total grey matter volume and hence differences across patients reflect differences in regional atrophy as a proportion of total brain loss, rather than differences in actual regional size.
Statistical analysis
All potential predictors were obtained at baseline and for convenience we grouped them as clinical, neuropsychological and imaging predictors. The following baseline clinical predictors were assessed: age at onset, age at baseline assessment, gender, education, family history, CDR-SB, MMSE, all severity subscores from the NPI-Q, and apolipoprotein E ε4 carrier status. The baseline neuropsychological variables analysed were executive, language, memory and visuospatial composite scores. These scores were defined as the first principal component from a principal component analysis based on the two neuropsychological tests for each of the four domains. Prior to the principal components analysis, we square-root transformed any skewed measures. For the composite scores, higher values corresponded to better performance. The main imaging predictor was the anatomical subtype; 44 subjects meeting the inclusion criteria had been analysed and assigned to an anatomical subtype (i.e. cluster) in our previous manuscript (Whitwell et al., 2009d) and that subtype was used for the current study. For all other subjects with imaging data (n = 35), they were assigned to the subtype corresponding to the cluster they were nearest using Ward’s method. Specifically, for the four clusters reported in the previous manuscript, we first calculated the expected sum of squares for that cluster. Note that, in this context, the expected sum of squares for a single region of interest is the sum across all subjects in that cluster of the squared differences between a subject’s grey matter volume and the region of interest mean. The expected sum of squares for a cluster is the sum of these expected sum of squares values across all 26 regions of interest. We then calculated separately for each subject, a new expected sum of squares for each cluster based on including this subject in the cluster; i.e. we added the subject to the cluster and recalculated the expected sum of squares. A subject was assigned to the cluster that resulted in the smallest increase in expected sum of squares. In addition, we also assessed volumes of the eight regions of interest as predictor variables. This analysis was performed since anatomic subtype explains a high amount of variability in the region of interest volumes, although there remains interest in quantifying the effect of individual regions of interest and determining whether any specific regions of interest is superior to pattern of atrophy, as represented by anatomical subtype.
We used linear mixed-effects regression models (Pinheiro and Bates, 2002) to estimate mean CDR-SB over time and to estimate the effect of potential predictors on CDR-SB at any given time point (the ‘shift test’) and the rate of CDR-SB increase (the ‘slope test’). In these models, CDR-SB was treated as the dependent variable and disease duration was the time scale. All available CDR-SB evaluations were used in these mixed-effects regression models unless the subject reached the maximum score of 18. For these subjects only the first score of 18 was used with subsequent scores omitted from the analysis. Predictors were entered linearly as fixed effects and included in the models as an intercept term, which allows for a shift in baseline CDR-SB, and as an interaction with disease duration, which allows the rate of CDR-SB increase to depend on the predictor. All models included a random patient-specific intercept and a random patient-specific slope. These random effects account for patient heterogeneity in baseline CDR-SB and in the rate of increase in CDR-SB that is not explained by the predictors in the model. The random effects also account for correlation among repeated measurements on the same subjects and induce a correlation structure such that the correlation between two measures from the same subject decreases as the time interval between measures increases.
We summarized the effect of each predictor individually by reporting point estimates and 95% confidence intervals (CI) based on restricted maximum likelihood fitting. We performed significance tests of the predictor using a full versus reduced model approach based on chi-squared test comparing maximum likelihood fits.
We evaluated whether CDR-SB was changing linearly over time by comparing the fits when disease duration was entered linearly versus as a restricted cubic spline with knots at 2, 6 and 11 years representing the 10th, 50th and 90th percentiles of the duration distribution (Harrell, 2001) (Fig. 2A). Since the non-linear fit was not significant (P = 0.35) and more importantly because the non-linear fit was very similar to the linear fit, we treated duration as linear throughout. Potential predictors were evaluated three ways. We first fit a series of single-predictor models where the predictor was entered as an intercept term and an interaction with disease duration. We report results from significance tests of the intercept term omitting the interaction term and of the interaction term, assuming an intercept term in the smaller model. We next fit what might be termed subtype-adjusted models in which we added to the single-predictor models subtype-specific intercepts and subtype-specific interactions with disease duration (i.e. slopes). Finally, we fit what might be termed subtype-stratified models that extend the subtype-adjusted models to include a three-way interaction between the predictor of interest, duration and subtype. In this way, the model allows for subtype-specific effects of the predictor. Because of the exploratory nature of these subtype-stratified models, we do not report significance tests of these three-way interactions.
Figure 2.
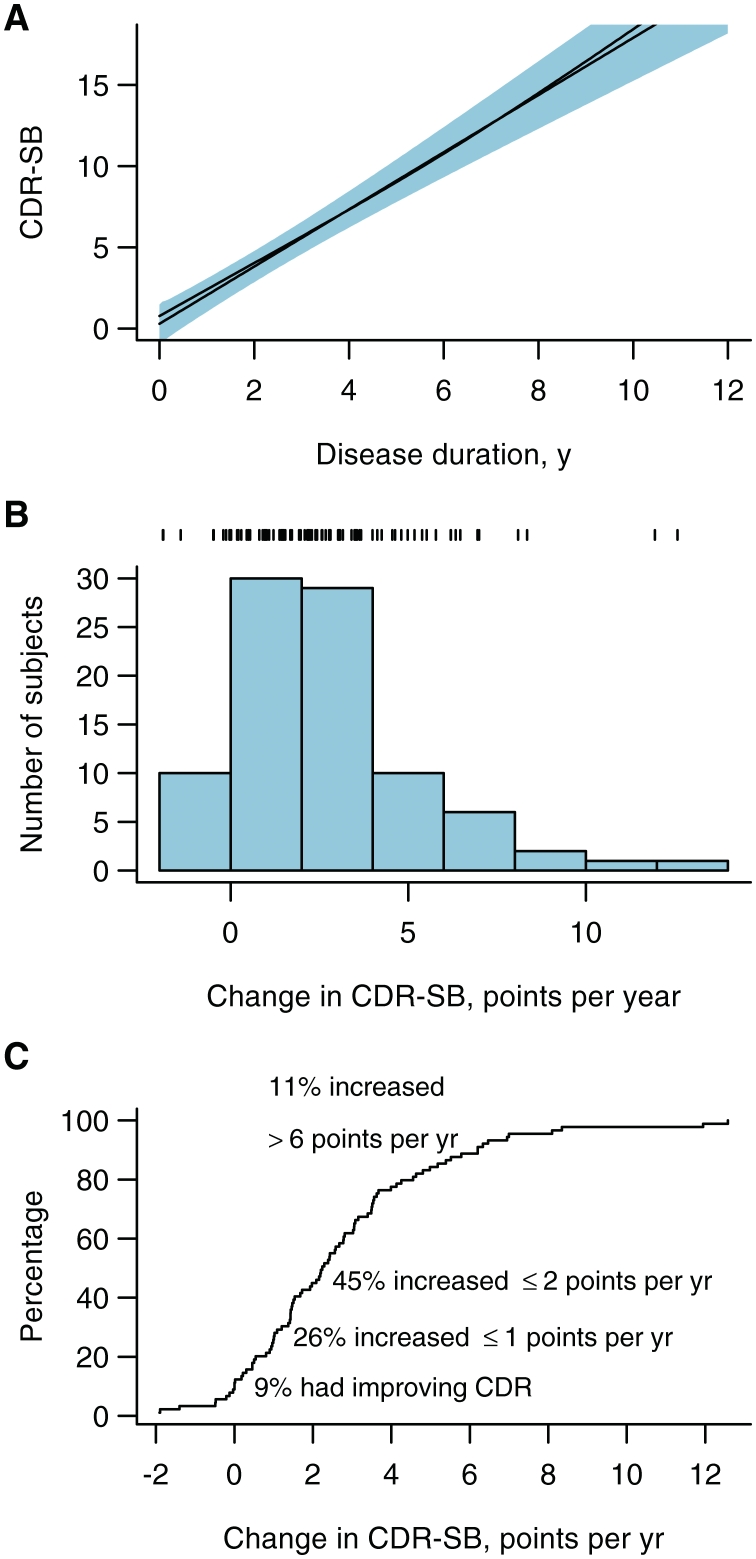
The distribution of annual rate of CDR-SB change across all 86 subjects in the study based on fitting a separate regression line for each subject. (A) Mean CDR-SB as a function of disease duration. The black line represents a point estimate for the mean while the blue shaded region represents pointwise 95% CIs. The black curve that almost coincides with the line represents the estimated mean based on a restricted cubic spline fit with knots at 2, 6 and 11 years and did not differ significantly from the straight-line fit (P = 0.35). (B) Histogram of the distribution of annual change in CDR-SB by subject based on calculating a slope estimate for each subject. Tick marks at the top of the plot indicate individual subject values. (C) The empirical cumulative distribution plot of the data shown in B. For a given value on the x-axis, the y-axis shows the percentage of subjects with an annual change less than that amount.
As a way of quantifying the strength of the association between CDR-SB and a given predictor, we report a mixed effects model partial R2 statistic (Edwards et al., 2008). This statistic is based on the F-statistic for testing fixed effects and can be interpreted similarly to the partial R2 in linear regression in that it measures the marginal improvement or reduction in unexplained variability, in the model after accounting for time effects.
These analyses were not adjusted for multiple comparisons since adjustment would increase the type II errors for associations that are not null. Not adjusting for multiple comparisons has been advocated since it will result in fewer errors in interpretation when the data are not random numbers but actual observations on nature (Rothman, 1990). Adjusting our data could therefore result in missing important findings.
All computations were performed in R version 2.8.1 (R Development Core Team: A Language and Environment for Statistical Computing. 2008 http://www.r-project.org) with mixed effects models fit using version 3.1-89 of nlme package (Pinheiro and Bates, 2002).
Results
The subject characteristics of the cohort at baseline are shown in Table 1 and Fig. 1. A total of 86 subjects were included in this study. Seventy-nine subjects with baseline MRI were clustered into one of the four anatomically defined behavioural variant FTD subtypes. Forty-two per cent of subjects had a positive family history. Sixteen subjects from nine families screened positive for mutations in MAPT and four subjects from three families screened positive for mutations in GRN. Fourteen subjects had a family member that was also included in this study (five from two-person families plus one family of four subjects). Compared to 12% of subjects in the other three behavioural variant FTD subtypes, 64% of the subjects in the temporal dominant subtype had a MAPT mutation (P < 0.001). The four subjects with GRN mutations were split across three different anatomical subtypes.
The majority of subjects had a baseline CDR global score of 0.5 or 1, with a median CDR-SB score of 4. The median number of CDR-SB evaluations per subject was 4 (range: 2–18). Figure 2 summarizes the heterogeneity of the distribution of the annual rate of CDR-SB change across all subjects in the study based on fitting a separate regression line for each subject. The average functional decline as indicated by the CDR-SB scores was linear with an estimated average rate of increase of 1.8 points/year (95% CI 1.4–2.1) (Fig. 2A). A rate of change between 0 and 2 points per year was the mode observed in this cohort (Fig. 2B). Approximately half of the subjects showed rates of change of ≤2 points per year (Fig. 2C). At the ends of the spectrum, 9% of subjects were stable or showed an improvement in the CDR-SB and 11% showed a dramatic increase in CDR-SB of more than 6 points per year. Thirty per cent of the cohort reached a maximum CDR-SB score of 18 during follow-up. The median time from onset to CDR-SB of these 18 subjects was 7 years (range: 3–19). The median rate of CDR-SB increase was faster in those with GRN mutations (median rate 3.4 points/per year; 95% CI 2.0–4.9), but slower in those with MAPT mutations (median rate 1.4 points/per year; 95% CI 1.0–1.9), compared to the median rate of increase across the remainder of the cohort (median rate 1.8 points/per year; 95% CI 1.4–2.1) (P = 0.01 for both).
Some of the predictors had a significant effect on the actual CDR-SB score estimated for any given time-point (Table 2, shift test). This effect is graphically represented by a vertical shift of the estimated trend-lines, as opposed to a rate-altering effect that is represented graphically by a change in the slope of the trend-lines (Fig. 3). These predictors included gender, CDR-SB at baseline, MMSE, executive composite score, visuospatial composite score, apathy/indifference and anatomical subtype. The plots in Fig. 3 illustrate this effect for those variables that also had a significant effect on rates of CDR-SB increase. At any given time-point, females had an overall higher baseline CDR-SB score compared to males, while higher baseline CDR-SB, lower MMSE and greater levels of apathy/indifference were associated with higher average CDR-SB scores.
Table 2.
Coefficients, partial R2 and P-values for the effect of predictors on CDR-SB score
| Shift test |
Slope test |
|||||
|---|---|---|---|---|---|---|
| Coef. (SE) | Partial R2 | P | Coef. (SE) | Partial R2 | P | |
| Male gender | −2.0 (0.99) | 0.05 | 0.05 | −0.31 (0.33) | 0.00 | 0.34 |
| Apolipoprotein E ε4 carrier | 1.6 (1.2) | 0.02 | 0.20 | 0.30 (0.41) | 0.00 | 0.47 |
| Positive family history | −0.49 (1.0) | 0.00 | 0.62 | 0.06 (0.33) | 0.00 | 0.84 |
| Age at onset | −0.02 (0.04) | 0.00 | 0.54 | 0.02 (0.01) | 0.01 | 0.09 |
| Education | −0.26 (0.17) | 0.03 | 0.12 | 0.02 (0.05) | 0.00 | 0.64 |
| MMSE at baseline | −0.28 (0.12) | 0.06 | 0.03 | −0.02 (0.03) | 0.00 | 0.63 |
| CDR-SB at baseline | 0.63 (0.14) | 0.19 | <0.001 | −0.04 (0.04) | 0.00 | 0.34 |
| Neuropsychiatric Inventory | ||||||
| Total | 0.17 (0.09) | 0.04 | 0.07 | −0.05 (0.03) | 0.01 | 0.09 |
| Delusions | 0.63 (0.52) | 0.02 | 0.25 | −0.30 (0.19) | 0.01 | 0.12 |
| Hallucinations | −0.92 (1.2) | 0.01 | 0.45 | −0.24 (0.34) | 0.00 | 0.49 |
| Agitation/aggression | 0.74 (0.53) | 0.03 | 0.17 | −0.42 (0.19) | 0.02 | 0.03 |
| Depression/dysphoria | −0.06 (0.66) | 0.00 | 0.91 | 0.06 (0.23) | 0.00 | 0.76 |
| Anxiety | 0.27 (0.59) | 0.00 | 0.64 | 0.03 (0.20) | 0.00 | 0.86 |
| Elation/euphoria | 0.05 (0.73) | 0.00 | 0.96 | −0.12 (0.25) | 0.00 | 0.64 |
| Apathy/indifference | 1.1 (0.55) | 0.05 | 0.05 | −0.03 (0.20) | 0.00 | 0.89 |
| Disinhibition | −0.04 (0.53) | 0.00 | 0.93 | −0.41 (0.17) | 0.02 | 0.02 |
| Irritability | 0.04 (0.58) | 0.00 | 0.95 | −0.19 (0.20) | 0.00 | 0.35 |
| Motor disturbance | 0.83 (0.54) | 0.03 | 0.12 | 0.08 (0.19) | 0.00 | 0.68 |
| Night-time behaviours | 0.44 (0.60) | 0.01 | 0.47 | −0.40 (0.18) | 0.02 | 0.03 |
| Appetite/eating | 0.64 (0.47) | 0.02 | 0.17 | −0.08 (0.17) | 0.00 | 0.64 |
| Composite scoresa | ||||||
| Executive | −1.4 (0.37) | 0.17 | <0.001 | −0.43 (0.14) | 0.04 | 0.003 |
| Language | 0.39 (0.44) | 0.01 | 0.41 | −0.34 (0.15) | 0.02 | 0.03 |
| Memory | −0.10 (0.47) | 0.00 | 0.78 | −0.17 (0.14) | 0.01 | 0.23 |
| Visuospatial | −1.1 (0.44) | 0.09 | 0.03 | −0.35 (0.14) | 0.03 | 0.02 |
| Anatomical subtype | 0.18 | 0.002 | 0.04 | 0.01 | ||
| Temporal dominant | −2.9 (1.3) | 1.4 (0.33) | ||||
| Temporofrontoparietal | −0.15 (0.83) | 1.3 (0.22) | ||||
| Frontotemporal | 1.7 (1.2) | 2.2 (0.38) | ||||
| Frontal dominant | 2.9 (1.0) | 2.6 (0.40) | ||||
SE = standard error.
aModels are adjusted for age at baseline and education.
Coefficients are based on models fit with restricted maximum likelihood. Partial R2 is the increase in R2 that results from adding the predictor. The R2 statistics can be interpreted as the per cent of total variability explained by the model, or the reduction in variability due to the model. P-values are based on likelihood ratio tests using models fit by maximum likelihood.
Figure 3.
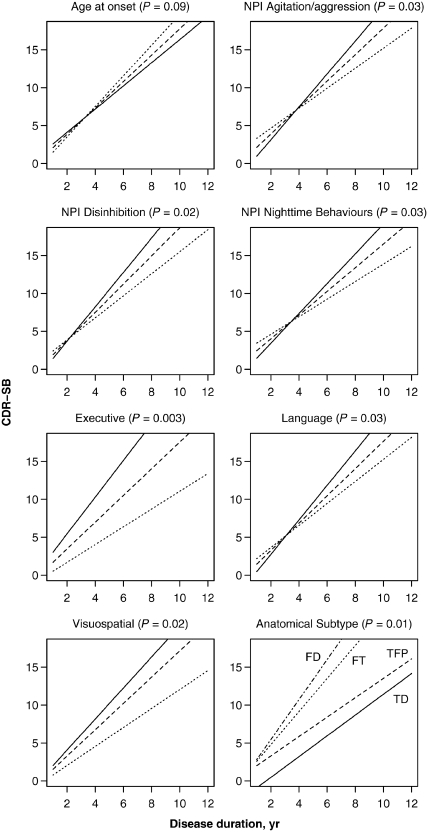
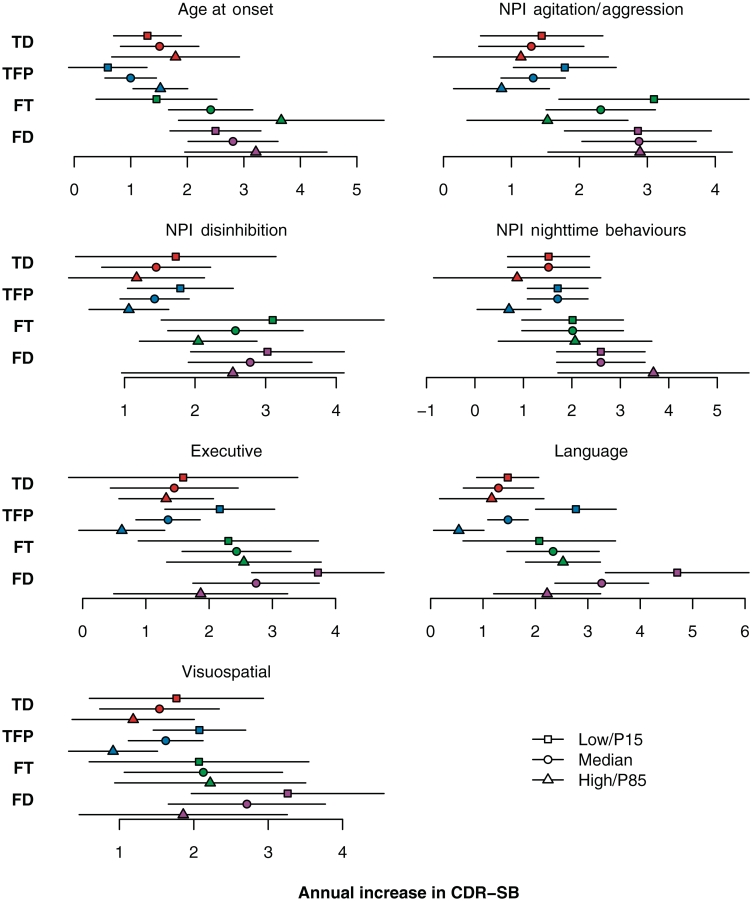
The effect of significant predictor variables on the rate of CDR-SB increase. Except for the anatomical subtype predictor, the solid line represents the estimated slope for a subject having a low value (defined as the 15th percentile), the dashed line represents the estimated slope for a subject having an average value (defined as the median), while the dotted line represents the estimated slope for a subject having a high value (defined as the 85th percentile). For age at onset, 15th percentile equates to age 45, median equates to age 56 and 85th percentile equates to age 70. For anatomical subtype, the solid line represents the estimated slope for the temporal dominant (TD) group, the dashed line represents the slope for the temporofrontoparietal (TFP) subtype, the dotted line represents the slope for the frontotemporal (FT) subtype, and the dotted–dashed line represents the slope for the frontal dominant (FD) subtype. The neuropsychological composite scores are adjusted for age at baseline and education and worse performance corresponds to a lower score and steeper slope. The significance of each predictor based on a test of the significance of the interaction term between duration and the predictor is shown in parentheses in each panel title. NPI = Neuropsychiatric Inventory.
The effect of predictors on the rate of CDR-SB increase is shown in Table 2 (slope test), Figs. 3 and 4. The only demographic feature that showed a trend for affecting the rate of CDR-SB increase was age at disease onset, with older subjects tending to decline at a faster rate. Poorer performance on executive, language and visuospatial composite scores was associated with a faster rate of CDR-SB increase, as was less disinhibition, less agitation/aggression and less night-time behaviours. The anatomical subtype significantly affected the rate of CDR-SB increase. The fastest rates of CDR-SB increase were observed in the frontal and frontotemporal subtypes, with the slowest rates of CDR-SB increase observed in the temporal and temporofrontoparietal subtypes.
Figure 4.
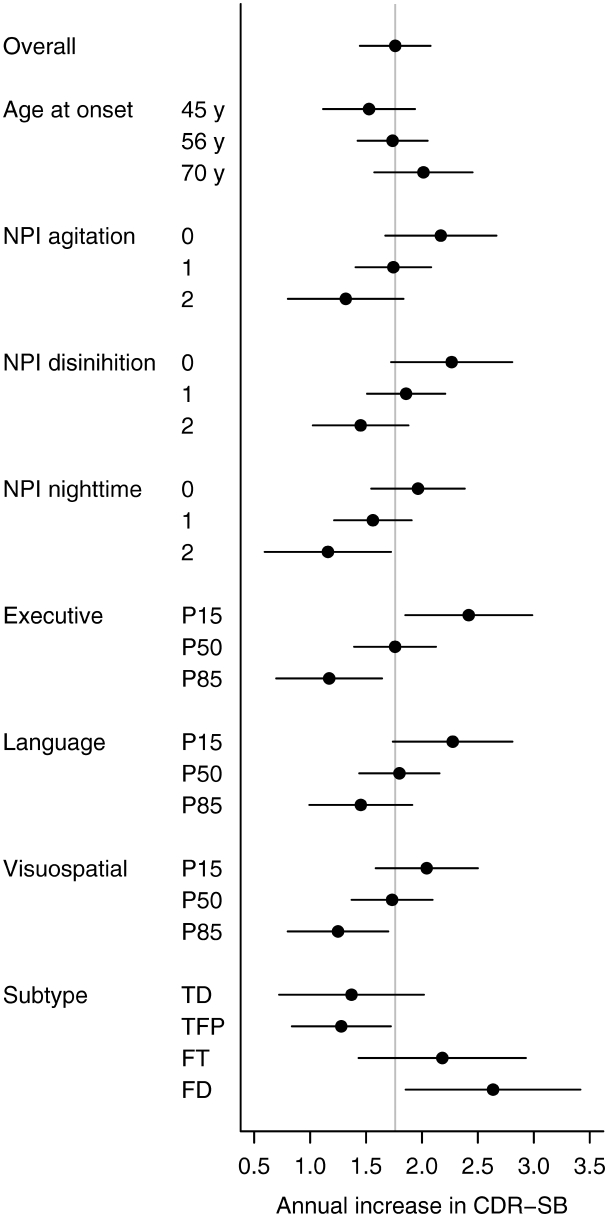
The estimated annual CDR-SB increase and 95% confidence intervals by predictor compared with each other. We define ‘low’ to be the 15th percentile and ‘high’ to be the 85th percentile. The executive, language and visuospatial composite scores are adjusted for age at baseline and education and worse performance corresponds to a lower score and greater annual increase. The grey vertical line represents the overall estimated annual increase of 1.8 points per year. FD = frontal dominant subtype; FT = frontotemporal subtype; NPI = Neuropsychiatric Inventory; TD = temporal dominant subtype; TFP = temporofrontoparietal subtype.
We also investigated the effects of the predictors on the rate of CDR-SB increase within each of the four anatomical subtypes (Fig. 5). Age at onset showed a similar trend within each of the four subtypes to that observed in the overall group, with older age associated with faster rate of CDR-SB increase. Executive, language and visuospatial composite scores showed a similar trend across the temporofrontoparietal, temporal dominant and frontal dominant subtypes to the overall association, with poorer performance being associated with a faster rate of CDB-SB increase. Disinhibition showed a similar trend within each of the four subtypes to the overall association, with less disinhibition associated with faster rate of CDR-SB increase, while agitation/aggression showed a similar trend across all subtypes except for the frontal dominant subtype. A trend for lower rates of CDR-SB increase in subjects with a high severity score for night-time behaviours was only observed in the temporal dominant and temporofrontoparietal subtypes.
Figure 5.
The estimated annual CDR-SB increase and 95% confidence intervals by predictor stratified by anatomical subtype. We define ‘low’ to be the 15th percentile (P15) and ‘high’ to be the 85th percentile (P85). The executive, language and visuospatial composite scores are adjusted for age at baseline and education and worse performance corresponds to a lower score and greater annual increase as can be seen in the temporofrontoparietal (TFP) and frontal dominant (FD) subtype; a less dramatic trend was observed for the temporal dominant subtype (TD). FT = frontotemporal subtype; NPI = Neuropsychiatric Inventory.
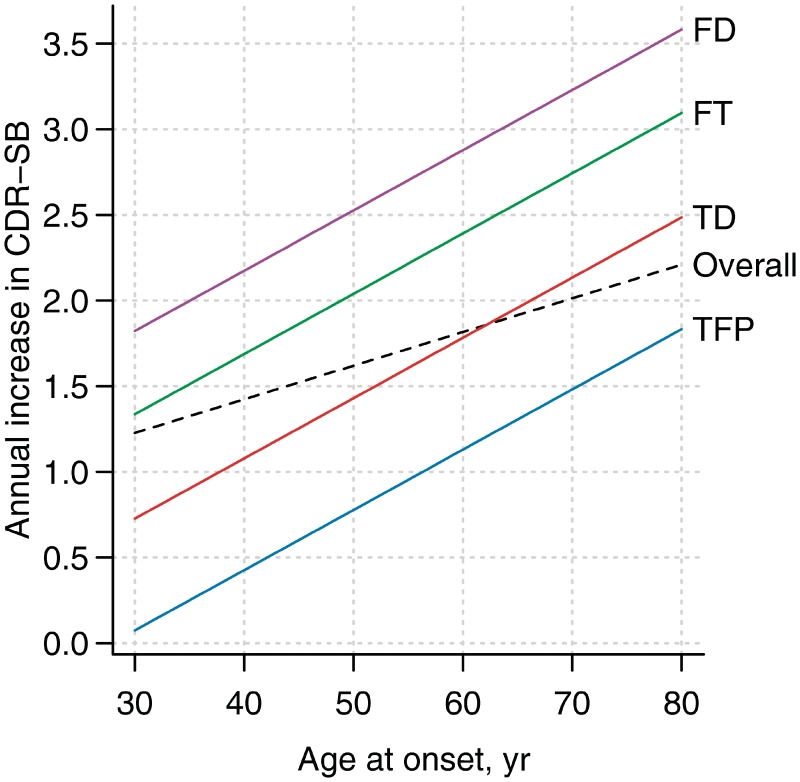
Given that we identified multiple predictor variables of rate of CDR-SB increase and that we have previously shown that many of these variables are associated with the anatomical subtypes of behavioural variant FTD (Whitwell et al., 2009d), we also investigated whether the addition of each of these variables had any significant effect on actual CDR-SB score at a given time-point, or on rate of CDR-SB increase, after adjusting for anatomical subtype. We found that CDR-SB at baseline and executive composite score improves our prediction of actual CDR-SB score; while age at onset, executive, language and visuospatial composite scores, and disinhibition severity improves our prediction of rate of CDR-SB increase (Table 3). The predictive effect of age at onset on the rate of CDR-SB increase dramatically improved (P = 0.002), compared to the predictive effect without adjusting for subtype (P = 0.09). The estimated annual increase in CDR-SB for a given age at onset, stratified by anatomical subtype is shown in Fig. 6. We also assessed whether anatomical subtypes improves prediction after first entering each neuropsychological composite score and each NPI-Q subscore in the model. Anatomical subtype always improved prediction in these models (P < 0.01).
Table 3.
Anatomical subtype adjusted model results
| Shift test |
Slope test |
|||||
|---|---|---|---|---|---|---|
| Coef. (SE) | Partial R2 | P | Coef. (SE) | Partial R2 | P | |
| Male gender | −1.3 (0.99) | 0.02 | 0.16 | −0.05 (0.33) | 0.00 | 0.86 |
| Apolipoprotein E ε4 carrier | 0.50 (1.2) | 0.00 | 0.68 | 0.12 (0.42) | 0.00 | 0.79 |
| Positive family history | −0.46 (1.0) | 0.00 | 0.67 | 0.00 (0.32) | 0.00 | 0.98 |
| Age at onset | −0.06 (0.04) | 0.02 | 0.17 | 0.04 (0.01) | 0.04 | 0.002 |
| Education | −0.18 (0.17) | 0.01 | 0.24 | 0.01 (0.05) | 0.00 | 0.76 |
| MMSE at baseline | −0.16 (0.13) | 0.02 | 0.17 | 0.00 (0.03) | 0.00 | 0.78 |
| CDR-SB at baseline | 0.43 (0.17) | 0.08 | 0.009 | −0.08 (0.05) | 0.01 | 0.08 |
| Neuropsychiatric Inventory | ||||||
| Total | 0.09 (0.10) | 0.01 | 0.34 | −0.07 (0.03) | 0.01 | 0.07 |
| Delusions | 0.05 (0.55) | 0.00 | 0.97 | −0.29 (0.19) | 0.01 | 0.13 |
| Hallucinations | −1.6 (1.3) | 0.02 | 0.19 | −0.28 (0.34) | 0.00 | 0.43 |
| Agitation/aggression | 0.39 (0.54) | 0.01 | 0.47 | −0.34 (0.19) | 0.01 | 0.08 |
| Depression/dysphoria | −0.47 (0.68) | 0.01 | 0.46 | 0.14 (0.22) | 0.00 | 0.47 |
| Anxiety | 0.29 (0.60) | 0.00 | 0.59 | 0.07 (0.20) | 0.00 | 0.70 |
| Elation/euphoria | −0.04 (0.72) | 0.00 | 0.93 | −0.14 (0.23) | 0.00 | 0.57 |
| Apathy/indifference | 0.57 (0.61) | 0.01 | 0.33 | −0.22 (0.19) | 0.01 | 0.26 |
| Disinhibition | 0.26 (0.53) | 0.00 | 0.66 | −0.36 (0.17) | 0.02 | 0.04 |
| Irritability | 0.02 (0.58) | 0.00 | 0.97 | −0.11 (0.20) | 0.00 | 0.66 |
| Motor disturbance | 0.62 (0.55) | 0.02 | 0.26 | 0.00 (0.19) | 0.00 | 1.00 |
| Night-time behaviours | 0.28 (0.61) | 0.00 | 0.64 | −0.32 (0.18) | 0.01 | 0.07 |
| Appetite/eating | 0.38 (0.48) | 0.01 | 0.42 | −0.10 (0.16) | 0.00 | 0.50 |
| Composite scoresa | ||||||
| Executive | −1.0 (0.39) | 0.10 | 0.008 | −0.37 (0.14) | 0.03 | 0.01 |
| Language | −0.05 (0.44) | 0.00 | 0.82 | −0.47 (0.14) | 0.05 | 0.001 |
| Memory | 0.24 (0.48) | 0.01 | 0.73 | −0.20 (0.15) | 0.01 | 0.18 |
| Visuospatial | −0.58 (0.42) | 0.03 | 0.18 | −0.39 (0.14) | 0.04 | 0.005 |
aModels are further adjusted for age at baseline and education.
Shift-related questions concern whether the predictor when entered as a ‘shift’ term is significant after including subtype-specific shift and slope terms in the model. Slope-related questions concern whether slope term is significant after including subtype-specific shift and slope terms. A positive shift coefficient indicates higher baseline CDR-SB while a positive slope coefficient indicated faster increase in CDR-SB (i.e. is worse).
SE = standard error.
Coefficients are based on models fit with restricted maximum likelihood. Partial R2 is the increase in R2 that results from adding the predictor. The R2 statistics can be interpreted as the per cent of total variability explained by the model, or the reduction in variability due to the model. P-values are based on likelihood ratio tests using models fit by maximum likelihood.
Figure 6.
Estimated annual increase in CDR-SB as a function of age of onset. This figure complements the top-left panel of Fig. 5 and can be used as a graphical ‘look-up table’ to estimate rates of CDR increase for a given age at onset overall and by anatomical subtype. The overall estimates are based on a slope model with age of onset as the predictor. The subtype-specific estimates are based on adding subtype-specific slopes to this model. FD = frontal dominant subtype; FT = frontotemporal subtype; TD = temporal dominant subtype; TFP = temporofrontoparietal subtype.
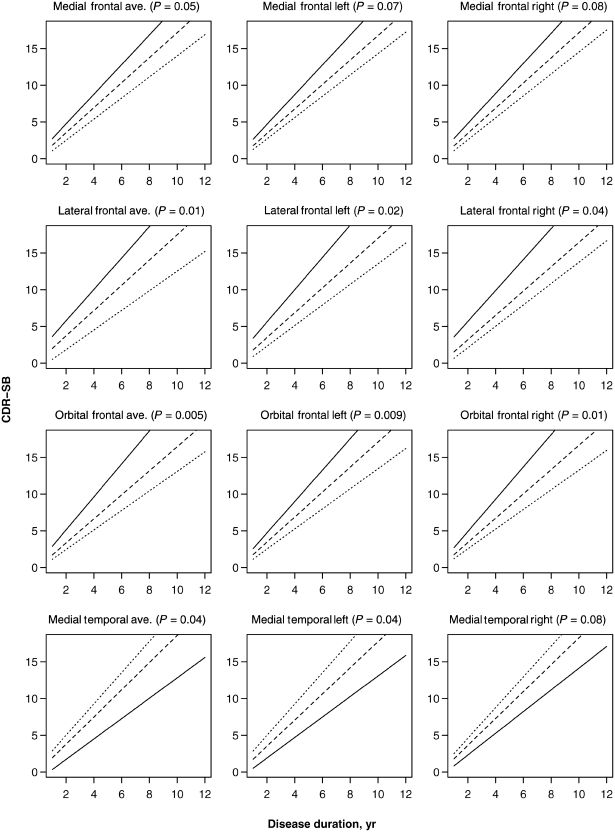
For the region of interest analysis, a shift effect was observed for all frontal and temporal volumes (Table 4). Smaller average frontal volumes were associated with lower average CDR-SB scores while smaller average temporal volumes were associated with higher average CDR-SB scores. These associations were observed for both left and right hemisphere volumes across all frontal and temporal regions of interest, except for the right lateral temporal lobe, which did not show a significant effect on shift. All three average frontal lobe regions of interest had a significant effect on the rate of CDR-SB increase, with smaller volumes associated with faster rates (Table 4, Fig. 7). When the left and right hemispheres were assessed separately, the left and right lateral and orbital frontal volumes showed a significant effect on the rate of CDR-SB increase and there was a trend for smaller left and right medial frontal volumes to also be associated with faster rates. The only average temporal lobe volume that had a significant effect on the rate of CDR-SB increase was the medial temporal lobe (Table 4, Fig. 7). However, unlike for the frontal regions, smaller medial temporal lobe volumes were associated with slower rates of CDR-SB increase (in other words, subjects with less temporal lobe volume loss showed faster rates of CDR-SB increase than those with more temporal lobe volume loss). This effect was only significant for the left medial temporal lobe with a trend observed for the right medial temporal lobe. Only average lateral frontal lobe volume improved our prediction of actual CDR-SB score beyond anatomical subtype but no region of interest volumes, average, left or right, improved the prediction of rate of CDR-SB increase beyond anatomical subtype (Table 5).
Table 4.
Coefficients, partial R2 and P-values for the effect of region of interest predictors on CDR-SB score
| Shift test |
Slope test |
|||||
|---|---|---|---|---|---|---|
| Coef. (SE) | Partial R2 | P | Coef. (SE) | Partial R2 | P | |
| Medial frontal | ||||||
| Average | −356 (149) | 0.07 | 0.02 | −90 (47) | 0.01 | 0.05 |
| Left | −273 (136) | 0.05 | 0.05 | −80 (43) | 0.01 | 0.07 |
| Right | −344 (144) | 0.07 | 0.02 | −81.7 (46) | 0.01 | 0.08 |
| Lateral frontal | ||||||
| Average | −322 (67) | 0.23 | <0.001 | −62.1 (25) | 0.02 | 0.01 |
| Left | −236 (64) | 0.15 | <0.001 | −54 (23) | 0.02 | 0.02 |
| Right | −280 (61) | 0.21 | <0.001 | −49.4 (24) | 0.02 | 0.04 |
| Orbital frontal | ||||||
| Average | −587 (199) | 0.10 | 0.008 | −177 (62) | 0.03 | 0.005 |
| Left | −499 (202) | 0.07 | 0.02 | −163 (62) | 0.03 | 0.009 |
| Right | −447 (168) | 0.08 | 0.01 | −143 (55) | 0.03 | 0.01 |
| Temporal pole | ||||||
| Average | 722 (248) | 0.10 | 0.004 | 70 (78) | 0.00 | 0.39 |
| Left | 651 (227) | 0.10 | 0.004 | 43 (75) | 0.00 | 0.58 |
| Right | 517 (224) | 0.06 | 0.02 | 75 (70) | 0.00 | 0.30 |
| Medial temporal | ||||||
| Average | 630 (177) | 0.14 | <0.001 | 126 (59) | 0.02 | 0.04 |
| Left | 600 (165) | 0.15 | <0.001 | 123 (58) | 0.02 | 0.04 |
| Right | 368 (149) | 0.07 | 0.02 | 92 (52) | 0.01 | 0.08 |
| Lateral temporal | ||||||
| Average | 229 (99) | 0.07 | 0.01 | 2.2 (33) | 0.00 | 0.98 |
| Left | 190 (77) | 0.07 | 0.01 | −5.6 (29) | 0.00 | 0.83 |
| Right | 103 (73) | 0.03 | 0.15 | 14 (26) | 0.00 | 0.62 |
| Caudate | ||||||
| Average | −91 (655) | 0.00 | 0.88 | −142 (227) | 0.00 | 0.53 |
| Left | 357 (538) | 0.01 | 0.51 | −72 (201) | 0.00 | 0.73 |
| Right | −578 (591) | 0.01 | 0.32 | −139 (195) | 0.00 | 0.47 |
| Parietal | ||||||
| Average | 0.86 (148) | 0.00 | 0.99 | −2.5 (50) | 0.00 | 0.98 |
| Left | 33 (207) | 0.00 | 0.89 | 52.6 (67) | 0.00 | 0.41 |
| Right | −3.8 (250) | 0.00 | 1.00 | −84 (87) | 0.00 | 0.33 |
Coefficients are based on models fit with restricted maximum likelihood. Partial R2 is the increase in R2 that results from adding the predictor. The R2 statistics can be interpreted as the per cent of total variability explained by the model, or the reduction in variability due to the model. P-values are based on likelihood ratio tests using models fit by maximum likelihood. SE = standard error.
Figure 7.
The effect of significant regions of interest predictor variables on the rate of CDR-SB increase. The solid line represents the estimated slope for a subject having a low value (defined as the 15th percentile), the dashed line represents the estimated slope for a subject having an average value (defined as the median), while the dotted line represents the estimated slope for a subject having a high value (defined as the 85th percentile). The significance of each predictor based on a test of the significance of the interaction term between duration and the predictor is shown in parentheses in each panel title.
Table 5.
Anatomical subtype adjusted model results for region of interest predictors
| Shift test |
Slope test |
|||||
|---|---|---|---|---|---|---|
| Coef. (SE) | Partial R2 | P | Coef. (SE) | Partial R2 | P | |
| Medial frontal | ||||||
| Average | −202 (250) | 0.01 | 0.38 | 33 (75) | 0.00 | 0.63 |
| Left | −47 (193) | 0.00 | 0.75 | 29 (60) | 0.00 | 0.60 |
| Right | −292 (238) | 0.02 | 0.21 | 23 (75) | 0.00 | 0.73 |
| Lateral frontal | ||||||
| Average | −264 (96) | 0.09 | 0.006 | −12 (32) | 0.00 | 0.82 |
| Left | −139 (91) | 0.03 | 0.11 | −14 (27) | 0.00 | 0.66 |
| Right | −240 (79) | 0.11 | 0.002 | −0.46 (30) | 0.00 | 0.91 |
| Orbital frontal | ||||||
| Average | −563 (298) | 0.05 | 0.06 | −97 (96) | 0.00 | 0.31 |
| Left | −263 (268) | 0.01 | 0.31 | −58 (80) | 0.00 | 0.46 |
| Right | −512 (240) | 0.06 | 0.04 | −99 (87) | 0.01 | 0.25 |
| Temporal pole | ||||||
| Average | 491 (364) | 0.02 | 0.17 | 64 (117) | 0.00 | 0.57 |
| Left | 360 (277) | 0.02 | 0.19 | −0.62 (98) | 0.00 | 0.99 |
| Right | 297 (328) | 0.01 | 0.36 | 96 (102) | 0.00 | 0.34 |
| Medial temporal | ||||||
| Average | 403 (241) | 0.04 | 0.10 | 119 (86) | 0.01 | 0.17 |
| Left | 355 (203) | 0.04 | 0.08 | 104 (77) | 0.01 | 0.17 |
| Right | 184 (190) | 0.01 | 0.34 | 70.0 (70) | 0.00 | 0.33 |
| Lateral temporal | ||||||
| Average | 8.0 (116) | 0.00 | 0.95 | −31 (38) | 0.00 | 0.38 |
| Left | 65 (84) | 0.01 | 0.43 | −30 (29) | 0.00 | 0.29 |
| Right | −65 (91) | 0.01 | 0.45 | −7.4 (29) | 0.00 | 0.79 |
| Caudate | ||||||
| Average | 868 (656) | 0.02 | 0.19 | 166 (232) | 0.00 | 0.42 |
| Left | 999 (514) | 0.05 | 0.05 | 119 (193) | 0.00 | 0.48 |
| Right | 120 (617) | 0.00 | 0.85 | 124 (206) | 0.00 | 0.50 |
| Parietal | ||||||
| Average | 102 (199) | 0.00 | 0.58 | −88 (71) | 0.01 | 0.19 |
| Left | 265 (239) | 0.02 | 0.26 | 42.4 (81) | 0.00 | 0.59 |
| Right | 136 (260) | 0.00 | 0.58 | −141 (88) | 0.01 | 0.09 |
Shift-related questions concern whether the predictor when entered as a ‘shift’ term is significant after including subtype-specific shift and slope terms in the model. Slope-related questions concern whether slope term is significant after including subtype-specific shift and slope terms. A positive shift coefficient indicates higher baseline CDR-SB while a positive slope coefficient indicated faster increase in CDR-SB (i.e. is worse). SE = standard error.
Coefficients are based on models fit with restricted maximum likelihood. Partial R2 is the increase in R2 that results from adding the predictor. The R2 statistics can be interpreted as the per cent of total variability explained by the model, or the reduction in variability due to the model. P-values are based on likelihood ratio tests using models fit by maximum likelihood.
Discussion
In this study we have identified baseline characteristics that are predictive of the rate of functional decline over time in subjects with behavioural variant FTD. As we had hypothesized, rate of functional decline was associated with anatomically defined behavioural variant FTD subtypes. Specifically, those with predominantly frontal and frontotemporal patterns of atrophy had faster rates of decline than those with temporofrontoparietal and temporal dominant patterns of atrophy. In addition, worse executive, language and visuospatial function, less severe disinhibition, agitation/aggression and night-time behaviours, and older age at onset, predicted faster rates of decline. In many instances, the effects of the predictor variables across all behavioural variant FTD subjects were preserved across each of the four anatomical subtypes, with some variables improving prediction after adjusting for subtype. In fact, anatomical subtype appears to be the strongest predictor (combined partial R2 of 0.22) and always improved prediction beyond cognitive and behavioural measures.
We had previously identified four specific anatomical subtypes of behavioural variant FTD using a hierarchical clustering approach (Whitwell et al., 2009d). The frontal dominant subtype was defined by predominant medial and lateral frontal lobe atrophy; the frontotemporal subtype by the presence of both frontal and temporal lobe atrophy; the temporal dominant subtype by predominant medial and lateral temporal lobe atrophy; and the temporofrontoparietal subtype by atrophy of the temporal lobes with some additional involvement of the frontal and parietal lobes. The 79 subjects with imaging in this study were categorized into the same four subtypes. As hypothesized, we found that anatomical subtype was a strong predictor of rate of CDR-SB increase. Interestingly, faster rates of CDR-SB increase were associated with the two variants that had the most severe frontal lobe involvement, i.e. the frontal dominant and frontotemporal subtypes, with the slowest rates observed in the temporofrontoparietal subtype. It is not surprising that the latter subtype showed the slowest rates since a small proportion of subjects in this subtype has been shown to have Alzheimer’s disease pathology (Whitwell et al., 2009d), and Alzheimer’s disease typically has a slower rate of functional decline than FTD (Rascovsky et al., 2005).
Examination of the region of interest volumes similarly showed that smaller frontal lobe volumes were associated with faster rates of CDR-SB increase. Conversely, subjects with less temporal lobe volume loss were associated with faster rates of CDR-SB increase than subjects with more temporal lobe volume loss. This paradoxical relationship can be explained by considering the anatomical subtypes, since subjects with less temporal lobe volume loss would have been clustered into the frontal dominant subtype and hence would have had severe frontal lobe volume loss. In fact, the Spearman correlation between combined temporal lobe volumes and combined frontal lobe volumes was −0.4 (P < 0.001). We also examined the predictive effect of each frontal and temporal lobe volume on rate of CDR-SB increase, adjusting for the anatomical subtype. These models showed no significant effect of volume beyond the predictive effect of anatomical subtype. Taking all these results into consideration, one would deduce that the overall pattern of involvement of all lobes (i.e. anatomical subtype) is far superior to the assessment of specific lobar volumes in predicting functional decline.
The temporal dominant subtype is interesting and requires separate discussion. This subtype has been shown to consist primarily of subjects with a mutation in the MAPT gene (Whitwell et al., 2009d). In this current study, a significantly higher proportion of subjects with mutations in MAPT were also observed in the temporal dominant subtype compared to the other behavioural variant FTD subtypes. The slow rate of decline in this subtype is therefore not surprising since we have demonstrated that the behavioural variant FTD subjects with a MAPT mutation in this cohort had a slower rate of functional decline compared to subjects without a MAPT mutation. The finding would also be in keeping with another study demonstrating longer survival in subjects with MAPT mutations compared to subjects without (Chiu et al., 2010). Indeed, the majority of subjects with a MAPT mutation have severe medial temporal and less lateral temporal and temporal pole volume loss (Josephs et al., 2009; Whitwell et al., 2009a, b), which explains the association between CDR-SB increase and medial temporal lobe volume.
In addition to the finding of anatomical subtype predicting functional decline, we also found that performance on neuropsychological tests of executive, language and visuospatial function could predict rate of CDR-SB increase. The results demonstrate that subjects with poorer performance on these tests at baseline have a faster rate of CDR-SB increase. In addition, these variables added to our ability to predict CDR-SB increase beyond the effect of anatomical subtype. Hence, this indicates that the effects of these neuropsychological variables are not being driven by anatomical subtype. It remains to be determined whether the trend observed across all subjects is preserved within each anatomical subtype, although there was strong evidence that the effect occurs in the frontal dominant and temporofrontoparietal subtypes; the largest two subtypes with increased power to detect effects. One previous survival study in behavioural variant FTD found that language performance, but not executive or memory performance, was associated with shorter survival (Garcin et al., 2009). However, it is difficult to compare these results to ours since it is unknown whether survival correlates to functional decline in behavioural variant FTD. It should be pointed out that, although it may be tempting to treat the neuropsychological measures as totally independent, there is overlap between specific tests used to assess the different domains. For example, we found that there was a moderate correlation between the Controlled Oral Word Association Test and category fluency tests (r = 0.38, P = 0.001) in our cohort. Therefore, although we aimed to reduce this overlap by using two neuropsychological tests to calculate a composite score for each domain, it would be impossible to eliminate all overlap due to the inter-relationship of these domains. This may help to explain why our visuospatial composite predictor variable was associated with rates of CDR increase, since visuospatial function has some dependency on executive function. Alternatively, the visuospatial association may be driven by a subset of behavioural variant FTD cases, but it is unlikely to be driven by differences across the anatomical subtypes since we previously showed that visuospatial function does not differ across subtypes (Whitwell et al., 2009d).
Three of the NPI-Q variables predicted rate of CDR-SB increase, including: disinhibition, agitation/aggression and night-time behaviours. Surprisingly, the data showed that subjects with less severe behaviours had a faster rate of CDR-SB increase, a pattern that appeared to hold true within each subtype for disinhibition and to a certain degree agitation/aggression. However, only the effect of disinhibition persisted after adjusting for anatomical subtype, suggesting that disinhibition is not being driven by anatomical subtype. It is therefore likely that this is a real effect and that patients who are less disinhibited at baseline will indeed have a faster rate of CDR-SB increase. It is unlikely however that disinhibition is protective, more likely that it is associated with some aspect, or a subtype, of the disease that progress more slowly, such as MAPT mutations, which have been shown to be associated with disinhibition (Pickering-Brown et al., 2008). In fact, a moderate correlation was observed between disinhibition scores and executive composite scores (r = 0.3, P = 0.009), with less disinhibition being associated with worse performance on executive testing. The interpretation of the disinhibition results are also complicated by the fact that behavioural features change over time in behavioural variant FTD. For example, there is some evidence that behavioural variant FTD subjects who are initially disinhibited commonly later develop features of apathy (Le Ber et al., 2006).
We found a trend for age at onset to be predictive of rate of functional decline; more specifically, for older subjects to have a faster rate of CDR-SB increase. A simple explanation for this could be that this result is being driven by the anatomical subtype since the temporal dominant subtype tends to be younger (Whitwell et al., 2009d) than the other subtypes and shows a slower rate of CDR-SB increase. This explanation is unlikely, however, as we found that the effect of age on CDR-SB increase was independent of anatomical subtype. Age at onset was also normally distributed and hence there were no outlier subjects that likely influenced the model. One previous survival study in behavioural variant FTD found that older age at onset was associated with poor prognosis (Chiu et al., 2010), which appears to support our result. We did not find education to be predictive of rate of functional decline as previously reported by one study that followed FTD subjects for 20 months on average (Perneczky et al., 2009). This discrepancy is probably driven by the fact that our study assessed functional change over many years using a mixed effects model in behavioural variant FTD subjects, as opposed to 20 months in all variants of FTD using linear regression.
A testament of how important anatomical subtype is clinically was the fact that anatomical subtype showed predictive value beyond all cognitive and behavioural variables. Furthermore, the anatomical subtype was the strongest predictor, since the combined partial R2 for the shift and slope effects was 0.22, hence accounting for a relative large per cent of the variability in CDR-SB scores over time. These findings suggest that imaging is equal, or possibly better, than clinical measures as a predictor of functional decline in behavioural variant FTD. It is likely however that the best prediction will result from a combination of demographic, clinical and imaging predictors.
Although we were primarily interested in identifying predictors of rate of CDR-SB increase we also assessed the effect of predictor variables on the actual CDR-SB score at any give time. This is referred to as a shift effect and is essentially represented by a vertical shift in the estimated trend lines in the direction of the y-axis. We identified multiple predictors that had a shift effect including: gender, CDR-SB at baseline, MMSE, executive composite, visuospatial composite, apathy/indifference, anatomical subtype and all frontal and temporal volumes. A number of these, as discussed above, also had an effect on the rate of CDR-SB increase. Gender did not affect the rate of CDR-SB increase but we demonstrate that for a given disease duration, females perform worse on CDR-SB than males. We also demonstrate an association between CDR-SB and MMSE, with poorer functional performance related to poorer cognitive performance. Similarly, poorer functional performance was related to more severe apathy/indifference. Relationships between functional, cognitive and behavioural measures have been previously reported in behavioural variant FTD (Rascovsky et al., 2005) and are likely to result from damage to the same frontal and temporal regions. Furthermore, functional decline may result from a combination of both cognitive and behavioural impairment. Anatomical subtype also had an effect on shift, in addition to slope. The frontal dominant and frontotemporal subtypes had the highest actual CDR-SB scores, as well as the fastest rates of CDR-SB increase. Although the rate of CDR-SB increase was higher in the temporal dominant subtype than the temporofrontoparietal subtype, the actual CDR-SB scores were lower in the temporal dominant subtype. This is possibly explained by the fact that the temporal dominant subtype has significant language impairment (Whitwell et al., 2009d) that unfortunately would not be detected with the CDR.
The average rate of CDR-SB increase in this cohort was 1.8 points per year and on an individual basis the majority of subjects showed <2 points increase per year. These estimates of CDR-SB change were calculated from multiple CDR assessments, up to 18. This calculation was based on statistically driven analyses that determined that a linear model was the best fit to explain our data points. Given that our institution is a tertiary care centre, our subjects had established disease by the time of initial presentation. It is unclear whether a non-linear trajectory would be observed if data were available earlier in the disease course. Other studies have reported somewhat similar rates of CDR-SB increase of 1.6–2.7 points per year in behavioural variant FTD. In these other studies however, rates were calculated as a change in CDR-SB over two time points only, usually one year apart (Brambati et al., 2007; Knopman et al., 2008; Gordon et al., 2010).
We also found that 11% of subjects had an increase of >6 points per year, and 9% had no change or a decrease, i.e. stable or improving, CDR-SB over time. The former suggests that there is a subset of subjects with behavioural variant FTD with a dramatic rate of functional decline, i.e. ‘fast progressors’, while the later suggests that a subset show no apparent functional decline over time, i.e. slow progressors. While the concept of slow progressors has been well described by one group in the literature (Davies et al., 2006), nothing has been previously reported on ‘fast progressors’. The results of this study shed light on features that will help to predict rate of functional decline in behavioural variant FTD and hence may be useful in identifying these ‘fast progressors’. Importantly, our cut-off of >6 points per year is not meant to be used to strictly define this group of subjects, but merely represents a reference point on the continuum to illustrate that some subjects with behavioural variant FTD progress very quickly. One subgroup of our subjects with behavioural variant FTD that showed a relatively faster rate of CDR-SB increase were the subjects with GRN mutations. This subgroup was actually declining, on average, twice as fast as the rest of the cohort.
We have identified clinical and imaging characteristics that are useful to predict rate of functional decline in behavioural variant FTD. These findings will have important scientific implications, as well as clinical implications to physicians, patients and their families, since they allow improved prognostic estimates, in a disease that severely affects the ‘young.’ The strengths of our study are the large number of subjects, especially for a relatively rare disease, the fact that the subjects were prospectively recruited and had standardized and validated independent clinical and neuropsychological assessments collected over a 15-year period, and the application of advanced statistical techniques. Most important, our estimate of CDR-SB change was based on multiple CDR scores per subject.
Funding
National Institutes of Health (NIH) Roadmap Multidisciplinary Clinical Research Career Development Award (K12/NICHD-HD49078), and NIH grants R01-AG037491, R01-DC010367, R21-AG38736, P50-AG16574, U01-AG06786 and R01-AG11378; The Dana Foundation; Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program of the Mayo Foundation; the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation; NIH Construction Grant (NIH C06 RR018898).
Acknowledgements
The authors would like to acknowledge Dr Rosa Rademakers and Mr Matthew Baker for performing the genetic testing.
Glossary
Abbreviations
- BA
Brodmann area
- CDR-SB
Clinical Dementia Rating Sum of Boxes
- FTD
frontotemporal dementia
- MMSE
Mini-Mental State Examination
- NPI-Q
Brief questionnaire form of the Neuropsychiatric Inventory
References
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. Manual. Iowa City: University of Iowa; 1989. Multilingual Aphasia Examination. [Google Scholar]
- Brambati SM, Renda NC, Rankin KP, Rosen HJ, Seeley WW, Ashburner J, et al. A tensor based morphometry study of longitudinal gray matter contraction in FTD. Neuroimage. 2007;35:998–1003. doi: 10.1016/j.neuroimage.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke WJ, Miller JP, Rubin EH, Morris JC, Coben LA, Duchek J, et al. Reliability of the Washington University Clinical Dementia Rating. Arch Neurol. 1988;45:31–2. doi: 10.1001/archneur.1988.00520250037015. [DOI] [PubMed] [Google Scholar]
- Chiu WZ, Kaat LD, Seelaar H, Rosso SM, Boon AJ, Kamphorst W, et al. Survival in progressive supranuclear palsy and frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2010;81:441–5. doi: 10.1136/jnnp.2009.195719. [DOI] [PubMed] [Google Scholar]
- Choi SH, Lee BH, Kim S, Hahm DS, Jeong JH, Yoon SJ, et al. Interchanging scores between clinical dementia rating scale and global deterioration scale. Alzheimer Dis Assoc Disord. 2003;17:98–105. doi: 10.1097/00002093-200304000-00008. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. J Psychosom Res. 1998;44:627–8. doi: 10.1016/s0022-3999(98)00034-8. [DOI] [PubMed] [Google Scholar]
- Davies RR, Kipps CM, Mitchell J, Kril JJ, Halliday GM, Hodges JR. Progression in frontotemporal dementia: identifying a benign behavioral variant by magnetic resonance imaging. Arch Neurol. 2006;63:1627–31. doi: 10.1001/archneur.63.11.1627. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, Muller KE, Wolfinger RD, Qaqish BF, Schabenberger O. An R2 statistic for fixed effects in the linear mixed model. Stat Med. 2008;27:6137–57. doi: 10.1002/sim.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillenbaum GG, Peterson B, Morris JC. Estimating the validity of the clinical Dementia Rating Scale: the CERAD experience. Consortium to Establish a Registry for Alzheimer’s Disease. Aging. 1996;8:379–85. doi: 10.1007/BF03339599. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Garcin B, Lillo P, Hornberger M, Piguet O, Dawson K, Nestor PJ, et al. Determinants of survival in behavioral variant frontotemporal dementia. Neurology. 2009;73:1656–61. doi: 10.1212/WNL.0b013e3181c1dee7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet. 2006;15:2988–3001. doi: 10.1093/hmg/ddl241. [DOI] [PubMed] [Google Scholar]
- Gordon E, Rohrer JD, Kim LG, Omar R, Rossor MN, Fox NC, et al. Measuring disease progression in frontotemporal lobar degeneration: a clinical and MRI study. Neurology. 2010;74:666–73. doi: 10.1212/WNL.0b013e3181d1a879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE, editor. Regression modelling stratergies. New York: Springer; 2001. [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer’s disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008a;27:685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008b;131:665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol. 2008;64:4–14. doi: 10.1002/ana.21426. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Tsuboi Y, Cookson N, Watt H, Dickson DW. Apolipoprotein E epsilon 4 is a determinant for Alzheimer-type pathologic features in tauopathies, synucleinopathies, and frontotemporal degeneration. Arch Neurol. 2004;61:1579–84. doi: 10.1001/archneur.61.10.1579. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Knopman DS, Boeve BF, Vemuri P, Senjem ML, et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology. 2009;73:1443–50. doi: 10.1212/WNL.0b013e3181bf9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–43. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–9. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Jack CR, Jr, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, et al. Brain and ventricular volumetric changes in frontotemporal lobar degeneration over 1 year. Neurology. 2009;72:1843–9. doi: 10.1212/WNL.0b013e3181a71236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131:2957–68. doi: 10.1093/brain/awn234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ber I, Guedj E, Gabelle A, Verpillat P, Volteau M, Thomas-Anterion C, et al. Demographic, neurological and behavioural characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain. 2006;129:3051–65. doi: 10.1093/brain/awl288. [DOI] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Graff-Radford NR, et al. Mayo’s older Americans normative studies: category fluency norms. J Clin Exp Neuropsychol. 1998;20:194–200. doi: 10.1076/jcen.20.2.194.1173. [DOI] [PubMed] [Google Scholar]
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173–6; discussion 177–8. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Pohl C, Bornschein S, Forstl H, Kurz A, Diehl-Schmid J. Accelerated clinical decline in well-educated patients with frontotemporal lobar degenerations. Eur Arch Psychiatry Clin Neurosci. 2009;259:362–7. doi: 10.1007/s00406-009-0004-6. [DOI] [PubMed] [Google Scholar]
- Pickering-Brown SM, Rollinson S, Du Plessis D, Morrison KE, Varma A, Richardson AM, et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: comparison with patients with MAPT and no known mutations. Brain. 2008;131:721–31. doi: 10.1093/brain/awm331. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM, editors. Mixed effects models in S and S-plus. New York: Springer-Verlag; 2002. [Google Scholar]
- Rascovsky K, Salmon DP, Ho GJ, Galasko D, Peavy GM, Hansen LA, et al. Cognitive profiles differ in autopsy-confirmed frontotemporal dementia and AD. Neurology. 2002;58:1801–8. doi: 10.1212/wnl.58.12.1801. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Lipton AM, Leverenz JB, DeCarli C, Jagust WJ, et al. Rate of progression differs in frontotemporal dementia and Alzheimer disease. Neurology. 2005;65:397–403. doi: 10.1212/01.wnl.0000171343.43314.6e. [DOI] [PubMed] [Google Scholar]
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–21. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- Rey A. L’examen Clinique en Psychologie. Paris, France: Universitaires de France; 1964. [Google Scholar]
- Rosen HJ, Narvaez JM, Hallam B, Kramer JH, Wyss-Coray C, Gearhart R, et al. Neuropsychological and functional measures of severity in Alzheimer disease, frontotemporal dementia, and semantic dementia. Alzheimer Dis Assoc Disord. 2004;18:202–7. [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6. [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65:249–55. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70:323–32. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1994;57:416–8. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- War Department. Army Individual Test Battery. Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale. New York, NY: Psychological Corp; 1981. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III. New York, NY: Psychological Corp; 1997. [Google Scholar]
- Whitwell JL, Jack CR, Jr, Boeve BF, Senjem ML, Baker M, Ivnik RJ, et al. Atrophy patterns in IVS10+16, IVS10+3, N279K, S305N, P301L, and V337M MAPT mutations. Neurology. 2009a;73:1058–65. doi: 10.1212/WNL.0b013e3181b9c8b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Boeve BF, Senjem ML, Baker M, Rademakers R, et al. Voxel-based morphometry patterns of atrophy in FTLD with mutations in MAPT or PGRN. Neurology. 2009b;72:813–20. doi: 10.1212/01.wnl.0000343851.46573.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Pankratz VS, Parisi JE, Knopman DS, Boeve BF, et al. Rates of brain atrophy over time in autopsy-proven frontotemporal dementia and Alzheimer disease. Neuroimage. 2008;39:1034–40. doi: 10.1016/j.neuroimage.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Senjem ML, Parisi JE, Boeve BF, Knopman DS, et al. MRI correlates of protein deposition and disease severity in postmortem frontotemporal lobar degeneration. Neurodegener Dis. 2009c;6:106–17. doi: 10.1159/000209507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain. 2009d;132:2932–46. doi: 10.1093/brain/awp232. [DOI] [PMC free article] [PubMed] [Google Scholar]