Abstract
For the past 150 years, neurobiological models of language have debated the role of key brain regions in language function. One consistently debated set of issues concern the role of the left inferior frontal gyrus in syntactic processing. Here we combine measures of functional activity, grey matter integrity and performance in patients with left hemisphere damage and healthy participants to ask whether the left inferior frontal gyrus is essential for syntactic processing. In a functional neuroimaging study, participants listened to spoken sentences that either contained a syntactically ambiguous or matched unambiguous phrase. Behavioural data on three tests of syntactic processing were subsequently collected. In controls, syntactic processing co-activated left hemisphere Brodmann areas 45/47 and posterior middle temporal gyrus. Activity in a left parietal cluster was sensitive to working memory demands in both patients and controls. Exploiting the variability in lesion location and performance in the patients, voxel-based correlational analyses showed that tissue integrity and neural activity—primarily in left Brodmann area 45 and posterior middle temporal gyrus—were correlated with preserved syntactic performance, but unlike the controls, patients were insensitive to syntactic preferences, reflecting their syntactic deficit. These results argue for the essential contribution of the left inferior frontal gyrus in syntactic analysis and highlight the functional relationship between left Brodmann area 45 and the left posterior middle temporal gyrus, suggesting that when this relationship breaks down, through damage to either region or to the connections between them, syntactic processing is impaired. On this view, the left inferior frontal gyrus may not itself be specialized for syntactic processing, but plays an essential role in the neural network that carries out syntactic computations.
Keywords: aphasia, Broca’s area, syntax, language networks, stroke
Introduction
Since Broca’s claim that language is a special faculty, instantiated in a specialized neural system primarily involving the left inferior frontal gyrus and posterior temporal cortex (Broca, 1861; Dronkers et al., 2004; Vigneau et al., 2006), there has been continued and heated debate about the precise role of the left inferior frontal gyrus in language. This debate focuses on core Broca’s area, defined as Brodmann area (BA) 45 and 44, and adjacent BA 47, and asks whether all or any of these regions are critical for the key linguistic function of syntactic parsing (Grodzinsky, 2000). The variation in tasks and stimuli used in previous studies of syntax may, in part, account for the lack of consensus on exactly which regions of the left inferior frontal gyrus are activated for syntactic processing, and how necessary this activation is to support syntactic function in general. Depending on the study, activation has been reported in BA 44 (Friederici et al., 2003), BA 45 (Hashimoto and Sakai, 2002; Marcus et al., 2003; Musso et al., 2003), BA 47 (Peelle et al., 2004; Caplan et al., 2008a), and both BA 44 and 45 (Dapretto and Bookheimer, 1999; Embick et al., 2000; Caplan et al., 2003; Kovelman et al., 2008; Weber and Indefrey, 2009; Tyler et al., 2010a). Moreover, it has also been argued that regions within the left inferior frontal gyrus support other linguistic functions (such as phonology, morphology and semantics; Bookheimer, 2002; Hagoort, 2005; Tyler et al., 2005b; Marslen-Wilson and Tyler, 2007) and that it plays a more general role in supporting cognitive functions that are not specific to language (Miller, 2000), such as memory retrieval, cognitive control mechanisms or processes of selection and/or competition (Thompson-Schill et al., 1999; Moss et al., 2005).
Classic neuropsychological data from aphasic patients have not been able to resolve this issue because of the absence of reliable lesion-deficit mapping (Dronkers et al., 2004). Functional imaging evidence for the involvement of the left inferior frontal gyrus in syntactic processing has been challenged on the grounds that studies typically involve task and stimulus demands that may activate brain regions that overlap with those involved in linguistic computations, making it difficult to differentiate between linguistic and non-linguistic processes (Kaan and Swaab, 2002). This is especially problematic for syntactic processing, where frontal cortex, including the left inferior frontal gyrus, is known to be activated both by linguistic variables and by task-related cognitive requirements. In a recent study where subjects listened to spoken stimuli and either made a lexical decision response to each stimulus or simply listened without making an overt response, we found that task effects and linguistic effects show up in closely adjacent regions in the left inferior frontal gyrus (Wright et al., 2011). Linguistic manipulations generated activity in left BA 44 whereas the lexical decision task was linked to activity in left BA 47. By not including an overt task in the current functional MRI study, we aim to avoid the potential confounds this may introduce.
In the present study, we ask directly whether the left inferior frontal gyrus plays a necessary role in syntactic parsing by combining evidence from functional imaging and the effects of brain lesions and relating both to performance. This allows much stronger inferences to be drawn about the brain regions that are essential for the performance of a given neurocognitive process (Chatterjee, 2005; Fellows et al., 2005; Price et al., 2006). Two complementary types of neuroimaging evidence were brought into play, cortical activation measured by functional MRI and correlational voxel-based lesion/behaviour analyses based on structural MRI. These are combined with data from behavioural manipulations known to elicit syntactic processing, applied to a neuropsychological population of chronic patients with left hemisphere damage and to matched controls. Focusing on the question of whether the left inferior frontal gyrus, especially BA 44, 45 and 47, plays an essential role in syntactic processing, this combination of methods allows us to determine not only which brain regions and networks are active during syntactic analysis but also whether their engagement is essential for preserved function.
To implement this approach, we carried out a functional MRI study that included manipulations designed to focus on the processing of syntax, which we anticipated, given previous studies, would elicit in healthy participants a network of left hemisphere fronto-temporal-parietal activity including Broca’s area (Binder et al., 1997; Keller et al., 2001; Friederici, 2002; Kaan and Swaab, 2002; Demonet et al., 2005; Caplan et al., 2008b; Tyler and Marslen-Wilson, 2008; Rodd et al., 2010; Tyler et al., 2010a). This network would provide a baseline for evaluating the effect of left hemisphere brain damage on functional activation associated with syntactic processing in the patient group, bringing together functional, structural and behavioural data. As noted above, participants listened to spoken sentences without performing an explicit task (Scott et al., 2000; Crinion et al., 2003; Coleman et al., 2007; Davis et al., 2007; Vannest et al., 2009) in order to avoid the potential confounds due to extra-linguistic task demands.
The key syntactic manipulation in the functional MRI study was the use of sentences that contained syntactic ambiguities, which are a normal and frequent aspect of human language, together with matched unambiguous sentences. These ambiguities consisted of phrases such as ‘bullying teenagers’, which occurred in sentences such as ‘The newspaper reported that bullying teenagers…’ where either ‘teenagers’ or ‘bullying’ can be the head of the ambiguous phrase. The ambiguous phrase was disambiguated by the verb that immediately followed it (e.g. ‘… are a problem for the local school’ or ‘… is bad for their self-esteem’). In functional MRI studies on healthy subjects, using similar stimuli in the same passive listening paradigm, we found that syntactically ambiguous sentences activated the left inferior frontal gyrus (including BA 44, 45 and 47) and the middle and posterior portions of the left middle temporal gyrus compared with matched unambiguous sentences (Rodd et al., 2010). Results such as these show that regions of the left inferior frontal gyrus are engaged during syntactic processing, but not whether their involvement is essential. By carrying out a similar functional MRI study on a group of chronic left hemisphere patients (with varying degrees of syntactic impairment) we can address this question directly, using correlational techniques to delineate the core neurocognitive networks critical for intact syntactic performance.
To be able to relate patients’ impairments directly to their performance in the functional MRI study and to their structural magnetic resonance analyses, as well as to confirm that these impairments were specifically syntactic in nature, we obtained complementary behavioural data from three different tests of syntactic function conducted outside the scanner. The first test used an acceptability judgement task involving the same stimuli as in the functional MRI experiment. This provided a behavioural assessment of each patient’s ability to syntactically interpret the ambiguous phrases, which could be correlated both with neural activity and with neural integrity.
Two further tests provided entirely independent measures of the patients’ syntactic function. The first of these was a sentence-picture matching task using semantically reversible sentences. This is a standard measure that has been used extensively to test for syntactic impairments (Caramazza and Zurif, 1976; Saffran et al., 1980; van der Lely and Harris, 1990; Berndt et al., 1996, 2004). Participants hear a spoken sentence and choose the picture that matches the sentence out of a three-picture array. The sentences are all semantically ‘reversible’ in that either entity can perform the action specified in the sentence (e.g. ‘The boy kissed the girl’) so that participants must rely on syntactic cues to interpret the sentences correctly. The second additional measure of syntactic performance, also obtained from patients in a separate testing session, was a word-monitoring task in which patients pressed a response key when they heard a pre-specified target word in a spoken sequence. The spoken sequences differentially loaded on syntax and semantics, providing a measure of each patient’s ability to conduct successful on-line sentential syntactic and semantic analyses (Marslen-Wilson and Tyler, 1980; Tyler et al., 2010b).
Patients’ performance on both these tasks was also related, using correlational techniques, to neural activity in the functional MRI study and to structural magnetic resonance analyses of whole brain neural integrity. This combination of inputs from the three behavioural tasks with the two types of neural measure is a strong test of the central hypothesis that there is a core left perisylvian network that supports syntactic function in the undamaged adult brain. Any form of brain damage that disrupts the functioning of this network will affect performance on any task where correct responses depend on the successful syntactic analysis of the relevant linguistic inputs. This holds independently of the specific operations required to perform each task and implicates instead the shared processing substrate tapped into by each task. On this basis, we expect that decreased tissue integrity as a result of brain damage in specific regions—most likely in the left inferior frontal gyrus—should correlate with poorer syntactic processing, as measured in each of these tests of syntactic function. The presence or absence of damage in these syntax-critical regions should also correlate with changes in functional activity during the processing of syntactically ambiguous sentences, since these put a load on syntactic analysis.
The hypothesis that the left inferior frontal gyrus is critically involved in syntactic processing also makes key predictions for activity outside the left inferior frontal gyrus, both in other left hemisphere regions and in homologous right hemisphere regions, as measured in the functional MRI context. If patients’ processing of syntactically ambiguous sentences activates neighbouring or homologous regions that are not activated in controls—and which therefore do not support syntactic function in the intact brain—then activity in these regions should not be associated with preserved syntactic processing. Such activity may be generally compensatory and may improve overall communicative function, but will not reinstate purely syntactic functionality (Tyler et al., 2010a). If, in contrast, the left inferior frontal gyrus is not uniquely essential for syntactic processing, then damage here may generate additional activity in neighbouring or homologous regions that is correlated with preserved syntactic processing. This would show that syntactic functions can indeed successfully reorganize when the left inferior frontal gyrus is damaged.
Materials and methods
Participants
Patients were recruited from the Centre for Speech, Language and the Brain’s panel of volunteers and from local stroke groups. All patients had been discharged from hospital, were stable at the time of testing and were tested a minimum of 1.5 years post-stroke (85% were tested 3.5 years or more post-stroke; mean 7 years). Patient selection was based upon the following criteria: ability to give informed consent and understand task instructions, native language was British English, lesions only involved the left hemisphere, right-handed prior to stroke and no MRI contraindications. These criteria were met in 14 patients (three female) aged 34–77 years (mean 56 years), who participated in the study after giving informed consent (Suffolk Research Ethics Committee). In 13 patients, lesions were caused by stroke and in one patient by post-surgical haematoma. Across patients, damage covered a wide area of the left hemisphere including left inferior and middle frontal gyri, superior and middle temporal gyri, superior and inferior parietal lobule, insula and basal ganglia. We tested 15 healthy control participants (eight female) aged 46–74 years (mean 58 years), who gave informed consent (Suffolk Research Ethics Committee). All were right-handed native British English speakers with no history of neurological illness or head injury and were free of psychiatric illness or psychoactive medication for at least 1 year prior to scanning. No participant (patient or control) had audiometer measurements indicating severe hearing impairment and none were cognitively impaired [25 or higher on Mini-Mental State Examination and/or above 25th percentile for adults aged 55–64 on Ravens Coloured Progressive Matrices, (a score of 26/36 or higher; Raven, 1995)].
The patients were not selected on the basis of the presence/absence or type of language deficit. Rather than looking for a specific level of deficit, we were looking for variation across patients in their level of performance. Their language function was subsequently tested in a variety of tests probing phonology, semantics, lexical processing and syntax. As Table 1 shows, although most patients were able to process the phonology and semantics of words without difficulty, many had problems in processing syntax. Their syntactic error rates ranged from 0–47% in the sentence-picture matching test but they made very few semantic errors (0–6%). Many of the patients also had difficulties with a sentence grammaticality test where errors ranged from 0–42%.
Table 1.
Behavioural tests for patients (number of errors)
| Patient | Age | Sentence level comprehension |
Word level comprehension |
Production |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Sentence-picture matchinga (n = 34) |
Sentence grammaticalityb (n = 24) | Lexical decisionc (n = 20) | Phonological discriminationd (n = 20) | Semantic categorisatione (n = 20) | Sentence repetitionf (n = 10) | Word repetitionf (n = 0) | |||
| Syntactic errors | Lexical errors | ||||||||
| 1 | 46 | 16 | 2 | 7 | 0 | 0 | 0 | 10 | 0 |
| 2 | 37 | 14 | 0 | 3 | 0 | 1 | 0 | 3 | 0 |
| 3 | 55 | 13 | 1 | 9 | 2 | 3 | 0 | 6 | 0 |
| 4 | 72 | 12 | 2 | 6 | 1 | 9 | 0 | 9 | 3 |
| 5 | 57 | 10 | 0 | 4 | 1 | 0 | 0 | 5 | 0 |
| 6 | 64 | 5 | 0 | 6 | 3 | 1 | 0 | 10 | 0 |
| 7 | 77 | 4 | 1 | 1 | 2 | 1 | 0 | 0 | 1 |
| 8 | 65 | 4 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 9 | 35 | 4 | 0 | 5 | 1 | 0 | 0 | 0 | 0 |
| 10 | 54 | 2 | 1 | 10 | 1 | 1 | 0 | 0 | 0 |
| 11 | 64 | 2 | 0 | 2 | 3 | 0 | 0 | 0 | 0 |
| 12 | 61 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13 | 42 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 14g | 50 | 0 | 0 | – | 2 | – | – | – | – |
| Controls (mean) | 58 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Controls (SD) | 11 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
a Refer to text for more details on the sentence-picture matching task.
b Grammaticality judgement to spoken sentences.
c Word/non-word discrimination to spoken words.
d Same/different judgement to spoken word pairs (e.g. bat/bat versus bat/bad).
e Living/non-living discrimination of spoken concrete nouns.
f Repetition of spoken words/sentences.
g The patient did not return to complete these tests.
Stimuli and functional magnetic resonance imaging study
In the scanner, subjects listened to spoken sentences without performing an explicit task. The stimuli consisted of 42 sentence-pairs each of which contained a two-word phrase of the form [verb + ‘ing’ plural noun]. An example is the phrase ‘bullying teenagers’, heard in the context of either ‘The newspaper reported that bullying teenagers are a problem for the local school’ or ‘The newspaper reported that bullying teenagers is bad for their self-esteem’. These phrases were syntactically ambiguous between different syntactic roles. In the sentences above, ‘bullying teenagers’ can either be a noun phrase, functioning as the complex subject of the embedded clause ‘… are a problem for the local school’, or it can be a verb phrase where ‘bullying’ functions as a gerund and itself is the subject of the embedded sentence ‘… is bad for their self-esteem’. This ambiguity can only be resolved when the listener hears the verb that immediately follows the ambiguous phrase (here ‘is’ or ‘are’) and which is consistent with one interpretation or the other—though it is important to emphasize that both readings were fully acceptable and grammatical up to the point of disambiguation. Behavioural studies have shown that listeners are sensitive to the presence of this type of syntactic ambiguity, and this is reflected in responses to the disambiguating word when it follows an ambiguous phrase compared with an unambiguous phrase (Tyler and Marslen-Wilson, 1977).
There were also 42 syntactically unambiguous sentences matched in structure to the ambiguous sentences (e.g. ‘The teacher knew that “rehearsing plays” is necessary for a good performance’). The two words in the phrases and disambiguating words were matched in frequency (taken from CELEX; Baayen et al., 1995) across conditions. We also controlled for the animacy of the noun phrase and the duration of the sentences; half of the sentences in each condition were disambiguated with is/was or are/were to ensure that grammatical constructions were matched over conditions.
Even though the ambiguous phrases were presented in a neutral context, subjects have preferences for one syntactic interpretation over another, which affect the ease with which ambiguities can be resolved (Rayner and Duffy, 1986). We obtained preferences for our stimuli from a sentence completion pre-test in which 23 subjects, who did not take part in the functional MRI study, heard each sentence up to the end of the ambiguous phrase and were asked to write down a plausible continuation. Preference scores for the two possible interpretations of the ambiguous phrases were calculated as the proportion of the continuations consistent with that interpretation given by the pre-test participants; for the ambiguous items, the dominant interpretation was the one that received the highest preference score. Both dominant- and subordinate-interpretation sentences for each ambiguous phrase were included in the experimental stimuli (42 of each, in the dominant and subordinate conditions, respectively). The mean [standard deviation (SD)] preference scores of the ambiguous items were 84% (11%) for the dominant and 16% (11%) for the subordinate interpretation. The unambiguous sentences were all rated as unambiguous, i.e. they had a preference score of 100%.
The functional MRI paradigm also included 126 filler sentences which did not include the phrase [verb + ‘ing’ noun] and 42 baseline items consisting of acoustic stimuli that were constructed to share the complex auditory properties of speech without triggering phonetic interpretation. This was envelope-shaped ‘musical rain’ (Uppenkamp et al., 2006) in which the long-term spectrotemporal distribution of energy is matched to that of the corresponding speech stimuli. Stimuli were digitally recorded by a female native speaker of British English, and presented in the scanner via magnetic resonance compatible headphones. Stimulus presentation was cued using E-Prime v.2.0 (Psychology Software Tools, Inc., Pittsburgh, PA) running on a PC.
Stimuli were presented in a pseudorandom order during three separate sessions. There were equal numbers of items in each condition in each session, each lasting 12–13 min. Two versions of the stimuli were presented, such that for a given pair of items, if the subordinate form was presented before the dominant in version one, the dominant would be presented first in version two and vice versa. Apart from this, the item ordering was the same in both versions. Volunteers were assigned alternating versions. To improve the detectability of response in functional MRI, the interstimulus interval was jittered according to a geometric distribution with mean 3250 ms (range from 2000–7000 ms; Burock et al., 1998).
Behavioural post-tests
Ambiguity acceptability study
A minimum of 1 month from functional MRI scanning, participants were tested in a behavioural version of the imaging study, using the stimuli from the functional MRI study plus an additional 24 stimuli in each condition. Participants heard the sentences up to the end of the ambiguous phrase, spoken by a female speaker, each of which was followed by the disambiguating word spoken by a male speaker. Participants listened to each sentence and pressed a response button to indicate whether the disambiguating word was an acceptable continuation of the sentence fragment they had heard. Because all of the test sentences had acceptable continuations, 132 unambiguous sentences were included with clearly unacceptable continuations. Although there is a larger proportion of acceptable compared with unacceptable continuations, any potential response bias arising from this should decrease the proportion of unacceptable judgements to the subordinate sentences, thereby biasing against our prediction that this condition should generate the largest number of unacceptable responses. Stimulus presentation and recording of responses was carried out using E-Prime v.1.1.
Performance in this task provides a measure of participants’ sensitivity to syntactic information during the processing of a spoken sentence. When participants reject the disambiguating word as an acceptable continuation, they are indicating their sensitivity to a local, and temporary, syntactic ambiguity, which is disambiguated in a way that is inconsistent with the representation that they have incrementally developed up to that point.
Sentence-picture matching task
A second measure of syntactic function in sentence comprehension was obtained from a sentence-picture matching task (Ostrin and Tyler, 1995), in which a spoken sentence was presented, either in the active or the passive voice, which describes two participants engaged in an event (e.g. ‘The horse chases the boy’ or ‘The boy is chased by the horse’). Sentences were ‘semantically reversible’ in that either participant could perform the action and they varied in syntactic complexity. The subject’s task was to match the sentence to the appropriate picture out of an array of three pictures (all line drawings), only one of which was correct. The other two pictures contained either: (i) a lexical distractor involving a change of meaning, which always involved a change of verb (e.g. a picture of a boy riding a horse) or (ii) a reverse role distractor in which the agent of the action became its recipient (e.g. a picture of a boy chasing a horse). These foil pictures were included so that when a patient made reverse role errors and few lexical distractor errors, this indicated difficulties with syntax in the presence of intact semantics. There were 34 sentences, half in the active voice, and half in the passive voice. In keeping with previous neuropsychological research, we expected passive sentences to generate more reverse role errors since they cannot be interpreted using a canonical word order heuristic.
Word monitoring task
In this third study, we obtained a behavioural measure of the ability to construct online syntactic and semantic representations of spoken language. Participants were asked to press a response key when they heard a prespecified target word occurring in different kinds of spoken sequences which differentially load on syntactic or semantic information (see Supplementary material for details). Importantly, the word monitoring task is a task that patients with brain damage can reliably perform—eliciting fast reaction times and few errors—and involves minimal working memory demands (Friederici, 1985; Tyler, 1992; Tyler et al., 2010b).
Lesion detection
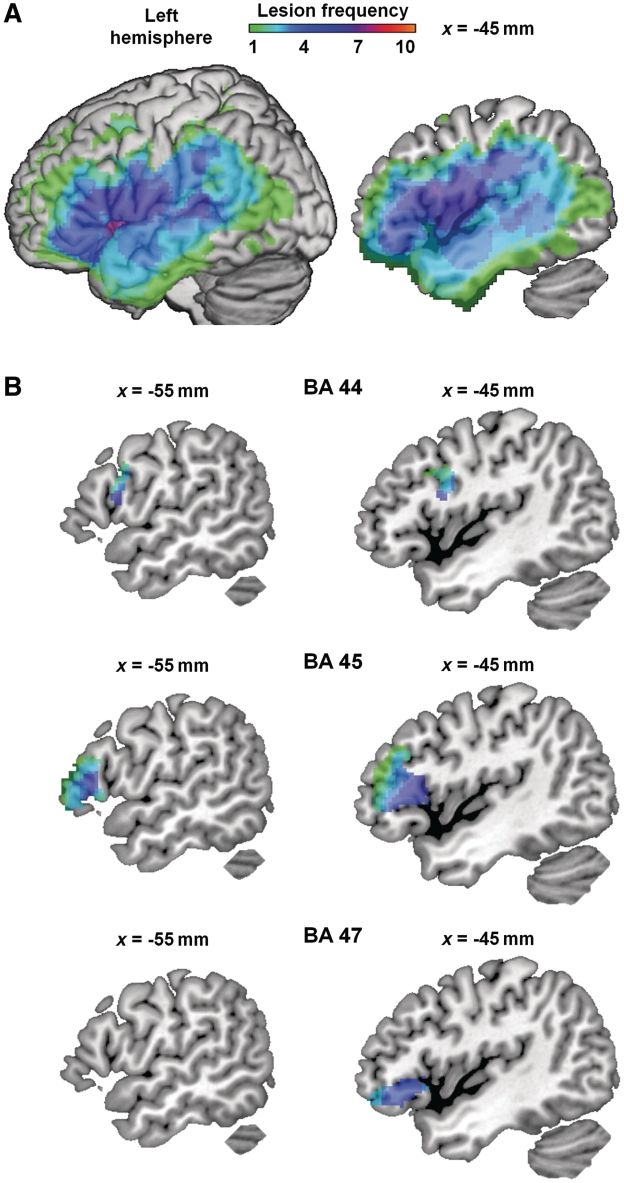
We identified damaged tissue using an automatic procedure (Stamatakis and Tyler, 2005). The normalized structural images were skull-stripped using the canonical brain mask in statistical parametric mapping (SPM), then smoothed using a Gaussian kernel of 10 mm full width half maximum. Each patient’s structural image was entered into a two-sample t-test with images from a set of age-matched controls, using non-sphericity correction for unbalanced group sizes. Voxels were identified as damaged if their intensity in the structural image (T1 signal) was significantly lower in the patients than controls (having accounted for global signal differences). The voxel-level and cluster size thresholds were adjusted on an individual basis to avoid enlarged sulci near intact tissue being classified as lesion. With this technique, we produced binarized lesion maps for each patient that were used to mask individual patients’ functional MRI data, but were not used in the lesion-deficit correlations. Figure 1A shows the lesion frequency map, which describes the extent and variability of lesions across the patients. Given our interest in the left inferior frontal gyrus, we also report the lesion frequency within each subregion of the left inferior frontal gyrus. We defined regions of interest in BA 44, 45 and 47 using the Brodmann atlas from MRIcron (http://www.cabiatl.com/mricro/mricro/lesion.html#brod; Drury et al., 1999). Lesion frequencies in each region are shown in Fig. 1B. The majority of voxels in each subregion were damaged in 4–6 patients, confirming a comparable distribution of damage across the three left inferior frontal gyrus subregions. The same regions were later used for region of interest analysis (see below).
Figure 1.
Lesion frequency map. (A) Whole-brain view. Across patients, damage covers left hemisphere regions including inferior and middle frontal gyri, superior and middle temporal gyri, superior and inferior parietal lobules, insula and basal ganglia. Colour indicates number of patients with damage at each voxel. Left = surface of left hemisphere. Right = sagittal section at MNI x = −45 mm. (B) Lesion frequency shown in separate subregions of left inferior frontal gyrus: BA 44, 45 and 47. Damage was comparable between subregions, with most voxels having a lesion frequency of 4–6. Regions were defined using the Brodmann atlas from MRIcron. Sagittal sections at MNI x = −45 mm (left column), slightly more medial sagittal sections at MNI x = −55 mm (right column).
Imaging methods and analysis
Participants were scanned at the Medical Research Council Cognition and Brain Sciences Unit, Cambridge with a Siemens 3T Tim Trio MRI scanner (Siemens Medical Solutions, Camberley, UK). Functional images comprised 32 oblique axial slices angled away from the eyes, each 3 mm thick with interslice gap of 0.75 mm and in-plane resolution of 3 mm and field of view = 192 mm × 192 mm. Repetition time = 2 s, echo time = 30 ms and flip angle = 78°. We acquired T1-weighted structural images at 1 mm isotropic resolution in the sagittal plane, using an MPRAGE sequence with repetition time = 2250 ms, inversion time = 900 ms, echo time = 2.99 ms and flip angle = 9°.
Pre-processing of the functional MRI data (using SPM5 software, Wellcome Institute of Imaging Neuroscience, London, UK) comprised realignment, spatial normalization and spatial smoothing. Movement parameters (translations and rotations in x, y and z directions) were included as nuisance variables in the model to account for residual movement effects. Spatial normalization used unified normalization, which combines grey matter segmentation with non-linear warping of the image to a template in Montreal Neurological Institute (MNI) space (Ashburner and Friston, 2005). In patients, normalization used a high warping regularization value of 100 to prevent the algorithm from warping the lesion, an approach that has produced reliable normalization in previous studies on patients and has been shown to be more reliable than the alternative method of cost function masking (Tyler et al., 2005a; Crinion et al., 2007). In one patient, increased regularization prevented the algorithm fitting the image to the template, so images from this patient were renormalized using standard regularization and cost function masking (Brett et al., 2001). Spatial smoothing was applied using a Gaussian kernel of 8 mm full-width half maximum (Friston et al., 2007).
We mapped neural responses using a general linear model in SPM5. The model comprised predicted response time series to stimuli in each experimental condition, the six movement parameters calculated during realignment and a high-pass filter with a cut-off of 128 s. We designed the model to maximize sensitivity to ambiguity by testing only the period during the sentence at which the effects of ambiguity would occur—i.e. in the second half of the sentence following the ambiguous phrase. To do this, we defined the onset of each condition separately as the offset of the ambiguous phrase and then included an extra variable of no interest with onset at the start of the sentence and duration up to the end of the ambiguous phrase, combining all sentence types. This model tested for effects in the second half of the sentence while controlling for effects in the first half of the sentence. Unambiguous sentences and filler items were modelled as separate conditions. For these conditions the second half of the sentence was defined according to similarities in sentence structure with the ambiguous sentences. In each subject, the model was applied to the time series at each voxel in the brain image, yielding a parameter estimate for each experimental condition. The differences between pairs of parameter estimates were calculated, giving a map of differences between experimental conditions, or contrast image.
To test for effects in each group, individual subject’s contrast images were entered into second-level analyses in SPM. In individual patients, voxels identified as damaged (refer to ‘Lesion detection’ section) were set to zero in the contrast images before these were entered into the group analysis. This maximizes available information by excluding damaged voxels from the group analysis on a patient-by-patient basis. The group-level statistical parametric maps were constrained using a voxel-level minimum statistic threshold, and a cluster size threshold. In order to balance false positive detection with reduced signal-to-noise in data from mature and brain-damaged individuals (D’Esposito et al., 2003) we used thresholds at voxel-level P < 0.01 uncorrected and cluster-level P < 0.05 corrected (except where noted). Based on previous evidence (Jung-Beeman, 2005; Vigneau et al., 2006; Rodd et al., 2010) we focused on bilateral fronto-temporo-parietal regions as the volume of interest for the analyses, including inferior frontal gyrus, superior and middle temporal gyri, transverse temporal gyrus, insula, supramarginal gyrus, angular gyrus and inferior parietal lobule, as defined by the Talairach Daemon atlas (Lancaster et al., 2000) using the Wake Forest University PickAtlas toolbox for SPM (Maldjian et al., 2003). These regions have consistently been identified as being involved in spoken language function (Dronkers et al., 2004; Vigneau et al., 2006; Hickok and Poeppel, 2007). For each cluster, peak voxel locations are reported in MNI coordinates. The anatomical extent of each cluster was identified using the Automatic Anatomic Labelling tool for SPM (Tzourio-Mazoyer et al., 2002) and the Brodmann atlas implemented in MRIcron (http://www.cabiatl.com/mricro/mricro/lesion.html#brod; Drury et al., 1999).
Correlations between performance and activity
We used voxel-wise correlations to test which brain regions were associated with syntactic function in patients. Because the patients showed a range of performance and damage, standard functional MRI contrasts looking at group mean activation are likely to be insensitive to highly variable activity in key areas. We exploited this variability by testing for correlations between performance and activity. We correlated activity with the percent of unacceptable judgements in the acceptability task, given the variability of reaction times for patients. In the ambiguity study, sensitivity to syntax was indicated by a greater difference in unacceptable responses between syntactically ambiguous and unambiguous sentences, and between subordinate and dominant conditions. Correlations were tested using the group-level multiple regression in SPM, with each performance measure being regressed voxel-by-voxel onto corresponding contrast images (e.g. ambiguous-unambiguous). Results were subject to the same statistical thresholds as one-sample t-tests.
Structure-function relationships
We have shown in earlier research that voxel-based correlational methods, which correlate continuous measures of neural tissue integrity (in both grey and white matter) with continuous measures of behavioural performance, are remarkably sensitive to brain-behaviour relationships (Tyler et al., 2005a; Bright et al., 2007; Taylor et al., 2009). We applied this method to the current dataset to investigate structure-function relationships by correlating performance on each of the behavioural measures of syntactic processing with T1 signal using voxel based statistics (Tyler et al., 2005a). Ischaemic and surgical lesions typically affect both grey and white matter, leading to reduced T1 signal in affected voxels.
Normalized, skull-stripped, smoothed T1 structural images (refer to ‘Lesion detection’ section) were entered into multiple regression analyses with behavioural scores as the variable of interest. We controlled for scan-to-scan variability in T1 signal by including the global mean as a confound. The resulting statistical parametric maps showed the significance of regional correlations between tissue integrity (T1 signal) and performance. The significance of correlated clusters was calculated as for functional MRI.
Region of interest analysis in left inferior frontal gyrus
A main focus of our research was to investigate the contribution of different subregions of the left inferior frontal gyrus to syntactic processing. Since patients who have a selective lesion in BA 44, 45 or 47 are rare, we investigated differences between subregions of the left inferior frontal gyrus at the group level using region of interest analysis. Since voxel-wise analysis includes correction for multiple comparisons and is more conservative than region of interest analysis (Poldrack, 2007), we report our region of interest analysis as an extension of our main voxel-wise analysis. Regions of interest were defined in BA 44, 45, and 47 using the Brodmann atlas from MRIcron (http://www.mricro.org/mricro/lesion.html#brod). We first ensured that damage within each of these regions was fairly evenly distributed across our patient group to establish that the power of tissue integrity analyses for each region would be comparable (refer to ‘Lesion detection’ section). We then extracted data from each region of interest using the Marsbar toolbox for SPM (Brett et al., 2002) to take the mean value of all voxels in each region. Values for activity were taken from contrast images and values for tissue integrity from T1-weighted structural images. Structural data were adjusted by regressing out the global mean. We then carried out Pearson correlations on the extracted data to examine the relationships between structure, function and performance.
Results
Behavioural data
Behavioural data were collected from three different tasks, to provide complementary measures of the range of variation in syntactic impairment across patients. This variation in performance could then be related to measures of neural activity and neural integrity across patients.
Ambiguity acceptability judgements
We obtained measures of syntactic function from each participant’s responses in the acceptability judgement task, noting the number of unacceptable judgements in each condition. This was to enable variation in syntactic performance to be related not only to voxel-by-voxel neural integrity, based on structural MRI data, but also to functional brain activity as measured using functional MRI. The focus here was not on overall differences between control and patient groups, but on characterizing the range of syntactic impairment exhibited by the patient group.
The control group (Table 2) show a strong effect of condition [F(2,28) = 43.92, P < 0.001], with a high rate of unacceptable judgements for the continuations in the subordinate condition, a much lower rate in the dominant condition, and almost no unacceptable judgements in the unambiguous condition (a similar pattern was also observed for the reaction times). This strongly differentiated pattern across conditions indicates the effectiveness of syntactic cues in driving the processing behaviour of the controls, and their sensitivity to syntax. They initially base their analysis on the dominant syntactic interpretation (e.g. ‘bullying teenagers’ as a subject noun phrase) and then have to revise this interpretation when the disambiguating word (e.g. ‘is’) turns out to force an interpretation that is inconsistent with the dominant reading and consistent with the subordinate reading. This requirement to reinterpret leads to many items being judged as unacceptable.
Table 2.
Behavioural data from the acceptability test (percent unacceptable judgements)
| Percent unacceptable judgements | SD | |
|---|---|---|
| Controls | ||
| Unambiguous | 2.9 | 5.03 |
| Subordinate | 42.4 | 22.33 |
| Dominant | 8.8 | 7.75 |
| Patients | ||
| Unambiguous | 20.1 | 32.75 |
| Subordinate | 36.2 | 27.59 |
| Dominant | 26.0 | 32.25 |
The patients show a much higher level of unacceptable judgements overall (Table 2). Although as a group they show a significant effect of condition [F(2,26) = 11.47, P < 0.001), there was a less clear cut differentiation between the rate of unacceptable judgements in the subordinate conditions than in the dominant or unambiguous conditions compared with controls (Table 2). The difference in rate of unacceptable judgements between subordinate and unambiguous sentences was larger in controls (39.5%) than patients [15%; F(1,27) = 10.48, P = 0.003], as was the difference between subordinate and dominant sentences [controls: 33.6%; patients: 10%, F(1,27) = 14.14, P < 0.001]. This reduced differentiation between conditions, which varies markedly within the patient group, reflects individual patients’ difficulty with syntactically-based processing. For subsequent analyses involving neural measures we summarized their performance by calculating the difference in percent of unacceptable judgements between subordinate and dominant sentences (dominance effect) and between ambiguous and unambiguous sentences (ambiguity effect). High numbers of unacceptable judgements to ambiguous items but few to unambiguous items is consistent with the effective processing of the syntactic cues present in the utterance. Similarly, higher rates of unacceptable judgements for subordinate than dominant sentences also reflect sensitivity to syntax. This is the pattern shown by the controls (Table 2). To the extent that a patient has intact syntactic processing capacities, they will similarly make more unacceptable judgements to subordinate than dominant sentences and to ambiguous than unambiguous sentences. To the extent that they have disrupted syntactic function, there should be less difference between ambiguous versus unambiguous sentences, since they may not be able to achieve sufficient syntactic analysis of the ‘verb + ing noun’ phrase (in either condition) to detect whether it is ambiguous or not.
Sentence-picture matching test
This test, using semantically reversible sentences, provides a standard measure of syntactic impairment that is independent of the other tests of syntactic processing function used with the patients. The performance of the controls was almost error-free, with a maximum of two out of 34 syntactic errors (reverse role errors) and no lexical distractor errors (see Table 1 for scores on this task). The patients made between 0 and 16 (out of 34) reverse role errors, most of which were on passive sentences (mean = 8.9; SD = 8.9) and few in active sentences (mean = 3.3, SD = 3.9). They only rarely made a lexical distractor error (most making one error or less). This confirms that the patients with left hemisphere damage varied in their degree of syntactic impairment, and identified the individuals with better or worse performance. Moreover, reverse role errors correlated with performance on the acceptability judgement task (difference in unacceptable judgements between syntactically ambiguous and unambiguous; r = −0.71, P < 0.01)—an important confirmation of consistency across tasks in identifying participants with greater or lesser syntactic impairments.
Word monitoring task
This test, using different types of sentence [normal prose, anomalous prose (syntactically correct, but semantically meaningless) and unrelated strings of words (random word order)] tests patients’ ability to process syntax in the absence of semantic support (see Supplementary material for details). Patients as a group showed a normal pattern of performance on normal prose sentences, indicating that they do not have a generalized language processing deficit. In contrast, their performance was abnormal on sentences that loaded on syntax (Supplementary Table 1), consistent with their performance on the sentence-picture matching and ambiguity studies.
Functional magnetic resonance imaging data
Controls
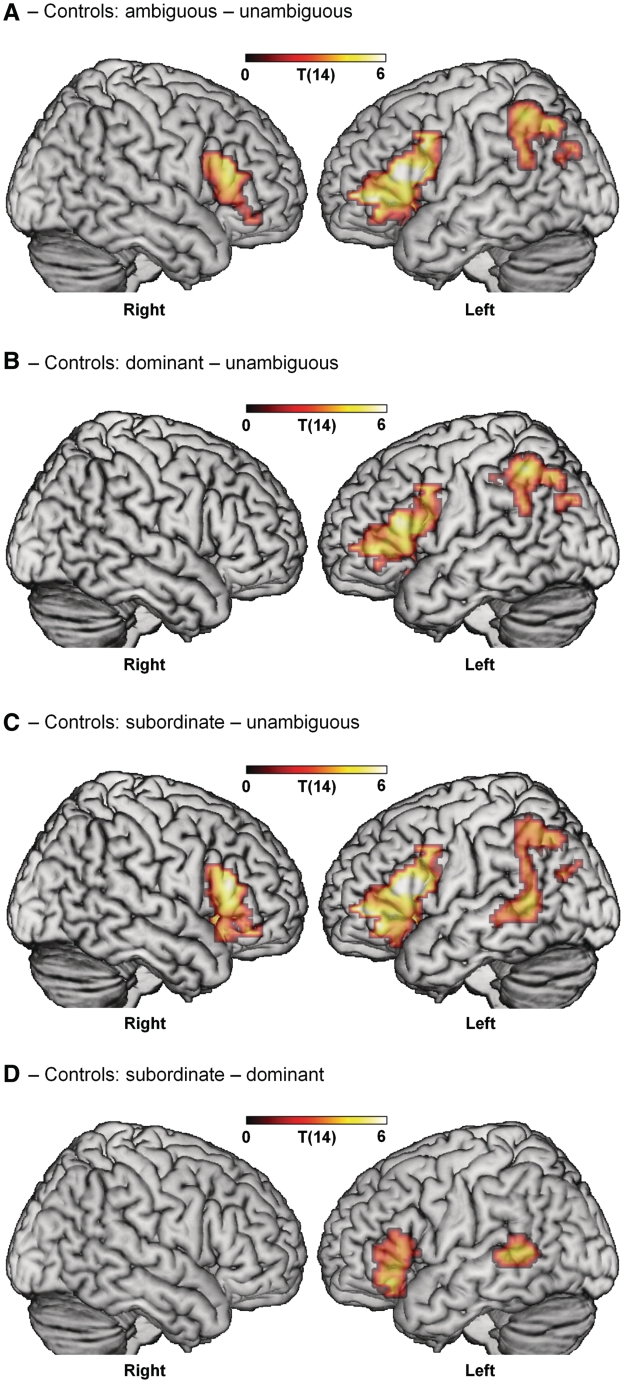
We first looked at the overall effect for sentences containing syntactic ambiguities compared with those that did not (Table 3, Fig. 2). Syntactic ambiguity generated bilateral inferior frontal gyrus activity, primarily in BA 45 (extending into BA 47 and 44 in the left hemisphere) and left inferior parietal lobule, angular gyrus and supramarginal gyrus (Fig. 2A). Activity in these regions was significantly correlated (all r ≥ 0.6, P < 0.05). Similar neural effects of syntactic ambiguity were seen in both the dominant and subordinate conditions. The contrast between dominant and unambiguous sentences produced increased activity primarily in left BA 45 (extending to BA 44 and 47) and left inferior parietal lobule, angular gyrus and supramarginal gyrus (Fig. 2B). When the disambiguating word was inconsistent with the initially preferred reading (in the subordinate sentences) additional activity was seen in right BA 45 (and 47) and left posterior middle temporal gyrus (Fig. 2C). No regions responded more strongly to unambiguous sentences. In previous studies, we have also found bilateral frontal activity in syntactic comprehension in older participants, with right inferior frontal gyrus activity correlated with decreasing integrity in left inferior frontal gyrus in the context of preserved syntax. We have argued that this may reflect functional compensation where right frontal activity can support (but does not replace) the functional role of the left inferior frontal gyrus in syntax in the face of age-related neural change (Tyler et al., 2010a).
Table 3.
Activation statistics for controls
| Contrast | Cluster |
Peak voxel |
||||
|---|---|---|---|---|---|---|
| Region | Pcorrected | Extent | x | y | z | Z-score |
| Ambiguous > unambiguous | ||||||
| LIFG BA 45 (47, 44) | <0.001 | 21.6 | −45 | 12 | 15 | 4.63 |
| RIFG BA 45 | 0.018 | 6.8 | 42 | 27 | 12 | 4.25 |
| LIPL/AG/SMG | 0.001 | 11.4 | −33 | −60 | 42 | 3.83 |
| Dominant > unambiguous | ||||||
| LIFG BA 45 (44, 47) | <0.001 | 15.3 | −48 | 24 | 18 | 4.16 |
| LIPL/AG/SMG | <0.001 | 12.0 | −45 | −48 | 51 | 3.94 |
| Subordinate > unambiguous | ||||||
| LIFG BA 45 (47, 44) | <0.001 | 22.5 | −48 | 12 | 15 | 4.74 |
| RIFG BA 45 (47) | 0.001 | 11.7 | 42 | 27 | 12 | 4.22 |
| LIPL/AG/SMG | 0.034 | 5.5 | −33 | −60 | 42 | 3.80 |
| LpMTG | 0.022 | 6.1 | −63 | −51 | 9 | 3.47 |
| Subordinate > dominant | ||||||
| LIFG BA 45 & 47 | 0.030 | 4.5 | −39 | 30 | −9 | 3.87 |
| LpMTG | 0.041 | 4.2 | −60 | −45 | 9 | 3.53 |
Cluster statistics corrected for multiple comparisons using random field theory. Extent given in cm3. Voxel-level threshold: P < 0.01 uncorrected. BA = Brodmann area; IPL/AG/SMG = inferior paretial lobule, angular gyrus and supramarginal gyrus; LIFG = left inferior frontal gyrus; LpMTG = left posterior middle temporal gyrus; RIFG = right inferior frontal gyrus. Parentheses indicate subsidiary extension of the main cluster.
Figure 2.
Effects of syntactic ambiguity in controls. (A) Controls show an overall effect of ambiguity in bilateral inferior frontal gyrus and left inferior parietal lobule, angular gyrus and supramarginal gyrus. (B) Sentences with the dominant continuation of the ambiguous phrase activate left inferior frontal gyrus and left inferior parietal lobule, angular gyrus and supramarginal gyrus only. (C) Sentences using the subordinate continuation activate bilateral inferior frontal gyrus, left inferior parietal lobule, angular gyrus, supramarginal gyrus and left posterior middle temporal gyrus. (D) Subordinate sentences elicit stronger activity than dominant in left inferior frontal gyrus and left posterior middle temporal gyrus. Voxel-level threshold P < 0.01; cluster-level threshold P < 0.05, corrected for multiple comparisons.
The strongest test of sensitivity to syntactic manipulations rests on the effects of syntactic dominance where activity is modulated by the strength of the preference for one reading over another, with increased left fronto-temporal activation correlating with increasing dominance (Rodd et al., 2010). In a direct comparison between the subordinate and dominant conditions (Fig. 2D), we found greater activation for subordinate compared with dominant sentences in left inferior frontal gyrus (BA 45 and 47) and left posterior middle temporal gyrus, replicating previous results (Rodd et al., 2010). Moreover, activity in these two regions was significantly correlated (r = 0.44, P < 0.05). This increased activation plausibly reflects the processes supporting the reinterpretation of the preferred (i.e. dominant) syntactic analysis when listeners encounter the disambiguating word, which triggers a re-analysis of the ambiguous pair of words (e.g. bullying teenagers) to find an alternative syntactic reading that is compatible with their lexical properties (Rodd et al., 2010).
In summary, the controls show that syntactic manipulations engage the left inferior frontal gyrus, left middle temporal gyrus and left inferior parietal lobule, angular gyrus and supramarginal gyrus with activity in these regions being significantly correlated. Within this network, the left inferior frontal gyrus and inferior parietal lobule cluster are maximally activated by the temporary presence of multiple syntactic representations. Although the specific functional role of the inferior parietal lobule and associated regions is not well understood, in this context it may reflect increased processing requirements involved in maintaining multiple representations. Syntactic dominance, which is associated with increased syntactic activity triggered by the need to reanalyse the ambiguous phrase, does not involve the left inferior parietal lobule but instead engages primarily left BA 45/47 and the left posterior middle temporal gyrus. These patterns of fronto-temporal-parietal activity for syntax seen in the controls provide a template against which to relate syntactic performance with functional MRI activity and tissue integrity in the patients.
Patients
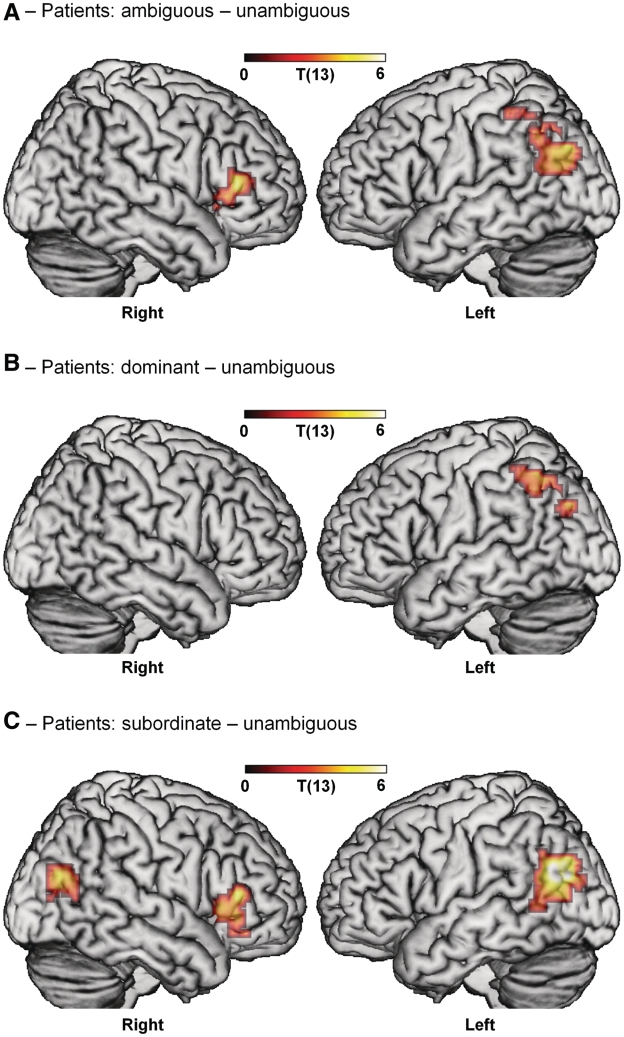
The group analyses for the patients, in contrast to the results for the controls, are poorly adapted to reveal the core set of left hemisphere regions that support syntactic processing, due to the variability of the patients’ syntactic performance. Unlike the controls, no significant activation is seen either in left inferior frontal gyrus or in left posterior middle temporal gyrus (Table 4 and Fig. 3A–C). As revealed in the group analyses, the regions that are activated in common by the patients most plausibly reflect, instead, those aspects of their sentence processing abilities that remain intact in the context of left hemisphere lesions that disrupt core syntactic function. In the overall comparison between syntactically ambiguous and unambiguous sentences, the patient group analyses show increased activity in left inferior parietal lobule, angular gyrus, supramarginal gyrus and right BA 45, extending into 47. These largely overlapped with the controls’ activity for this contrast (Table 3 and Fig. 2A), though without the strong left inferior frontal gyrus effects shown by the controls. A similar left inferior parietal lobule, angular gyrus and supramarginal gyrus cluster was seen for the dominant condition and an overlapping, more ventral region in left posterior middle temporal gyrus and angular gyrus for the subordinate condition (Fig. 3B and C). This pattern of effects suggests that for patients, like the controls, the presence of an ambiguous phrase activated multiple representations that may have placed increased demands on working memory. The patients also activated the right inferior frontal gyrus in the same ambiguous-unambiguous and subordinate-unambiguous contrasts as the controls. However, unlike the controls, the patients did not show any differences in activity for the subordinate compared with the dominant condition. No regions responded more strongly to unambiguous sentences.
Table 4.
Activation statistics for patients
| Contrast | Cluster |
Peak voxel |
||||
|---|---|---|---|---|---|---|
| Region | Pcorrected | Extent | x | y | z | Z-score |
| Ambiguous > unambiguous | ||||||
| RIFG BA 45 (47) | 0.031 | 4.4 | 48 | 33 | 9 | 3.91 |
| LIPL/AG/SMG | <0.001 | 10.2 | −36 | −72 | 33 | 3.64 |
| Dominant > unambiguous | ||||||
| LIPL/AG/SMG | 0.011 | 6.2 | −30 | −54 | 45 | 3.87 |
| Subordinate > unambiguous | ||||||
| LpMTG (AG) | <0.001 | 9.8 | −51 | −66 | 27 | 4.49 |
| RIFG BA 45 (47) | 0.014 | 4.6 | 48 | 33 | 9 | 3.75 |
| RpMTG | 0.031 | 3.8 | 36 | −72 | 21 | 3.71 |
Cluster statistics corrected for multiple comparisons using random field theory. Extent given in cm3. Voxel-level threshold: P < 0.01 uncorrected. Parentheses indicate subsidiary extension of the main cluster. AG = angular gyrus; LIPL = left inferior parietal lobule; LpMTG = left posterior middle temporal gyrus; RIFG = right inferior frontal gyrus; SMG = supramarginal gyrus.
Figure 3.
Effects of syntactic ambiguity in patients. (A) Patients show an overall effect of ambiguity in right inferior frontal gyrus (BA 45 extending to BA 47) and left inferior parietal lobule, angular gyrus and supramarginal gyrus. (B) Sentences using the dominant continuation of the ambiguous phrase activate left inferior parietal lobule, angular gyrus and supramarginal gyrus. (C) Sentences using the subordinate continuation activate right inferior frontal gyrus (BA 45 extending to BA 47) and bilateral posterior middle temporal gyrus (extending to angular gyrus). (Note: there were no significant differences between subordinate and dominant sentences in patients). Voxel-level threshold P < 0.01; cluster-level threshold P < 0.05, corrected for multiple comparisons.
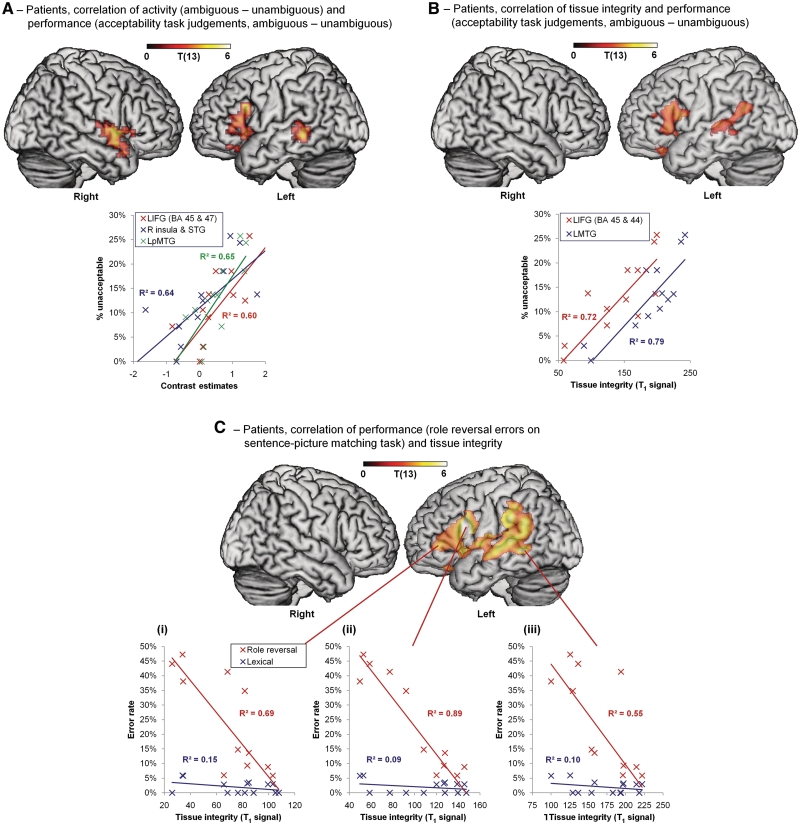
To use the patient data to determine the regions that are essential for syntactic processing, we need to turn instead to correlational analyses that relate performance measures of syntactic processing to activity and tissue integrity within the patient group and which exploit the variation across patients in lesion location, activity and performance. We therefore correlated activity for each patient with performance on the three behavioural measures of syntactic function. For the acceptability judgement task, we found that increasing sensitivity to syntactic ambiguity (i.e. more unacceptable judgements in ambiguous compared with unambiguous sentences) was associated with increasing activity primarily in left BA 45 (extending into BA 47) and right anterior superior temporal gyrus and insula (Table 5 and Fig. 4A). Performance also correlated with activation in left posterior middle temporal gyrus, an important region for language function, although this was not significant after correction for multiple comparisons. Intact functional connectivity between frontal and temporal regions has been claimed to be essential for successful syntactic processing (Caplan et al., 1996). These left hemisphere fronto-temporal regions—which did not show up in any of the patient group mean analyses—are similar to those shown in the controls for the syntactically-demanding subordinate-dominant contrast (Fig. 2). Complementing the results with the acceptability task, a similar pattern of fronto-temporal activation was found in correlations between neural activity in the ambiguity study and the other two behavioural measures—reverse role errors on the sentence-picture matching task (Supplementary Fig. 1) and syntactic performance in the word monitoring task (Supplementary Fig. 2).
Table 5.
Correlation statistics in patients
| Contrast | Cluster |
Peak voxel |
||||
|---|---|---|---|---|---|---|
| Region | Pcorrected | Extent | x | y | z | Z-score |
| Activity (ambiguous–unambiguous) by performance (unacceptable judgements in ambiguity acceptability task, ambiguous–unambiguous) | ||||||
| LIFG BA 45 (& 47) | 0.003 | 6.6 | −54 | 18 | 24 | 3.84 |
| RSTG & insula | 0.014 | 4.9 | 60 | 6 | 3 | 3.53 |
| LpMTG | [0.039] | 1.6 | −54 | −42 | −6 | 3.43 |
| Tissue integrity by performance (unacceptable judgements in continuation acceptability task, ambiguous–unambiguous) | ||||||
| LMTG | 0.015 | 7.3 | −56 | −29 | 5 | 3.29 |
| LIFG BA 47 | [0.042] | 2.4 | −27 | 30 | −16 | 3.23 |
| LIFG BA 45 (44 & 47) | <0.001 | 11.2 | −48 | 20 | 23 | 3.11 |
| Tissue integrity by performance (sentence–picture matching task, role reversal errorsa) | ||||||
| LIFG (BA 45, 44 & 47), pMTG, STG, insula, SMG | <0.001 | 69.3 | −65 | −18 | 18 | 4.95 |
Cluster statistics corrected for multiple comparisons using random field theory (results which are in italics and in square brackets indicate uncorrected cluster statistics). Extent given in cm3. Voxel-level threshold: P < 0.01.
aPartial correlation controlling for lexical errors. LIFG = left inferior frontal gyrus; pMTG = posterior middle temporal gyrus; SMG = supramarginal gyrus; STG = superior middle temporal gyrus. Parentheses indicate subsidiary extension of the main cluster.
Figure 4.
Correlations between performance, activity and tissue integrity in patients. (A) Activity in left inferior frontal gyrus (BA 45 extending to BA 47), right insula, superior temporal gyrus and left posterior middle temporal gyrus correlates with performance on the acceptability task (difference in unacceptable judgements between ambiguous and unambiguous sentences). Plot: performance over cluster mean activity for each region. (B) Tissue integrity in left inferior frontal gyrus (BA 45 and 47) and left posterior middle temporal gyrus correlates with performance on the acceptability task (as in A). Plot: performance over cluster mean tissue integrity for each region. (C) Tissue integrity in corresponding regions correlates with syntactic impairment on the sentence-picture matching task (partial correlation with role reversal errors controlling for lexical errors). Plots: performance over tissue integrity at voxels in left inferior frontal gyrus BA 45 (i; MNI −51, 39, 3), BA 44 (ii; MNI −54, 12, 20) and left posterior middle temporal gyrus (iii; MNI −59, −44, −2). All effects shown voxel-level P < 0.01, (A) and (B) cluster-level P < 0.05 uncorrected, (C) cluster-level P < 0.05 corrected. See ‘Results’ section for explanation of thresholds. LIFG = left inferior frontal gyrus; LpMTG = left posterior middle temporal gyrus; LMTG = left middle temporal gyrus; MNI = Montreal Neurological Institute coordinates; R = right; STG = superior temporal gyrus.
Although the patients were sensitive to the presence of a syntactic ambiguity, they were not sensitive to the effect of dominance; there were no significant correlations between activity and dominance scores (either subordinate or dominant). The lack of a dominance effect in the correlations between activity and behaviour reflects the reduced differentiation between subordinate and dominant conditions compared with the controls, which in turn reflects impairments in the patients’ capacity to compute well-differentiated syntactic analyses of sentential inputs.
Turning to the data from structural MRI, we looked at the relationship between damaged tissue and performance by correlating voxel-based measures of neural integrity using the T1 images with measures of syntactic performance (refer to ‘Structure-function relationships’ in ‘Imaging methods and analysis’ section). Increasing tissue integrity was associated with increasing sensitivity to syntax as measured by performance in the acceptability task in left BA 45 (extending into BA 44 and 47) and left posterior middle temporal gyrus, (Table 5 and Fig. 4B). Uncorrected cluster-level statistics revealed a second left inferior frontal gyrus cluster in left BA 47 that also correlated with performance. [We report this cluster in Table 5 and Fig. 4B to clarify the relationship between these voxel-wise analyses, which show only suprathreshold voxels, and the region of interest analyses (below), which were based on a priori anatomically-determined regions and are not subject to a statistical threshold.] A similar pattern was obtained when we used instead the different measure of syntactic performance provided by the sentence-picture matching task. The number of reverse role errors on this task correlated with tissue integrity in the entire left inferior frontal gyrus (but primarily BA 45), left superior temporal gyrus, insula and left posterior middle temporal gyrus, extending into left supramarginal gyrus (Fig. 4C). We confirmed these regions’ relationship with syntax by correlating tissue integrity with reverse role errors to active and passive sentences separately (Supplementary Fig. 3). The correlation in these regions is driven by passive sentences, which place greater reliance on syntax. These different analyses suggest that the left inferior frontal gyrus, most robustly left BA 45, is critically involved in syntactic comprehension, though almost always in conjunction with left posterior middle temporal gyrus.
It is notable that none of these correlational analyses, delineating the key areas for successful syntactic performance, implicated the posterior parietal regions that were prominent in the group analyses (Fig. 3). This is consistent with the view that while the left inferior parietal lobule does play a substantive role in sentence comprehension—perhaps, as several studies suggest, when multiple representations are temporarily active—it does not form part of the core left perisylvian network that must be present for syntactic functions to be spared.
Finally, we examined in detail the contribution of BA 44, 45 and 47 to activity and performance by correlating activity, tissue integrity and performance for the patients in regions of interest of each region. Activity for the ambiguous-unambiguous contrast correlated with performance in left BA 45 and 47, but not in 44 (BA 44: r = 0.35, P = 0.22; BA 45: r = 0.61, P < 0.05; BA 47: r = 0.69, P < 0.01). Increased tissue integrity significantly correlated with increased sensitivity to syntax as measured by ambiguous-unambiguous judgements in all three regions (all r ≥ 0.6, P < 0.05). There were no significant correlations between activity or tissue integrity and performance for the subordinate-dominant contrast. We also correlated tissue integrity in each of the three regions of interest with reverse role errors on the sentence picture matching task and found that increasing issue integrity in all three regions was significantly correlated with decreasing numbers of reverse role errors (all r > 6, P < 0.05).
Discussion
This study combined measures of syntactic comprehension, neural integrity and neural activity in patients with left hemisphere damage to determine which brain regions are essential for preserved syntactic comprehension, focusing on the left inferior frontal gyrus and its associated networks. This combination of functional imaging and lesion data from the same participants is necessary to support strong inferences about the causal role of patterns of neural activation in supporting a given neurocognitive function (Chatterjee, 2005; Fellows et al., 2005; Price et al., 2006; Tyler et al., 2010a). By using a task that minimized non-linguistic processing demands, we reduced the possibility of left inferior frontal gyrus (and related) activation being attributable to task-related activation that is unrelated to the linguistic manipulations of interest (Wright et al., 2011).
Is the left inferior frontal gyrus essential for preserved syntactic processing?
The starting point for our interpretation of the results is the demonstration that the left hemisphere patient group exhibits a range of severity in syntactic impairment, and that this variability, measured by the number of syntactic errors (reverse role errors) on the sentence-picture matching task, correlates strongly with tissue integrity in left inferior frontal gyrus, while also implicating functionally related regions in left posterior middle temporal gyrus. Patients with damage in left inferior frontal regions made greater numbers of reverse role errors primarily in the passive sentences, suggesting that they had difficulty determining the agent and recipient of the action in the sentences; a distinction that is made in semantically reversible sentences on the basis of syntactic information. In contrast, the patients showed no evidence of a more generalized auditory processing deficit; they made few semantic errors on the sentence-picture matching test and showed a normal word position effect in normal prose in the word monitoring task.
The key functional MRI result for the patients is not the group analysis, since this tells us what regions are commonly activated in each contrast, irrespective of the severity of each patient’s syntactic deficit. The critical functional MRI results are those that capitalize on the heterogeneity of the location of the patients’ damage and degree of syntactic deficit. It is these that allow us to relate performance to activity and tissue integrity. These correlational analyses all broadly converged on the same outcome—the involvement of the left inferior frontal gyrus in syntactic processing. First, we found that patients’ ability to process syntactic information appropriately was correlated with activity in left inferior frontal gyrus. Patients showing increased activation here were also more sensitive to the presence of a syntactic ambiguity with greater numbers of unacceptable judgements to ambiguous compared with unambiguous sentences. Second, increasing damage in the left inferior frontal gyrus was correlated with less sensitivity to syntactic information. The increase in unacceptable judgements for ambiguous sentences compared with unambiguous was smaller in patients with increased damage in the left inferior frontal gyrus. The control data confirmed the importance of the left inferior frontal gyrus in syntactic analysis. All of the contrasts between ambiguous and unambiguous sentences generated increased activity in the left inferior frontal gyrus.
With respect to the role of subregions of the left inferior frontal gyrus in syntactic processing, we found that better syntactic performance among the patients was consistently associated with increased tissue integrity and increased activity in left BA 45, with slightly weaker involvement of left BA 47. Activity in left BA 44 was not correlated with syntactic performance, although reduced tissue integrity in this region was associated with syntactic deficits. These results argue against strong functional segregation of the subregions of the left inferior frontal gyrus, at least with respect to syntax. While left BA 45 was consistently implicated in the particular syntactic manipulations we used in this study, there was more variation in the contribution of left BA 47, and particularly of left BA 44. Left BA 44’s contribution was primarily seen in the region of interest analyses, in which we correlated tissue integrity in each anatomically-defined subregion of the left inferior frontal gyrus with activity and performance. However, region of interest analyses are more liberal than voxel-wise analyses, as they are not corrected for multiple comparisons.
Previous studies have highlighted the role of left BA 44 in syntactic processing (Friederici et al., 2006), but discrepancies in the specific left inferior frontal gyrus regions involved in syntactic analysis are likely to be influenced by the task requirements and stimulus manipulations involved in different studies, since these are known to interact with a variety of cognitive functions subserved by the left inferior frontal gyrus (e.g. Gold and Buckner, 2002). Many functional MRI studies of syntactic comprehension involve stimuli and/or tasks that potentially involve cognitive control mechanisms, factors that are known to involve BA 44 (Thompson-Schill and Kan, 2001; Fincham et al., 2002; Fiebach et al., 2005). In the current study, we attempted to minimize these factors by avoiding the use of a specific task and having the participants simply listen to the sentences. Moreover, since it is plausible that syntax does not consist of a single, uniform computation, it may be the case that different subregions of the left inferior frontal gyrus contribute differentially to different aspects of syntactic processing (Friederici, 2002). Future studies may need to more systematically investigate the contribution of different syntactic variables as well as differentiating between linguistic and non-linguistic components in order to determine the extent to which regions of the left inferior frontal gyrus have specialized functional roles.
The left inferior frontal gyrus in the broader language network
Syntactic processing does not, however, implicate the left inferior frontal gyrus alone, but always involves other regions. In the studies and analyses reported here, a variety of brain areas were co-activated with the left inferior frontal gyrus, including the right inferior frontal gyrus, bilateral superior temporal gyrus, left middle temporal gyrus and a more posterior temporo-parietal cluster including left inferior parietal lobule, left angular gyrus and left supramarginal gyrus. Within this broader set of brain areas, all potentially related to the general process of language comprehension, an important advantage of the current study is that it makes it possible to identify which of these regions are part of the core left inferior frontal gyrus-based network that must be in place to support syntactic function and which regions are less critical. Evidence from the patients, in particular, argues strongly that the critical region linked to the left inferior frontal gyrus is the left posterior middle temporal gyrus.
As noted earlier, it is the patient correlational analyses that pick out the brain areas that are necessary to support a given neurocognitive function. All of these analyses, whether involving functional activity or tissue integrity, and for each of the three behavioural measures of the patients’ syntactic capacities, have in common the co-identification of left inferior frontal gyrus and left posterior middle temporal gyrus (Fig. 4A–C and Supplementary Figs 1–3) as constituting the necessary processing substrate for successful syntactic performance. No other sets of brain regions show up consistently across these several analyses. The focus on this constrained perisylvian circuit is supported by a wide range of other research and by the neuroanatomical organization of the brain, with left inferior frontal gyrus and posterior temporal cortex being linked by major dorsal and ventral white matter tracts (the arcuate fasciculus and the extreme capsule; Frey et al., 2008; Makris and Pandya, 2009). In recent research we have shown that the integrity of both of these tracts, which provide direct connections between the left inferior frontal gyrus and the left posterior middle temporal gyrus, is essential for preserved syntax (Griffiths et al., 2009).
In the present study, the functional roles of the main left fronto-temporal components of this circuit are illuminated by the syntactic dominance effects. In the controls the subordinate compared with the dominant condition produced activity in left inferior frontal gyrus and left posterior middle temporal gyrus, consistent with previous studies (Mason et al., 2003; Rodd et al., 2010)—a pattern of left fronto-temporal activity for syntax that has been reported in several studies for a variety of syntactic manipulations and paradigms (Caplan et al., 1996; Just et al., 1996; Tyler and Marslen-Wilson, 2008; Tyler et al., 2010a).
The dominance effect may be interpreted within a lexicalist framework in which the syntactic, semantic and phonological properties of each word are activated and integrated into the existing sentential representation (Marslen-Wilson and Tyler, 1980; Marslen-Wilson, 1987), and where this interplay between lexical properties and syntactic analysis is mediated by the interaction between left frontal and posterior temporal structures. When normal listeners hear a syntactically ambiguous phrase, they activate multiple representations in parallel with each having a probabilistic weighting (MacDonald, 1994). The representation with the highest weight is the preferred (i.e. dominant) interpretation. If further incoming speech (the disambiguating word) is inconsistent with the preferred interpretation, a temporary discrepancy occurs that is rapidly resolved by the listener revising their analysis in order to re-establish a coherent structural representation of the sentence. The present results show that co-activation of left inferior frontal gyrus and left posterior middle temporal gyrus in the subordinate condition is associated with this revision of the alternative syntactic interpretation of the ambiguous phrase (e.g. whether the noun or the verb is interpreted as the head of the phrase) in order to integrate the disambiguating word into a coherent syntactic representation, and with selection processes which resolve competition between activated candidate syntactic interpretations (Thompson-Schill et al., 2005). The patients do not show comparable effects of dominance, since their syntactic deficits compromise their ability to conduct the syntactic computations that result in strong preferences for one syntactic interpretation over another. Their preferences are necessarily weaker, resulting in a less marked difference between the processing of dominant and subordinate sentences compared with controls, and perhaps generally less stable syntactic representations—possibly contributing to the left inferior parietal lobule activations discussed below.
Although our study cannot address the timing of processes of syntactic revision, previous studies using EEG or magnetoencephalography show that resolving syntactic ambiguity with non-preferred continuations is accompanied by a late positivity effect (P600; Osterhout and Holcomb, 1992; Osterhout and Holcomb, 1993; Kaan et al., 2000). The results from our study suggest that the P600 effect may arise from the interaction of the left inferior frontal gyrus and left posterior middle temporal gyrus during syntactic reinterpretation. Whatever the exact mechanism, the process of resolving the ambiguity is rapid and obligatory, in the sense that listeners cannot tolerate conflicting syntactic representations and seek to resolve them immediately.
The results from the patients suggest that, in the face of left hemisphere damage, preserved syntactic performance requires intact functionality in left middle temporal gyrus, as well as left inferior frontal gyrus—with the connectivity between them also likely to be a crucial factor. Some evidence supporting this comes from a case study of a single patient who had a lesion in left posterior middle temporal gyrus, no damage at all to the left inferior frontal gyrus and disrupted white matter tract connections between left posterior middle temporal gyrus and left inferior frontal gyrus (Tyler and Marslen-Wilson, 2008). Tested on an earlier version of the functional MRI ambiguity study run here, the patient showed no activity in the left inferior frontal gyrus. Instead, activation was located in the right inferior frontal gyrus during syntactic processing even though the left inferior frontal gyrus was intact. This activity in right inferior frontal gyrus however, was not accompanied by preserved syntactic performance since the patient continued to have a persistent syntactic deficit (Tyler and Marslen-Wilson, 2008). This illustrates the point that an intact left inferior frontal gyrus does not guarantee preserved syntactic function; this may well require connectivity between left inferior frontal gyrus and an intact left middle temporal gyrus to be preserved as well.
In contrast to the left inferior frontal gyrus-left posterior middle temporal gyrus network, co-activation of the left inferior frontal gyrus and left inferior parietal lobule cluster does not seem to be required for core syntactic functions. In the controls, both the left inferior frontal gyrus and left inferior parietal lobule, angular gyrus and supramarginal gyrus were activated by the presence of a syntactic ambiguity. It has been claimed that when listeners encounter a syntactically ambiguous phrase they temporarily activate both syntactic interpretations and this increases working memory load (MacDonald et al., 1992). It is plausible that activity in the left inferior parietal lobule in the context of syntactic ambiguity reflects increased working memory demands and is consistent with other findings implicating the left inferior parietal lobule in working memory (Jonides et al., 1998; Champod and Petrides, 2010). The patients as a group also showed increased activation in the left inferior parietal lobule, angular gyrus and supramarginal gyrus when they encountered a syntactically ambiguous phrase. This reflects some sensitivity to the presence of a syntactic ambiguity, but without the highly differentiated on-line discrimination between preferred readings seen in the controls. Moreover, this activity in the left inferior parietal lobule, as well as the associated activity seen in the right hemisphere, did not correlate with measures of syntactic performance. The functions of these posterior temporo-parietal regions are unlikely, however, to be restricted to working memory. Since the early 20th century, damage to these regions—with emphasis on the angular gyrus (Dejerine, 1914) and the angular gyrus and supramarginal gyrus together (Marie and Foix, 1917)—has been associated with language comprehension deficits. More recently, functional MRI studies have shown that these regions are also activated in language comprehension, together with the left inferior parietal lobule. Determining the exact roles of these regions within the neural language system requires further study.
Finally, although our findings suggest that the left inferior frontal gyrus plays a key role in syntactic computation, this does not necessarily imply that the left inferior frontal gyrus is specialised for syntactic processing. Given that activity in left inferior frontal gyrus typically co-occurs with activity in other regions known to be involved in language—most saliently the left posterior middle temporal gyrus—these results show that the left inferior frontal gyrus plays an essential role within the neural language network, and that differential modulation within this network underpins different types of linguistic computations. This suggests, in turn, that instead of regions of the frontal cortex being functionally specialized, their involvement in a specific cognitive function depends on the inputs they receive.
Supplementary Material
Acknowledgements
We warmly thank all our participants and the radiographers at the MRC-CBU for their contributions to this research. We are grateful to Prof. C. Price for referring one of the patients to us.
Funding
Medical Research Council (UK) programme grant (G0500842 to L.K.T.); Medical Research Council Cognition and Brain Sciences Unit funding (U.1055.04.002.00001.01 to W.D.M.-W.).
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- BA
Brodmann area
- SPM
statistical parametric mapping
References
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Pipenbrook R, Gulikers L. The CELEX Lexical database. Linguistic Data Consortium. Philadelphia: Philadelphia Linguistic Data Consortium, University of Pennsylvania; 1995. [Google Scholar]
- Berndt RS, Mitchum C, Burton M, Haendiges A. Comprehension of reversible sentences in aphasia: the effects of verb meaning. Cogn Neuropsychol. 2004;21:229–45. doi: 10.1080/02643290342000456. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Mitchum CC, Haendiges AN. Comprehension of reversible sentences in ‘agrammatism’: a meta-analysis. Cognition. 1996;58:289–308. doi: 10.1016/0010-0277(95)00682-6. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–62. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–88. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16 Abstract 497. [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Bright P, Moss HE, Longe O, Stamatakis EA, Tyler LK. Conceptual structure modulates anteromedial temporal involvement in processing verbally presented object properties. Cereb Cortex. 2007;17:1066–73. doi: 10.1093/cercor/bhl016. [DOI] [PubMed] [Google Scholar]
- Broca PP. Perte de la parole, ramollissement chronique et destruction partielle du lobe antérieur gauche du cerveau. Bulletin de la Société Anthropologique. 1861;2:235–8. [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 1998;9:3735–9. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Caplan D, Chen E, Waters G. Task-dependent and task-independent neurovascular responses to syntactic processing. Cortex. 2008a;44:257–75. doi: 10.1016/j.cortex.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Hildebrandt N, Makris N. Location of lesions in stroke patients with deficits in syntactic processing in sentence comprehension. Brain. 1996;119:933–49. doi: 10.1093/brain/119.3.933. [DOI] [PubMed] [Google Scholar]
- Caplan D, Stanczak L, Waters G. Syntactic and thematic constraint effects on blood oxygenation level dependent signal correlates of comprehension of relative clauses. J Cogn Neurosci. 2008b;20:643–56. doi: 10.1162/jocn.2008.20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Waters G, Alpert N. Correlates of syntactic processing in sentence comprehension. Hum Brain Mapp. 2003;19:112–31. doi: 10.1002/hbm.10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza A, Zurif EB. Dissociation of algorithmic and heuristic processes in language comprehension - evidence from Aphasia. Brain Lang. 1976;3:572–82. doi: 10.1016/0093-934x(76)90048-1. [DOI] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociation within the frontoparietal network in verbal working memory: a parametric functional magnetic resonance imaging study. J Neurosci. 2010;30:3849–56. doi: 10.1523/JNEUROSCI.0097-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. A madness to the methods in cognitive neuroscience? J Cogn Neurosci. 2005;17:847–9. doi: 10.1162/0898929054021085. [DOI] [PubMed] [Google Scholar]
- Coleman MR, Rodd JM, Davis MH, Johnsrude IS, Menon DK, Pickard JD, et al. Do vegetative patients retain aspects of language comprehension? Evidence from fMRI. Brain. 2007;130:2494–507. doi: 10.1093/brain/awm170. [DOI] [PubMed] [Google Scholar]
- Crinion J, Ashbumer J, Leff A, Brett M, Price C, Friston K. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. Neuroimage. 2007;37:866–75. doi: 10.1016/j.neuroimage.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion JT, Lambon-Ralph MA, Warburton EA, Howard D, Wise RJS. Temporal lobe regions engaged during normal speech comprehension. Brain. 2003;126:1193–201. doi: 10.1093/brain/awg104. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the bold FMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–72. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Bookheimer SY. Form and content: dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24:427–32. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Davis MH, Coleman MR, Absalom AR, Rodd JM, Johnsrude IS, Matta BF, et al. Dissociating speech perception and comprehension at reduced levels of awareness. Proc Natl Acad Sci USA. 2007;104:16032–7. doi: 10.1073/pnas.0701309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejerine J. Sémiologie des affections du système nerveux. Paris: Masson et Cie; 1914. [Google Scholar]
- Demonet JF, Thierry G, Cardebat D. Renewal of the neurophysiology of language: functional neuroimaging. Physiol Rev. 2005;85:49–95. doi: 10.1152/physrev.00049.2003. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–77. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Drury HA, Van Essen DC, Corbetta M, Snyder AZ. Surface-based analyses of the human cerebral cortex. In: Toga A, editor. Brain Warping. San Diego, CA: Academic Press; 1999. pp. 337–63. [Google Scholar]
- Embick D, Marantz A, Miyashita Y, O'Neil W, Sakai KL. A syntactic specialization for Broca's area. Proc Natl Acad Sci USA. 2000;97:6150–4. doi: 10.1073/pnas.100098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Heberlein AS, Morales DA, Shivde G, Waller S, Wu DH. Method matters: an empirical study of impact in cognitive neuroscience. J Cogn Neurosci. 2005;17:850–8. doi: 10.1162/0898929054021139. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD. Revisiting the role of Broca's area in sentence processing: Syntactic integration versus syntactic working memory. Hum Brain Mapp. 2005;24:79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham JM, Carter CS, van Veen V, Stenger VA, Anderson JR. Neural mechanisms of planning: a computational analysis using event-related fMRI. Proc Natl Acad Sci USA. 2002;99:3346–51. doi: 10.1073/pnas.052703399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Campbell JSW, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28:11435–44. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Levels of processing and vocabulary types: evidence from on-line processing in normals and agrammatics. Cognition. 1985;19:133–66. doi: 10.1016/0010-0277(85)90016-2. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Fiebach CJ, Schlesewsky M, Bornkessel ID, von Cramon DY. Processing linguistic complexity and grammaticality in the left frontal cortex. Cereb Cortex. 2006;16:1709–17. doi: 10.1093/cercor/bhj106. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Ruschemeyer S-A, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13:170–7. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD. Statistical parametric mapping. London, UK: Academic Press; 2007. [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–12. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Griffiths JD, Stamatakis EA, Tyler LK. Damage to dorsal and ventral frontotemporal white matter pathways impairs syntactic aspects of language comprehension: a DTI tractography study. Neuroimage. 2009;47:S143. [Google Scholar]
- Grodzinsky Y. The neurology of syntax: language use without Broca's area. Behav Brain Sci. 2000;23:1–21. doi: 10.1017/s0140525x00002399. [DOI] [PubMed] [Google Scholar]
- Hagoort P. On Broca, brain, and binding: a new framework. Trends Cogn Sci. 2005;9:416–23. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Sakai KL. Specialization in the left prefrontal cortex for sentence comprehension. Neuron. 2002;35:589–97. doi: 10.1016/s0896-6273(02)00788-2. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, et al. The role of parietal cortex in verbal working memory. J Neurosci. 1998;18:5026–34. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends Cogn Sci. 2005;9:512–8. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–6. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kaan E, Harris A, Gibson E, Holcomb P. The P600 as an index of syntactic integration difficulty. Lang Cogn Process. 2000;15:159–201. [Google Scholar]
- Kaan E, Swaab TY. The brain circuitry of syntactic comprehension. Trends Cogn Sci. 2002;6:350–6. doi: 10.1016/s1364-6613(02)01947-2. [DOI] [PubMed] [Google Scholar]
- Keller TA, Carpenter PA, Just MA. The neural bases of sentence comprehension: a fMRI examination of syntactic and lexical processing. Cereb Cortex. 2001;11:223–37. doi: 10.1093/cercor/11.3.223. [DOI] [PubMed] [Google Scholar]
- Kovelman I, Baker SA, Petitto LA. Bilingual and monolingual brains compared: a functional magnetic resonance imaging investigation of syntactic processing and a possible “neural signature” of bilingualis. J Cogn Neurosci. 2008;20:153–69. doi: 10.1162/jocn.2008.20011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald MC. Probabilistic constraints and syntactic ambiguity resolution. Lang Cognitive Proc. 1994;9:157–201. [Google Scholar]
- MacDonald MC, Just MA, Carpenter PA. Working memory constraints on the processing of syntactic ambiguity. Cognit Psychol. 1992;24:56–98. doi: 10.1016/0010-0285(92)90003-k. [DOI] [PubMed] [Google Scholar]
- Makris N, Pandya DN. The extreme capsule in humans and rethinking of the language circuitry. Brain Struct Funct. 2009;213:343–58. doi: 10.1007/s00429-008-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marcus GF, Vouloumanos A, Sag IA. Does Broca's play by the rules? Nat Neurosci. 2003;6:651–2. doi: 10.1038/nn0703-651. [DOI] [PubMed] [Google Scholar]
- Marie P, Foix C. Les aphasies de guerre. Rev Neurol (Paris) 1917;24:53–87. [Google Scholar]
- Marslen-Wilson WD. Functional parallelism in spoken word-recognition. Cognition. 1987;25:71–102. doi: 10.1016/0010-0277(87)90005-9. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson WD, Tyler LK. The temporal structure of spoken language understanding. Cognition. 1980;8:1–71. doi: 10.1016/0010-0277(80)90015-3. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson WD, Tyler LK. Morphology, language and the brain: the decompositional substrate for language comprehension. Philos Trans R Soc Lond B Biol Sci. 2007;362:823–36. doi: 10.1098/rstb.2007.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RA, Just MA, Keller TA, Carpenter PA. Ambiguity in the brain: what brain imaging reveals about the processing of syntactically ambiguous sentences. J Exp Psychol Learn Mem Cogn. 2003;29:1319–38. doi: 10.1037/0278-7393.29.6.1319. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Moss HE, Abdallah S, Fletcher P, Bright P, Pilgrim L, Acres K, et al. Selecting among competing alternatives: Selection and retrieval in the left inferior frontal gyrus. Cereb Cortex. 2005;15:1723–35. doi: 10.1093/cercor/bhi049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso M, Moro A, Glauche V, Rijntjes M, Reichenbach J, Buchel C, et al. Broca's area and the language instinct. Nat Neurosci. 2003;6:774–81. doi: 10.1038/nn1077. [DOI] [PubMed] [Google Scholar]
- Osterhout L, Holcomb PJ. Event-related brain potentials elicited by syntactic anomaly. J Mem Lang. 1992;31:785–806. [Google Scholar]
- Osterhout L, Holcomb PJ. Event-related potentials and syntactic anomaly - evidence of anomaly detection during the perception of continuous speech. Lang Cogn Proc. 1993;8:413–37. [Google Scholar]
- Ostrin RK, Tyler LK. Dissociations of lexical function: semantics, syntax and morphology. Cogn Neuropsychol. 1995;12:345–89. [Google Scholar]
- Peelle JE, McMillan C, Moore P, Grossman M, Wingfield A. Dissociable patterns of brain activity during comprehension of rapid and syntactically complex speech: evidence from fMRI. Brain Lang. 2004;91:315–25. doi: 10.1016/j.bandl.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. SCAN. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Crinion J, Friston KJ. Design and analysis of fMRI studies with neurologically impaired patients. J Magn Reson Imaging. 2006;23:816–26. doi: 10.1002/jmri.20580. [DOI] [PubMed] [Google Scholar]
- Raven JC. Colored Progressive Matrices. Oxford: Oxford Psychologists Press Ltd.; 1995. [Google Scholar]
- Rayner K, Duffy SA. Lexical complexity and fixation times in reading - effects of word-frequency, verb complexity, and lexical ambiguity. Mem Cognit. 1986;14:191–201. doi: 10.3758/bf03197692. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Longe OA, Randall B, Tyler LK. The functional organisation of the fronto-temporal language system: evidence from syntactic and semantic ambiguity. Neuropsychologia. 2010;48:1324–35. doi: 10.1016/j.neuropsychologia.2009.12.035. [DOI] [PubMed] [Google Scholar]
- Saffran E, Schwartz E, Marin O. The word order problem in agrammatism: 11. Production. Brain Lang. 1980;10:263–80. doi: 10.1016/0093-934x(80)90056-5. [DOI] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJS. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–6. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis EA, Tyler LK. Identifying lesions on structural brain images - validation of the method and application to neuropsychological patients. Brain Lang. 2005;94:167–77. doi: 10.1016/j.bandl.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Taylor KI, Stamatakis EA, Tyler LK. Crossmodal integration of object features: voxel-based correlations in brain-damaged patients. Brain. 2009;132:671–83. doi: 10.1093/brain/awn361. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Aguirre GK, d'Esposito M, Farah MJ. A neural basis for category and modality specificity of semantic knowledge. Neuropsychologia. 1999;37:671–6. doi: 10.1016/s0028-3932(98)00126-2. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15:219–24. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Kan IP. Perceptual and conceptual sources of priming on a word generation task. Mem Cognit. 2001;29:698–706. doi: 10.3758/bf03200472. [DOI] [PubMed] [Google Scholar]
- Tyler LK. Spoken language comprehension: an experimental approach to disordered and normal processing. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- Tyler LK, Marslen-Wilson WD. The on-line effects of semantic context on syntactic processing. J Verb Learn Verb Beh. 1977;16:683–92. [Google Scholar]
- Tyler LK, Marslen-Wilson WD. Fronto-temporal brain systems supporting spoken language comprehension. Philos Trans R Soc Lond B Biol Sci. 2008;363:1037–54. doi: 10.1098/rstb.2007.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Marslen-Wilson WD, Stamatakis EA. Differentiating lexical form, meaning, and structure in the neural language system. Proc Natl Acad Sci USA. 2005a;102:8375–80. doi: 10.1073/pnas.0408213102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Shafto MA, Randall B, Wright P, Marslen-Wilson WD, Stamatakis EA. Preserving syntactic processing across the adult life span: the modulation of the frontotemporal language system in the context of age-related atrophy. Cereb Cortex. 2010a;20:352–64. doi: 10.1093/cercor/bhp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Post B, Randall B, Marslen-Wilson WD. Temporal and frontal systems in speech comprehension: an fMRI study of past tense processing. Neuropsychologia. 2005b;43:1963–74. doi: 10.1016/j.neuropsychologia.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Wright P, Randall B, Marslen-Wilson WD, Stamatakis EA. Reorganization of syntactic processing following LH brain damage: does right-hemisphere activity preserve function? Brain. 2010b;133:3396–408. doi: 10.1093/brain/awq262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uppenkamp S, Johnsrude IS, Norris D, Marslen-Wilson W, Patterson RD. Locating the initial stages of speech-sound processing in human temporal cortex. Neuroimage. 2006;31:1284–96. doi: 10.1016/j.neuroimage.2006.01.004. [DOI] [PubMed] [Google Scholar]
- van der Lely HKJ, Harris M. Comprehension of reversible sentences in specifically language impaired children. J Speech Hear Disord. 1990;55:101–17. doi: 10.1044/jshd.5501.101. [DOI] [PubMed] [Google Scholar]
- Vannest JJ, Karunanayaka PR, Altaye M, Schmithorst VJ, Plante EM, Eaton KJ, et al. Comparison of fMRI data from passive listening and active-response story processing tasks in children. J Magn Reson Imaging. 2009;29:971–6. doi: 10.1002/jmri.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–32. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Weber K, Indefrey P. Syntactic priming in German-English bilinguals during sentence comprehension. Neuroimage. 2009;46:1164–72. doi: 10.1016/j.neuroimage.2009.03.040. [DOI] [PubMed] [Google Scholar]
- Wright P, Randall B, Marslen-Wilson WD, Tyler LK. Dissociating linguistic and task-related activity in LIFG. J Cogn Neurosci. 2011;23:404–13. doi: 10.1162/jocn.2010.21450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.