Abstract
We previously reported that foetal valproate exposure impairs intelligence quotient. In this follow-up investigation, we examined dose-related effects of foetal antiepileptic drug exposure on verbal and non-verbal cognitive measures. This investigation is an ongoing prospective observational multi-centre study in the USA and UK, which has enrolled pregnant females with epilepsy on monotherapy from 1999 to 2004. The study seeks to determine if differential long-term neurodevelopmental effects exist across four commonly used drugs (carbamazepine, lamotrigine, phenytoin and valproate). This report compares verbal versus non-verbal cognitive outcomes in 216 children who completed testing at the age of three years. Verbal and non-verbal index scores were calculated from the Differential Ability Scales, Preschool Language Scale, Peabody Picture Vocabulary Test and Developmental Test of Visual-Motor Integration. Verbal abilities were lower than non-verbal in children exposed in utero to each drug. Preconceptional folate use was associated with higher verbal outcomes. Valproate was associated with poorer cognitive outcomes. Performance was negatively associated with valproate dose for both verbal and non-verbal domains and negatively associated with carbamazepine dose for verbal performance. No dose effects were seen for lamotrigine and phenytoin. Since foetal antiepileptic drug exposure is associated with lower verbal than non-verbal abilities, language may be particularly susceptible to foetal exposure. We hypothesize that foetal drug exposure may alter normal cerebral lateralization. Further, a dose-dependent relationship is present for both lower verbal and non-verbal abilities with valproate and for lower verbal abilities with carbamazepine. Preconceptional folate may improve cognitive outcomes. Additional research is needed to confirm these findings, extend the study to other drugs, define the risks associated with drug treatment for seizures in the neonates, and understand the underlying mechanisms.
Keywords: antiepileptic drugs, child development, cognitive neurology, epilepsy, pregnancy
Introduction
Animal studies have demonstrated that foetal exposure to some antiepileptic drugs (AEDs), at doses lower than those that result in structural malformations, can produce behavioural and cognitive deficits, alter neurochemistry and reduce brain weight (Fisher and Vorhees, 1992; Gaily and Meador, 2007). Further, some AEDs can produce widespread neuronal apoptosis in the immature brain similar to alcohol (Bittigau et al., 2002, 2003; Glier et al., 2004; Manthey et al., 2005; Katz et al., 2007; Kim et al., 2007; Stefovska et al., 2008). The effect is dose dependent, occurs at therapeutically relevant blood levels, and requires only relatively brief exposure. The effect has been related to reduced expression of neurotrophins and levels of protein kinases that promote neuronal growth and survival. These observations suggest that certain AEDs might produce similar adverse effects in children exposed in utero or in the neonatal period. In fact, some AEDs have been associated with reduced cognitive abilities (e.g., IQ) in children exposed in utero (Adab et al., 2004; Gaily et al., 2004; Thomson et al., 2008, Meador et al., 2009, Brombley et al., 2010). In this report, we examine the effects of foetal AED exposure on specific cognitive functions rather than global cognitive function. Verbal and non-verbal abilities were assessed at 3 years of age in children of females with epilepsy.
Participants and methods
Design
The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study is a prospective observational study examining possible behavioural teratogenesis of AEDs. We enrolled pregnant females with epilepsy, who were on one of four AED monotherapies (carbamazepine, lamotrigine, phenytoin or valproate) from October 1999 through to February 2004, across 25 epilepsy centres in the USA and UK. We recently reported on IQ outcomes in these children at 3 years of age (Meador et al., 2009). Here, we contrast verbal and non-verbal cognitive outcomes at the age of 3 years and examine dose-dependent effects.
Participants
Consent from participants was obtained according to the Declaration of Helsinki. Institutional review boards at each centre approved the study, and written informed consent was obtained prior to enrolment. Pregnant females with epilepsy, taking carbamazepine, lamotrigine, phenytoin or valproate monotherapy, were enrolled. These four AED monotherapies were the most frequently employed during the enrolment time period. Other AEDs were not included because of insufficient numbers. Polytherapy was not included because of its association with poorer outcomes (Kluger and Meador, 2008; Harden et al., 2009a). A non-exposed control group was not included at the direction of an NIH review panel. Mothers with IQ < 70 were excluded to avoid floor effects and because maternal IQ is the major predictor of child IQ in population studies (Sattler, 1992). Other exclusion criteria included positive syphilis or HIV serology, progressive cerebral disease, other major disease (e.g. diabetes), exposure to teratogenic agents other than AEDs, poor AED compliance, drug abuse in the prior year, or drug abuse sequelae.
Procedures
Information was collected on potentially confounding variables, including maternal IQ, age, education, employment, race, seizure/epilepsy types and frequency, AED dosages, compliance, socio-economic status (Hollingshead, 1975), UK/USA site, preconception folate use, use of alcohol, tobacco or other drugs during pregnancy, unwanted pregnancy, abnormalities/complications in the present pregnancy or prior pregnancies, enrolment and birth gestational age, birth weight, breastfeeding and childhood medical diseases. Children were classified as ‘breastfed’ if they were breastfeeding at the time of the 3 month follow-up phone call, after delivery. Cognitive outcomes were evaluated by assessors (blinded to AED) using the Differential Ability Scales (Elliott, 1990), Preschool Language Scale (4th edition) (Zimmerman et al., 2002), Peabody Picture Vocabulary Test (fourth edition) (Dunn and Dunn, 2007) and Developmental Test of Visual-Motor Integration (fifth edition) (Beery and Beery, 2004). Testing was conducted at 36–45 months of age; standardized scores were calculated. Separate investigations with very similar designs in the USA and UK were merged after initiation. Maternal IQs were determined by different measures due to the later merger; these measures included the Test of Non-Verbal Intelligence (third edition) (Brown et al., 1996) in 267 mothers (67 UK), Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) in 20 (all UK) and National Adult Reading Test (Nelson and Willison, 1991) in 17 (all UK). Training and monitoring of neuropsychological evaluations were conducted to assure quality and consistency. As part of the training, workshops were conducted for all neuropsychological test batteries annually. In addition, each assessor was required to identify errors and provide appropriate correction for videotaped testing sessions containing errors in administration and scoring. Assessors were also required to submit their own videotape of a practice test session with record forms to the Neuropsychology Core Director for review, feedback and approval. If assessors failed, they submitted additional video assessments for approval prior to testing children in the study.
To allow comparisons across AEDs, the dosages were standardized (Meador et al., 2009). Average AED dose during pregnancy was standardized relative to ranges observed within each AED group in the intent-to-treat population by the following calculation:
Statistical analysis
The primary analysis in this substudy included 216 children with complete verbal and non-verbal outcomes at 3 years of age. Multivariate regression models were used to examine group differences, adjusting for covariates. The a priori hypothesis was that the specific AED, dose and maternal IQ were important covariates, and so these were included as predictors in a linear model with child verbal and non-verbal indices as outcomes. Other covariates were added individually to the model and were included if found to be significant (P < 0.05), or if they did not exhibit co-linearity with existing predictors. The covariates in the final model were AED, maternal IQ, pregnancy average AED dose, maternal age, race (four categories), alcohol use during pregnancy (yes/no) and preconception folate. The effects of multiple additional covariates were also examined, which included: gestational age at enrolment, gestational age at birth, epilepsy type (localization related, generalized and generalized tonic clonic for which it was unknown if localization related or generalized), seizure types (convulsive, non-convulsive), seizure frequency during pregnancy, education, employment, socio-economic status, paternal IQ, USA/UK site, tobacco use during pregnancy, birth weight, unwanted pregnancy, breastfeeding, prior pregnancy birth defects and complications, present pregnancy complications and AED compliance.
The dependent variables were verbal and non-verbal outcomes at the age of 3 years. A verbal index score was created by averaging the expressive communication and the auditory comprehension subtests of the Preschool Language Scale (Zimmerman et al., 2002), the naming vocabulary and the verbal comprehension subtests of the Differential Abilities Scales (Elliott, 1990), and the Peabody Picture Vocabulary Test (Dunn and Dunn, 2007). A non-verbal index score was the average of the block building subtest of the Differential Ability Scales (Elliott, 1990) and the Developmental Test of Visual-Motor Integration (Beery and Beery, 2004).
Multivariate regression models were adjusted for AED group, maternal IQ, standardized AED dose, maternal age at delivery, race, alcohol use during pregnancy and preconception folate. These covariates were found to be significant predictors in the multivariate model, at the 0.05 level of significance. Secondary analyses examined the following: (i) relative relationships of AED exposures to verbal versus non-verbal abilities; (ii) correlations of the individual index scores to the standardized AED doses; (iii) sensitivity of results to baseline differences in covariates; and (iv) sensitivity of results to missing data. Analyses were performed at the NEAD Data and Statistical Centre using Statistical Analysis System (SAS 9.2).
To investigate whether baseline differences in AED groups affected the results, subgroup analyses were conducted in which subgroups were defined by propensity scores (Rosenbaum and Rubin, 1983; D’Agostino, 1998). Propensity scores are predicted probabilities of receiving a treatment given baseline covariate values. Covariates are approximately equally distributed within propensity score subgroups. Propensity scores were estimated using predicted probabilities from a logistic regression model with AED treatment group (valproate versus non-valproate) as outcome. Variables related to valproate group membership were predictors in the propensity score model along with variables significantly related to age 3 verbal and non-verbal outcomes (Brookhart et al., 2006). The predictors in the propensity score model included dose, maternal IQ, maternal and gestational age, preconception folate, USA/UK site, epilepsy type, alcohol use during pregnancy, tobacco use during pregnancy, education, socio-economic status, employment status, unwanted pregnancy and race.
To investigate sensitivity of primary results to missing data (i.e. missing age 3 verbal and non-verbal outcomes), analyses were also conducted using the intent-to-treat sample (n = 310 live births including six twin pairs). One or both index scores were missing in 94 (30%); 78 (25%) had missing non-verbal scores; and 91 (29%) had missing verbal scores. Monotone data Markov Chain Monte Carlo methods were used in secondary analyses to impute missing age 3 outcomes (Li, 1988; Schafer, 1997; Little and Rubin, 2002). Variables in the imputation model included IQ scores at 2 and 3 years of age [Bailey Scales of Infant Development (Bailey, 1993); Differential Ability Scales (Elliott, 1990)], AED, dose, maternal IQ and age, gestational age at delivery, preconception folate, socio-economic status, race, tobacco use during pregnancy, employment status, education, unwanted pregnancy and alcohol use during pregnancy. Least squares mean index scores were estimated for the AED groups adjusting for maternal IQ, AED group, maternal age, dose, alcohol use during pregnancy, race and preconception folate. Standard errors and confidence intervals (CIs) of parameter estimates incorporated imputation uncertainty.
Results
The primary analysis included 211 mothers and 216 children (five sets of twins). Mean gestational age at enrolment was 17.5 weeks (range = 3–19), which did not differ across the different AEDs. Baseline maternal characteristics are shown in Table 1. The statistical results for the primary analysis are presented in Table 2. Higher maternal IQ was associated with higher verbal (r = 0.40; P < 0.0001) and non-verbal (r = 0.26; P < 0.0001) index scores in the child. Maternal age was associated with higher verbal (r = 0.37; P < 0.0001) and non-verbal (r = 0.17; P = 0.01) index scores; this was due to lower scores in children with the youngest mothers. Overall, scores were higher in children whose mothers took preconception folate. Mean (95% CI) for verbal index score adjusted for factors in the primary analysis model was 94 (91:98) for children exposed to preconception folate, which differed (P = 0.01) from children not exposed, who had mean of 89 (86:93). Adjusted means (95% CI) for non-verbal index were 102 (98:107) for children exposed to preconception folate versus 100 (96:104) for those who were not exposed, which did not differ statistically. Verbal index scores were higher for Caucasian than other races (P < 0.0001), but non-verbal index scores did not differ across race. Children exposed to alcohol during pregnancy (n = 18) had a mean verbal index score of 97, which did not differ from the mean (97) for those not exposed (n = 198); however, non-verbal index scores differed (P = 0.02) with means for exposed (99) and unexposed (105). Note that means by race and alcohol history category used in the follow-up analyses were unadjusted due to small sample sizes in some of the subgroups.
Table 1.
Demographics and IQ results for mothers of children with complete verbal and non-verbal index scores
| Antiepileptic drug | Carbamazepine | Lamotrigine | Phenytoin | Valproate | Totala | Missingb |
|---|---|---|---|---|---|---|
| Mothers (n) | 59 | 70 | 39 | 43 | 211 | 93 |
| Mean maternal IQs (95% CI) | 100 (96:105) | 102 (98:106) | 92 (87:98) | 95 (91:100) | 98 (96:101) | 97 (94:100) |
| Mean maternal ages (95% CI) | 31 (29:32) | 31 (30:32) | 30 (28:32) | 28 (26:30) | 30 (29:31) | 29 (28:30) |
| Mean dosec (95% CI) (mg/day) | 781 (676:887) | 489 (430:547) | 401 (363:438) | 1012 (819:1204) | N/A | N/A |
| Standardized dosed (95% CI) | 32 (28:37) | 38 (33:43) | 49 (43:54) | 25 (20:31) | 36 (33:38) | 32 (28:37) |
| Gestational age at birth (95% CI) (weeks) | 38 (38:39) | 39 (39:40) | 39 (38:39) | 39 (39:40) | 39 (39:39) | 39 (38:39) |
| Preconception folate n (%) | 34 (58) | 41 (59) | 17 (44) | 28 (65) | 120 (57) | 53 (57) |
| Alcohol usee during pregnancy n (%) | 6 (10) | 6 (9) | 2 (5) | 4 (9) | 18 (9) | 6 (6) |
| Epilepsy typesf | ||||||
| Localization related n (%) | 51 (86) | 39 (56) | 28 (72) | 8 (19) | 126 (60) | 57 (61) |
| Idiopathic generalized n (%) | 5 (8) | 24 (34) | 7 (18) | 32 (74) | 68 (32) | 29 (31) |
| GTCSfn (%) | 3 (5) | 7 (10) | 4 (10) | 3 (7) | 17 (8) | 7 (8) |
| Non-convulsive seizures n (%) | 18 (33) | 23 (37) | 14 (37) | 12 (28) | 67 (34) | 21 (25) |
| Convulsionsg | ||||||
| None n (%) | 46 (85) | 46 (75) | 28 (76) | 33 (79) | 153 (79) | 71 (87) |
| >5 Convulsions n (%) | 3 (6) | 0 (0) | 3 (8) | 1 (2) | 7 (4) | 1 (1) |
| Race | ||||||
| Caucasian | 51 (86) | 62 (89) | 23 (59) | 38 (88) | 174 (82) | 70 (75) |
| Black | 3 (5) | 1 (1) | 3 (8) | 1 (2) | 8 (4) | 6 (6) |
| Hispanic | 2 (3) | 3 (4) | 12 (31) | 2 (5) | 19 (9) | 12 (13) |
| Other | 3 (5) | 4 (6) | 1 (3) | 2 (5) | 10 (5) | 5 (5) |
a Total across antiepileptic drugs for mothers whose children had age 3 verbal index or non-verbal index scores.
b Missing = mothers for whom their children’s verbal index or non-verbal index scores are missing.
c Average dose for pregnancy.
d Refer to ‘Partcipants and methods’ section for description of how dosages were standardized.
e Any alcohol use during pregnancy (yes/no).
f Epilepsy types: localization-related (includes cryptogenic and symptomatic); idiopathic generalized (includes absence, juvenile myoclonic, genetic and other idiopathic generalized not otherwise classified).
g Convulsions = number (%) of mothers without convulsions or >5 during pregnancy. Seizure frequency during pregnancy not reported for n = 17 mothers.
GTCS = generalized tonic clonic seizures (unknown if generalized or secondary generalized); N/A = not applicable.
Table 2.
Statistical results for multivariate analysis with verbal and non-verbal index scores as dependent variables
| Effect | F-valuea | df | P-value |
|---|---|---|---|
| AED group | 5.36 | 6 | <0.0001 |
| Maternal IQ | 9.37 | 2 | 0.0001 |
| Standardized dose | 8.61 | 2 | 0.0003 |
| Maternal age | 6.98 | 2 | 0.0012 |
| Preconception folate | 3.33 | 2 | 0.0379 |
| Race (four categories) | 3.89 | 6 | 0.0009 |
| Any alcohol during pregnancy | 3.05 | 2 | 0.0497 |
Based on age 3 completer population (n = 216).
a F-value = exact F-statistic for Wilk’s Lambda.
df = degrees of freedom.
Cognitive outcomes differed across AEDs. Mean verbal and non-verbal index scores for children exposed in utero to valproate were significantly lower than all other AEDs combined (P ≤ 0.0001) and across all individual pair-wise comparisons: carbamazepine (P = 0.0009), lamotrigine (P ≤ 0.0001) and phenytoin (P = 0.0006). Adjusted age 3 mean verbal and non-verbal index scores along with 95% CIs for each AED group are listed in Table 3. Overall, verbal abilities were significantly more affected than non-verbal abilities; the mean difference between verbal and non-verbal index scores across all drugs was −5.36 (95% CI = 3.5:7.2), P < 0.0001 from a paired t-test. Verbal index scores were also significantly lower than non-verbal for each AED. Using a linear model controlling for maternal IQ, dose, age of mother, alcohol use, race and folate, the mean differences (95% CI) between non-verbal and verbal indices for each AED group were: carbamazepine: 6.6 (2.1, 11.1), P = 0.0044; lamotrigine: 9.7 (5.2, 14.2), P < 0.0001; phenytoin: 6.2 (1.1, 11.2), P = 0.0171; and valproate: 14.6 (9.3, 19.8), P < 0.0001.
Table 3.
Adjusteda mean age 3 cognitive scores (95% CI) for verbalb and non-verbalc index scores for each antiepileptic drug
| Carbamazepine | Lamotrigine | Phenytoin | Valproate | Differences of valproate with other AEDsd | |
|---|---|---|---|---|---|
| Verbal indexb | 93.0 (88.6:97.3) | 96.6 (92.3:100.9) | 95.9 (91.0:100.8) | 83.9 (78.8:89.0) | 9.0–12.7 |
| Non-verbal indexc | 99.6 (95.0:104.2) | 106.3 (101.7:110.9) | 102.0 (96.9:107.2) | 98.5 (93.1:103.8) | 1.1–7.8 |
a Mean age 3 index scores adjusted for maternal IQ, maternal age, dose, race, alcohol use during pregnancy and folate.
b Verbal index included: (i) naming vocabulary and verbal comprehension subtests from the Differential Ability Scales (Elliott, 1990); (ii) expressive communication and auditory comprehension subscales from the Preschool Language Scale (Zimmerman et al., 2002); and (iii) the Peabody Picture Vocabulary Test (Dunn and Dunn, 2007).
c Non-verbal index included: (i) block building subtest from the Differential Ability Scales (Elliott, 1990) and (ii) the Developmental Test of Visual-Motor Integration (Beery and Beery, 2004).
d Mean difference between non-verbal and verbal index scores across all drugs was 5.36 (95% CI = 3.5:7.2), P < 0.0001 from a paired t-test.
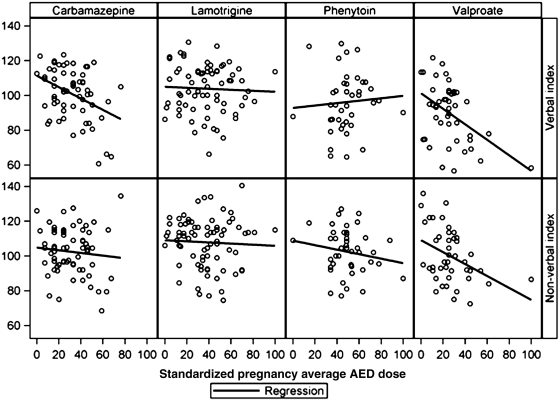
Partial Pearson correlations for verbal and non-verbal indices to pregnancy average standardized dose, controlling for maternal IQ, are listed in Table 4, and scatter plots are depicted in Fig. 1. Dose-dependent effects for valproate were seen for both indices. Dose-dependent effects for carbamazepine were seen for the verbal index. Partial correlations for each of the individual subtest scores are given in Table 5. Similar results were found for separate analyses using either the first or third trimester dosages. Median dosages (in mg) for the whole pregnancy average dose were: carbamazepine, 800; lamotrigine, 500; phenytoin, 400; and valproate, 1000. Means (ranges) of whole pregnancy average dosages (in mg) were: carbamazepine, 781 (33–1800); lamotrigine, 489 (50–1217); phenytoin, 401 (67–750); and valproate, 1012 (133–3583).
Table 4.
Partial Pearson correlations (significant P-values) for cognitive indices to pregnancy average standardized dose, controlling for maternal IQ
| Carbamazepine | Lamotrigine | Phenytoin | Valproate | |
|---|---|---|---|---|
| Verbal index | −0.35 (0.007) | −0.07 | 0.09 | −0.48 (0.001) |
| Non-verbal ndex | −0.05 | −0.08 | −0.20 | −0.39 (0.010) |
| N | 60 | 71 | 41 | 44 |
Significant findings in bold.
Figure 1.
Scatter plots of verbal index and non-verbal index versus standardized dose for each antiepileptic drug during pregnancy.
Table 5.
Partial Pearson correlations (significant P-values) for seven subtests to pregnancy average standardized dose, controlling for maternal IQ
| Carbamazepine | Lamotrigine | Phenytoin | Valproate | |
|---|---|---|---|---|
| Preschool Language Scale (expressive communication) | −0.40 (0.002) | −0.11 | −0.02 | −0.41 (0.006) |
| Preschool Language Scale (auditory comprehension) | −0.29 (0.03) | −0.005 | −0.008 | −0.51 (0.0004) |
| Differential Ability Scales (naming vocabulary) | −0.22 | −0.11 | 0.29 | −0.45 (0.003) |
| Differential Ability Scales (verbal comprehension) | −0.19 | −0.007 | 0.21 | −0.38 (0.01) |
| Peabody Picture Vocabulary | −0.34 (0.008) | −0.07 | −0.12 | −0.39 (0.01) |
| Differential Ability Scales (block building) | −0.07 | −0.11 | −0.11 | −0.47 (0.002) |
| Developmental Test of Visual-Motor Integration | −0.02 | −0.04 | −0.23 | −0.23 |
Significant findings in bold.
The propensity score analyses demonstrated that the results are not due to differences in baseline variables related to either the child IQ outcome or chances of belonging to the valproate group. The analysis examining sensitivity of results to missing data demonstrates that the results cannot be explained by incomplete data. In the intent-to-treat sample, including 310 of the originally enrolled children, missing outcomes were imputed, and results were similar.
Discussion
The present study demonstrates reduced verbal compared with non-verbal abilities in children exposed in utero to each of four commonly employed AED monotherapies. Since the verbal and non-verbal indices are standard scores based upon normative data from healthy populations, the two index scores would be expected to be equivalent. We cannot rule out that the observation of consistently greater impairments in verbal abilities is due to genetic or other environmental factors, but there is a distinct possibility that they are due to foetal AED exposure. A prior study reported reduced language abilities in children of women with epilepsy, but did not assess individual AEDs (Thomas et al., 2007). This raises the question as to why language functions might be more susceptible. As noted earlier, some AEDs can produce widespread neuronal apoptosis in the immature brain (Bittigau et al., 2002, 2003; Glier et al., 2004; Manthey et al., 2005; Katz et al., 2007; Kim et al., 2007; Stefovska et al., 2008). However, it seems unlikely that the apoptosis occurs to a greater degree in language-related neurons. In the immature brain, alcohol can not only produce apoptosis (Ikonomidou et al., 2000), but can also impair neural physiology in the remaining neurons (Medina et al., 2003, 2005). AEDs may also impair the physiology of remaining neurons, but it seems unlikely that AED-induced physiological alterations at the neuronal level would be greater in language related neurons. We hypothesize that the foetal AED exposure alters cerebral lateralization, which has a more profound effect on the neural networks underlying language functions.
In addition, our study found that both verbal and non-verbal cognitive outcomes were reduced in children exposed in utero to valproate. The effect is dose-dependent, and the magnitude of the effect is greater for verbal than non-verbal abilities. Further, foetal exposure to carbamazepine was associated with a dose-related reduction in language abilities. Strengths of our study include its prospective design, blinded cognitive assessments using standardized measures, and detailed monitoring of multiple potential confounding factors. However, caution is advised due to study limitations that include a relatively small non-population based sample, loss of enrolled subjects to analysis, lack of randomization, lack of AED blood levels, absence of an unexposed control group during pregnancy, and the relatively young age of the children at this planned interim analysis. Because the NEAD study is not a randomized trial, it is possible that confounding factors related to baseline characteristics may have affected the child’s IQ. However, analyses adjusting for baseline characteristics, including the propensity analyses, suggest that the results cannot be explained by differences in baseline characteristics.
Recently, the American Academy of Neurology published new practice parameters concerning management issues for females with epilepsy (Harden et al., 2009a, b, c). With regards to cognitive teratogenesis of AED (Harden et al., 2009a), they concluded the following:
cognition is probably not reduced in children of females with epilepsy who are not exposed to AEDs in utero (two Class II studies);
there is insufficient evidence to determine if the children of females with epilepsy on AEDs in general are at increased risk for reduced cognition (conflicting Class II studies);
carbamazepine probably does not increase poor cognitive outcomes compared with unexposed controls (two Class II studies);
valproate is probably associated with poor cognitive outcomes compared with unexposed controls (two Class II studies);
cognitive outcomes are probably reduced in children exposed to valproate during pregnancy compared with carbamazepine (two Class II studies);
cognitive outcomes are possibly reduced in children exposed to valproate during pregnancy compared with phenytoin (one Class II study);
phenytoin is possibly associated with poor cognitive outcomes compared with unexposed controls (one Class II and two Class III studies);
phenobarbital is possibly associated with poor cognitive outcomes in male offspring of females with epilepsy compared with unexposed controls (two Class III studies); and
cognitive outcomes are probably reduced in children exposed to AED polytherapy as compared with monotherapy in utero (three Class II studies).
These practice parameters were formulated prior to the publication of the recent NEAD results. The present and previously published results from the NEAD study provide Class I evidence that cognitive outcomes are reduced in children exposed in utero to valproate compared with carbamazepine, lamotrigine and phenytoin. In addition, the present study supports two prior studies demonstrating an adverse effect of valproate on verbal abilities, which is greater in magnitude compared with its effect on non-verbal abilities (Adab et al., 2004; Gaily et al., 2004). The present finding of a dose-dependent effect of carbamazepine on verbal abilities is in contrast to the two prior studies (Adab et al., 2004; Gaily et al., 2004). The difference in results might be due to the fact that the verbal IQ measures used in those studies included some subtests without strong verbal factor loadings. For example, the verbal IQ from the third edition of the Wechsler Intelligence Scale for Children (Wechsler, 1991) used in the Adab et al. (2004) study, and the verbal IQ from the Revised Wechsler Intelligence Scale for Children (Wechsler, 1984) and the Revised Wechsler Preschool and Primary Scale of Intelligence (Wechlser, 1995) used in the Gaily et al. (2004) study include an arithmetic subtest. In addition, other contributing factors might include Type I error and differences across studies in AED dosage ranges and patient characteristics of the samples under investigation. As a result, further studies are needed to clarify this issue.
The majority of AED prescriptions in the USA are written for pain or psychiatric indications rather than epilepsy or seizures (Zito et al., 2006; Cascade et al., 2008). There are no published studies on cognitive outcomes in children of females taking AEDs for these other indications. However, one study found that congenital malformation rate was similar in these children compared with children of females taking AEDs for epilepsy (Holmes et al., 2001).
In clinical practice, females of childbearing potential who are prescribed AEDs with known risks should be informed of these risks. However, the observed risks of some AEDs to the child have to be balanced against the risks of seizures to the mother and child. Further complicating care, the risks for many AEDs are still poorly defined. The most consistent and severe risks for both anatomical and behavioural teratogenesis have been found for valproate (Meador et al., 2008; Harden et al., 2009a), so it seems a poor first choice for most females of childbearing potential. However, seizures in some females with generalized epilepsy may only be controlled on valproate (Marson et al., 2007). Thus, therapeutic decisions need to be individualized. Children exposed in utero to AEDs, especially valproate, should be considered at risk for cognitive deficits. Further, they may be at risk for behavioural abnormalities (Williams et al., 2001; Bromley et al., 2008; Vinten et al., 2009). Based on these findings, a raised index of suspicion should translate into more careful follow-up and evaluation of these children with appropriate referral for interventional therapies when indicated.
In conclusion, our results combined with the findings of other studies raise particular concerns that foetal valproate exposure imposes a risk of cognitive impairment, especially for higher doses and for language functions. The implications of the observed dose-dependent relationship of carbamazepine exposure to impaired verbal abilities in the present study is less clear given that this finding is in contrast to two prior studies. Additional research is needed to confirm these findings, examine risks of other AEDs, define the risks associated with AEDs in the neonate for treatment of seizures, and to understand the underlying mechanisms of adverse AED effects on the immature brain.
Funding
National Institutes of Health (2RO1-NS038455 to K.J.M., R01NS050659 to N.B.); UK Epilepsy Research Foundation (RB219738 to G.A.B.).
Conflict of interest
K.J.M. reports receiving research support from McKnight Brain Institute, MCG Foundation, Glaxo SmithKline, EISAI Medical Research, Myriad Pharmaceuticals, Marinus Pharmaceuticals, NeuroPace, SAM Technology, UCB Pharma, Epilepsy Foundation and National Institutes of Health; and also serves on the Professional Advisory Board for the Epilepsy Foundation. G.A.B. reports serving on paid advisory boards, receiving lecture fees from Pfizer, UCB and Janssen, receiving grant support from Sanofi-Aventis and Pfizer and has served as an expert witness in litigation related to neurodevelopment effects of antiepileptic drugs. N.B. and M.J.C. report that they have no significant disclosures. J.C.-S. has served as editor for Clinical Dysmorphology and as an expert witness in litigation related to neurodevelopment effects of antiepileptic drugs. L.A.K. reports consulting fee from Cyberonics, and grant support from Marinus and Medical College of Georgia. A.K. reports receiving lecture fees from GlaxoSmithKline, Ortho McNeil and UCB, consulting fees or paid advisory boards from GlaxoSmithKline, Ortho McNeil and Pfizer and grant support from GlaxoSmithKline, Novartis and Pfizer. J.D.L. that she is a member of the Professional Board of Epilepsy Foundation of Eastern PA and the speaker’s bureau for UCB, Pharma. P.B.P. has previously received grant support from UCB Pharma, Marinus Pharmaceuticals, Centers for Disease Control and National Institutes of Health. M.P. reports receiving consulting and advisory board fees from UCB, Johnson and Johnson and Epifellows Foundation; lecture fees from Pfizer and UCB; grant support from UCB, Ortho McNeil, National Institutes of Health and American Epilepsy Society. D.W.L. reports receiving consulting fees from UCB, NeuroPace and Sanofi-Aventis and grant support from Myriad Pharm, Sam Technology and Novartis. No other potential conflicts of interest are reported.
Supplementary Material
Acknowledgements
The investigators would like to thank the children and families who have given their time to participate in the NEAD Study.
Glossary
Abbreviations
- AED
antiepileptic drug
- NEAD
neurodevelopmental effects of antiepileptic drugs
Appendix I: NEAD study group
Arizona Health Sciences Centre, Tucson, Arizona: David Labiner MD, Jennifer Moon PhD, Scott Sherman MD; Baylor Medical Centre, Irving Texas: Deborah T. Combs Cantrell MD, University of Texas-Southwestern, Dallas Texas: Cheryl Silver PhD; Case Western Reserve University, Cleveland, Ohio: Monisha Goyal MD, Mike R. Schoenberg PhD; Columbia University, New York City, NY: Alison Pack MD, Christina Palmese PhD, Joyce Echo PhD; Emory University, Atlanta, Georgia: Kimford J. Meador MD, David Loring PhD, Page Pennell MD, Daniel Drane PhD, Eugene Moore BS, Megan Denham MAEd, Charles Epstein MD, Jennifer Gess PhD, Sandra Helmers MD, Thomas Henry MD; Georgetown University, Washington, DC: Gholam Motamedi MD, Erin Flax BS; Harvard-Brigham and Women’s, Boston, Massachusetts: Edward Bromfield MD, Katrina Boyer PhD, Barbara Dworetzky ScB, MD; Harvard–Massachusetts General, Boston, Massachusetts: Andrew Cole MD, Lucila Halperin BA, Sara Shavel-Jessop BA; Henry Ford Hospital, Detroit, Michigan: Gregory Barkley MD, Barbara Moir MS; Medical College of Cornell University, New York City, New York: Cynthia Harden MD, Tara Tamny-Young PhD; Medical College of Georgia, Augusta, Georgia: Gregory Lee PhD, Morris Cohen EdD; Minnesota Epilepsy Group, St. Paul, Minnesota: Patricia Penovich MD, Donna Minter EdD; Ohio State University, Columbus, Ohio: Layne Moore MD, Kathryn Murdock MA; Riddle Health Care, Media, Pennsylvania: Joyce Liporace MD, Kathryn Wilcox, BS; Rush University Medical Center, Chicago, Illinois: Andres Kanner MD, Michael N. Nelson PhD; The Comprehensive Epilepsy Care Center for Children and Adults, St. Louis, Missouri: William Rosenfeld MD, Michelle Meyer MEd; St. Mary’s Hospital, Manchester, England: Jill Clayton-Smith MD, George Mawer MD, Usha Kini MD; University Alabama, Birmingham, Alabama: Roy Martin PhD; University of Cincinnati, Cincinnati, Ohio: Michael Privitera MD, Jennifer Bellman PsyD, David Ficker MD; University of Kansas School of Medicine-Wichita, Wichita, Kansas: Lyle Baade PhD, Kore Liow MD; University of Liverpool, Liverpool, England: David Chadwick MD, Gus Baker PhD, Alison Booth BSc, Rebecca Bromley BSc, Sara Dutton BSc, James Kelly BSc, Jenna Mallows BSc, Lauren McEwan MSc, Laura Purdy BSC; University of Miami, Miami, Florida: Eugene Ramsay MD, Patricia Arena PhD; University of Southern California, Los Angeles, California: Laura Kalayjian MD, Christianne Heck MD, Sonia Padilla PsyD; University of Washington, Seattle, Washington: John Miller MD, Gail Rosenbaum BA, Alan Wilensky MD; University of Utah, Salt Lake City, Utah: Tawnya Constantino MD, Julien Smith PhD; Walton Centre for Neurology and Neurosurgery, Liverpool, England: Naghme Adab, MD, Gisela Veling-Warnke MD; Wake Forest University, Winston-Salem, North Carolina: Maria Sam MD, Cormac O’Donovan MD, Cecile Naylor PhD, Shelli Nobles MS, Cesar Santos MD. Executive Committee: Dartmouth Medical School, Hanover, New Hampshire: Gregory L. Holmes MD; Stanford University, Stanford, California: Maurice Druzin MD, Martha Morrell MD, Lorene Nelson PhD; Texas A & M University Health Science Center, Houston, Texas: Richard Finnell PhD; University of Oregon, Portland, Oregon: Mark Yerby MD; University of Toronto, Toronto, Ontario: Khosrow Adeli PhD, Peter Wells Pharm.D. Data and Statistical Centre: EMMES Corporation, Rockville, Maryland: Temperance Blalock AA, Nancy Browning PhD, Todd Crawford MS, Linda Hendrickson, Bernadette Jolles MA, Meghan Kelly Kunchai MPH, Hayley Loblein BS, Yinka Ogunsola BS, Steve Russell BS, Jamie Winestone BS, Mark Wolff PhD, Phyllis Zaia BS, Thad Zajdowicz MD, MPH.
References
- Adab N, Kini U, Vinten J, Ayres J, Baker G, Clayton-Smith J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75:1575–83. doi: 10.1136/jnnp.2003.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N. Bayley scales of infant development. 2nd. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- Beery K, Beery N. Developmental test of visuo-motor integration. 5th. Minneapolis, MN: Pearson Assessments; 2004. [Google Scholar]
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99:15089–94. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann NY Acad Sci. 2003;993:103–14. doi: 10.1111/j.1749-6632.2003.tb07517.x. ; discussion 23–4. [DOI] [PubMed] [Google Scholar]
- Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–56. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley RL, Mawer G, Clayton-Smith J, Baker GA. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurology. 2008;71:1923–4. doi: 10.1212/01.wnl.0000339399.64213.1a. [DOI] [PubMed] [Google Scholar]
- Bromley RL, Mawer G, Love J, Kelly J, Purdy L, McEwan L, et al. Early cognitive development in children born to women with epilepsy: a prospective report. Epilepsia. 2010;51:2058–65. doi: 10.1111/j.1528-1167.2010.02668.x. [DOI] [PubMed] [Google Scholar]
- Brown L, Sherbenou RJ, Johnsen SK. Test of non-verbal intelligence (TONI-3) 3rd. Austin, TX: Pro-Ed; 1996. [Google Scholar]
- Cascade E, Kalali AH, Weisler RH. Varying uses of anticonvulsant medications. Psychiatry. 2008;5:31–3. [PMC free article] [PubMed] [Google Scholar]
- D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statist Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Dunn L, Dunn D. Peabody picture vocabulary test. 4th. Minneapolis, MN: Pearson Assessments; 2007. [Google Scholar]
- Elliott CD. Differential abilities scales. 2nd. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- Fisher JE, Vorhees CV. Developmental toxicity of antiepileptic drugs: relationship to postnatal dysfunction. Pharmacol Res. 1992;26:207–21. doi: 10.1016/1043-6618(92)90210-3. [DOI] [PubMed] [Google Scholar]
- Gaily E, Kantola-Sorsa E, Hiilesmaa V, Isoaho M, Matila R, Kotila M, et al. Normal intelligence in children with prenatal exposure to carbamazepine. Neurology. 2004;62:28–32. doi: 10.1212/wnl.62.1.28. [DOI] [PubMed] [Google Scholar]
- Gaily E, Meador KJ. Neurodevelopmental effects. In: Engel J, Pedley TA, editors. Epilepsy: a comprehensive textbook. 2nd. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1225–33. [Google Scholar]
- Glier C, Dzietko M, Bittigau P, Jarosz B, Korobowicz E, Ikonomidou C. Therapeutic doses of topiramate are not toxic to the developing rat brain. Exp Neurol. 2004;187:403–9. doi: 10.1016/j.expneurol.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Harden CL, Hopp J, Ting TY, Pennell PB, French JA, Hauser WA, et al. Practice Parameter update: Management issues for women with epilepsy–focus on pregnancy (an evidence-based review): obstetrical complications and change in seizure frequency. Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009b;73:126–32. doi: 10.1212/WNL.0b013e3181a6b2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden CL, Meador KJ, Pennell PB, Hauser WA, Gronseth GS, French JA, et al. Practice Parameter update: Management issues for women with epilepsy–focus on pregnancy (an evidence-based review): Teratogenesis and perinatal outcomes. Report of the Quality Standards Subcommittee and Therapeutics and Technology Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009a;73:133–41. doi: 10.1212/WNL.0b013e3181a6b312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden CL, Pennell PB, Koppel BS, Hovinga CA, Gidal B, Meador KJ, et al. Practice Parameter update: Management issues for women with epilepsy–focus on pregnancy (an evidence-based review): Vitamin K, folic acid, blood levels, and breastfeeding. Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009c;73:142–9. doi: 10.1212/WNL.0b013e3181a6b325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Holmes LB, Harvey EA, Coull BA, Huntington KB, Khoshbin S, Hayes AM, et al. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001;344:1132–8. doi: 10.1056/NEJM200104123441504. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–60. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Katz I, Kim J, Gale K, Kondratyev A. Effects of lamotrigine alone and in combination with MK-801, phenobarbital, or phenytoin on cell death in the neonatal rat brain. J Pharmacol Exp Ther. 2007;322:494–500. doi: 10.1124/jpet.107.123133. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kondratyev A, Tomita Y, Gale K. Neurodevelopmental impact of antiepileptic drugs and seizures in the immature brain. Epilepsia. 2007;48 (Suppl. 5):19–26. doi: 10.1111/j.1528-1167.2007.01285.x. [DOI] [PubMed] [Google Scholar]
- Kluger BM, Meador KJ. Teratogenicity of antiepileptic medications. Semin Neurol. 2008;28:328–35. doi: 10.1055/s-2008-1079337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KH. Imputation using Markov chains. J Stat Comput Simul. 1988;30:57–79. [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. 2nd. New York: John Wiley & Sons Inc.; 2002. [Google Scholar]
- Manthey D, Asimiadou S, Stefovska V, Kaindl AM, Fassbender J, Ikonomidou C, et al. Sulthiame but not levetiracetam exerts neurotoxic effect in the developing rat brain. Exp Neurol. 2005;193:497–503. doi: 10.1016/j.expneurol.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1016–26. doi: 10.1016/S0140-6736(07)60461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador K, Reynolds MW, Crean S, Fahrbach K, Probst C. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1–13. doi: 10.1016/j.eplepsyres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1597–605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Coppola DM, Ramoa AS. Neonatal alcohol exposure induces long-lasting impairment of visual cortical plasticity in ferrets. J Neurosci. 2003;23:10002–12. doi: 10.1523/JNEUROSCI.23-31-10002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Ramoa AS. Early alcohol exposure induces persistent alteration of cortical columnar organization and reduced orientation selectivity in the visual cortex. J Neurophysiol. 2005;93:1317–25. doi: 10.1152/jn.00714.2004. [DOI] [PubMed] [Google Scholar]
- Nelson H, Willison J. National adult reading test (NART) Oxford, England: NFER-Nelson Publishing; 1991. [Google Scholar]
- Rosenbaum P, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- Sattler JM. Assessment of children. 3rd. San Diego: Jerome M. Sattler Pub. Inc.; 1992. [Google Scholar]
- Schafer JL. Analysis of incomplete multivariate data. New York: Chapman and Hall; 1997. [Google Scholar]
- Stefovska VG, Uckermann O, Czuczwar M, Smitka M, Czuczwar P, Kis J, et al. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol. 2008;64:434–45. doi: 10.1002/ana.21463. [DOI] [PubMed] [Google Scholar]
- Thomas SV, Ajaykumar B, Sindhu K, Nair MK, George B, Sarma PS. Motor and mental development of infants exposed to antiepileptic drugs in utero. Epilepsy Behav. 2008;13:229–36. doi: 10.1016/j.yebeh.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Thomas SV, Sukumaran S, Lukose N, George A, Sarma PS. Intellectual and language functions in children of mothers with epilepsy. Epilepsia. 2007;48:2234–40. doi: 10.1111/j.1528-1167.2007.01376.x. [DOI] [PubMed] [Google Scholar]
- Vinten J, Bromley RL, Taylor J, Adab N, Kini U, Baker GA. The behavioral consequences of exposure to antiepileptic drugs in utero. Epilepsy Behav. 2009;14:197–201. doi: 10.1016/j.yebeh.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WISC-R Käsikirja (Wechsler Intelligence Scale for Children—revised, Manual for the Finnish version) Helsinki: Psykologien Kustannus Oy; 1984. [Google Scholar]
- Wechsler D. The Wechsler intelligence scale for children. 3rd. San Antonio, Texas: The Psychological Corporation; 1991. [Google Scholar]
- Wechlser D. WPPSI-R Käsikirja (Wechsler Preschool and Primary Scales of Intelligence-revised, Manual for the Finnish adaptation) Helsinki: Psykologien Kustannus Oy; 1995. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence (WASI) San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Williams G, King J, Cunningham M, Stephan M, Kerr B, Hersh JH. Fetal valproate syndrome and autism: additional evidence of an association. Dev Med Child Neurol. 2001;43:202–6. [PubMed] [Google Scholar]
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale, Fourth Edition (PLS-4) 3rd. San Antonio, TX: Pearson Educatoin Inc.; 2002. [Google Scholar]
- Zito JM, Safer DJ, Gardner JF, Soeken KS, Ryu J. Anticonvulsant treatment for psychiatric and seizure indications among youths. Psychiatr Serv. 2006;57:681–5. doi: 10.1176/ps.2006.57.5.681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.