Abstract
Matrix metalloproteases (MMPs) are Zn-containing endopeptidases involved in the degradation of extracellular matrix components and are typically secreted in a latent (pro-MMP) form and activated either by proteolytic or oxidative disruption of a conserved cysteine switch. Several recent studies have suggested that nitric oxide (NO) can contribute to the activation of MMPs, but the mechanisms involved are incompletely understood. We investigated the ability of NO to regulate the activation of (pro)MMP-9 using a variety of NO-donor compounds and characterized modifications of the cysteine switch using a synthetic peptide (PRCGVPDLGR) representing the cysteine switch domain of MMP-9. Among the NO-donors used, only S-nitrosocysteine (SNOC) was found to be capable of modest activation of proMMP-9, but S-nitrosoglutathione (GSNO) or the NONOates, DEA-NO, SPER-NO, or DETA-NO, were ineffective. In fact, high concentrations of DETA-NO were found to inhibit MMP-9 activity, presumably by direct interaction with the active-site Zn2+. Analysis of chemical modifications within the Cys-containing peptide, PRCGVPDLGR, revealed rapid and transient S-nitrosylation by SNOC and GSNO, and formation of mixed disulfides and dimerized peptide as major final products. Similarly, NONOates induced transient S-nitrosylation and primarily peptide dimerization. Coordination of the peptide Cys with a synthetic Zn2+ complex, to more closely mimic the structure of the active site in proMMP-9, reduced peptide nitrosylation and oxidation by NONOates, but enhanced peptide nitrosylation by SNOC and GSNO. Collectively, our results demonstrate that NO is incapable of directly activating proMMP-9 and that S-nitrosylation of MMP-9 propeptide by NO-donors is unrelated to their ability to regulate MMP-9 activity.
Matrix metalloproteinases (MMPs) comprise a family of zinc-containing endopeptidases and play important roles in development, inflammation, tissue injury and repair, and tumor biology (1, 2). The MMP family consists of at least 26 members that share several structural characteristics including a highly conserved Cys-containing pro-peptide domain and a catalytic domain containing three His residues bound to a Zn(II) metal ion. MMPs are typically expressed at low levels in normal tissues, but in conditions of inflammation and/or tissue injury, MMP expression is generally increased by the action of pro-inflammatory cytokines, such as TNF-Α and TGF-β, or by other cellular mediators such as nitric oxide (NO) (2–4). Most MMPs are secreted in a latent (pro-MMP) form in which the conserved pro-domain Cys is ligated with the active site Zn2+, and require extracellular activation which involves disruption of the zinc-cysteine bond (5). The precise mechanisms by which secreted MMPs are activated are incompletely understood, but are typically thought to involve the proteolytic removal of the pro-domain by serine proteinases such as plasmin or other active MMPs (1, 2). In addition, a number of studies have indicated that oxidative modification of the pro-domain Cys may also activate MMPs (6–10). However, the precise chemical mechanisms by which oxidants activate MMPs and the in vivo significance of oxidative MMP activation still remain unclear.
Several recent studies have suggested that NO contributes to the activation of MMPs or related metalloproteinases by S-nitrosylation (often also referred to as S-nitrosation) of the pro-domain Cys residue. For example, MMP-9 activation in vivo was found to be associated with increased NOS activity, and the nitrosothiol S-nitrosocysteine is capable of activating pro-MMP-9 by a mechanism that appears to involve transnitrosylation (4, 11). Similarly, NO has been implicated in the activation of TNF-Α-converting enzyme (TACE; ADAM-17), a related zinc-containing metalloproteinase (12). Since NO is capable of inducing the release of Zn2+ from zinc-thiolate centers in, for example, metallothionein and zinc-finger proteins (13, 14), it is plausible that NO may similarly disrupt zinc-thiolate bonds in pro-MMPs. However, recent studies in various cell types demonstrated that NO-mediated increases in MMP-9 activity are primarily due to enhanced MMP-9 expression rather than direct MMP-9 activation (3, 15). Furthermore, a number of studies with purified MMPs have shown that NO itself is incapable of directly activating MMPs and that formation of more reactive intermediates such as nitrogen dioxide or peroxynitrite are required for oxidative MMP activation (3, 8, 9). Chemical analysis of pro-domain Cys modifications indicates that oxidative MMP activation, by, for example, hypochlorous acid or peroxynitrite, is associated with irreversible oxidation of the pro-domain Cys to sulfinic/sulfonic acids or a sulfenyl disulfide (6, 9). Similarly, recent observations of NO-associated MMP-9 activation during cerebral ischemia were associated with oxidation of the pro-domain Cys to sulfinic/sulfonic acids, without evidence for intermediate S-nitrosylation (4).
To address whether NO is capable of directly activating MMP-9 via S-nitrosylation under biologically relevant conditions, we performed experiments in cell-free systems with a range of NO-donor compounds and S-nitrosothiols, purified (pro)MMP-9, and a synthetic peptide resembling the Cys-containing pro-domain of MMP-9 to investigate their ability to activate MMP-9 in association with chemical modifications within the pro-domain region. Our results indicate that NO at physiologically relevant concentrations is incapable of activating MMP-9 and that the ability of S-nitrosothiols to activate MMP-9 is not strictly related to their ability to S-nitrosate the pro-domain Cys, but may also be limited by steric factors within proMMP-9.
EXPERIMENTAL PROCEDURES
Materials
Human ProMMP-9 was synthesized using a baculovirus system and purified by gelatin 4B column chromatography as described previously (16). Recombinant active MMP-9 and S-nitroso-glutathione (GSNO) were purchased from Calbiochem. Spermine NONO-ate (SPERNO), DETA NONOate (DETA-NO), and DEA NONOate (DEA-NO) were obtained from Cayman. S-Nitrosocysteine (CSNO) was prepared immediately prior to use, as previously reported (17). Briefly, equal volumes of 400 mM l-cysteine, 400 mM sodium nitrite, and 400 mM HCl were mixed for 5 min in the dark. The reaction was terminated by the addition of one volume of 400 mM NaOH and the resulting solution stored in the dark on ice. CSNO concentration was determined spectrophotometrically (ε334 = 900 M−1 cm−1). DQ Gelatin was purchased from Molecular Probes. The decapeptide PRCGVPDLGR, corresponding to the Cys-containing pro-peptide region of MMP-9, was synthesized at the University of Vermont Core Protein Facility, at >90% purity. All other chemicals were purchased from Sigma.
Reactions of proMMP-9 and Active MMP-9 with NONOates and S-Nitrosothiols
Stock solutions of NONOates were prepared in 10 mM NaOH, and S-nitrosothiol solutions were prepared in deionized water. All solutions were kept on ice and protected from light until use. A 2 µg/mL solution of either proMMP-9 or active MMP-9 was prepared in reaction buffer (50 mM Tris (pH 7.6), 150 mM NaCl, 5 mM CaCl2, and 0.2 mM sodium azide).
Reactions were conducted in a 96-well plate as follows. To each well, 75 µL of reaction buffer was added, followed by the addition of 10 µL of the appropriate NO stock solution or 10 mM NaOH as the blank control. The mercurial MMP activator, p-chloromercuribenzoic acid (pCMB), which activates MMPs by covalent Cys modification followed by autoproteolytic cleavage (18), was used as a positive control (10 µL of 2 mM stock solution in 10 mM NaOH). Immediately after the addition of the NO donors, 10 µL of the appropriate MMP-9 stock and 5 µL of DQ Gelatin (1 mg/mL) were added to each well. In the case of DETA NONOate, 10 µL of the appropriate stock was added 1.5 h prior to the other reactants, to allow for sufficient accumulation of NO before the start of the reaction. The gelatinase reaction was monitored as fluorescence increase (485 Ex/530 Em) in a microplate reader (Bio-Tek Synergy HT, Winooski, VT). The slope of the linear fluorescence increase over 2 h (Mean V) was determined and expressed relative to untreated controls.
Synthesis of Zn[TACN]
The synthesis of Zn[TACN] was adopted from Yang and Zompa (19), with several modifications. Briefly, 50 mg of 1,4,7-triazacyclononane (TACN)·3HCl (210 µmol, 1 equiv) was suspended in 1.5 mL of 95% ethanol, and 1.0 M KOH was added dropwise until a homogeneous solution was obtained. The final volume was adjusted to 2.1 mL with 95% ethanol, and 4.2 mL of 0.5 M ZnCl2 was added, and the solution was thoroughly mixed and allowed to stand for several days. Long rod shaped crystals of the 1:1 complex of Zn and TACN formed and were collected by filtration through a Buchner funnel and air-dried. Typical yield was 50%. Characterization: NMR {1H, D2O, δ ppm, chemical shift assigned from residual H} 2.58 (q, J = 7.1 Hz), 2.82 (q, J = 6.7 Hz).
Analysis of MMP-9 Prodomain Modifications by NO Donors
A 1 mM stock solution of the synthetic deca-peptide PRCGVPDLGR, representing the Cys-containing sequence within the MMP-9 prodomain, was prepared in reaction buffer (50 mM Tris (pH 7.6), 150 mM NaCl, 5 mM CaCl2, and 0.2 mM sodium azide), and 90 µL of peptide solution was mixed with 10 µL of appropriate NONOate or S-nitrosothiol stock solutions in a 0.5 mL tube, and the mixture immediately transferred to a glass autosampler insert for HPLC analysis. In some cases, the peptide was pretreated with Zn[TACN] by 1:1 mixing of peptide solution (2 mM) with a stock solution of Zn[TACN] (2 mM in water) for 1 h prior to the addition of NO-donors. Reaction mixtures were analyzed at several time points by HPLC using a Waters Alliance 2695 HPLC system interfaced with a Waters 2487 Dual Absorbance detector. Samples (10 µL) were injected on a Waters Symmetry C18 column (3.5 µm; 4.6 mm × 75 mm) and eluted by gradient elution. Initial solvent conditions were 100% A (0.1 trifluoroacetic acid (TFA)) with a linear gradient to 70% A:30% B (0.1% TFA in acetonitrile (ACN)) from 0–30 min with column equilibration (100%A) for 10 min. Peptide products were detected at 214 and 340 nm, the latter as an index of peptide S-nitrosylation (20). Peak areas at 214 nm were used for quantitation of relative product yields, and peaks eluting from the HPLC were collected for further characterization.
For product characterization by MALDI-TOF, fractionated peaks eluted from the HPLC were evaporated to dryness in a speedvac (Labconco) and resuspended in 5 µL of a 50/50 mixture of 0.1% TFA and ACN, and 1 µL of this solution was mixed with 1 µL of a saturated Α-cyano sinapinic acid matrix solution dissolved in 0.1% TFA/ACN (50/50) for spotting on a stainless steel sample plate and analysis by matrix-assisted laser desorption-time of flight mass spectrometer (MALDI-TOF; Applied Biosystems Voyager-DE Pro). MS spectra were acquired in positive ion reflector mode with accelerating voltage 20,000 V, grid voltage 75%, guide wire voltage 0.002%, and extraction delay time 175 ns. Masses were calibrated using an external standard (bradykinin fragment 1–7 M.W., 757.3997; angiotensin II M.W., 1046.5423; P14R M.W., 1533.8582; ACTH fragment 18–39 M.W., 2465.1989; insulin oxidized B chain M.W., 3494.6513). Reported spectra are an average of 10 spectra of 50 laser shots each (total 500 laser shots).
Alternatively, reaction mixtures were analyzed by LC ESI/MS using a Thermo Finnagan LCQ Deca XP ion trap mass spectrometer plus liquid chromatography using a Magic C18 packed capillary column (0.2 mm i.d., 50 mm length). Separations were accomplished using a binary separation gradient with solvent A (0.1% TFA) and solvent B (0.1% TFA in ACN) using a linear gradient from 0–40% B over 40 min. Products were identified by correlating retention time and mass obtained from MS analysis to predicted masses.
RESULTS
Activation of proMMP-9 by NONOates and S-Nitrosothiols
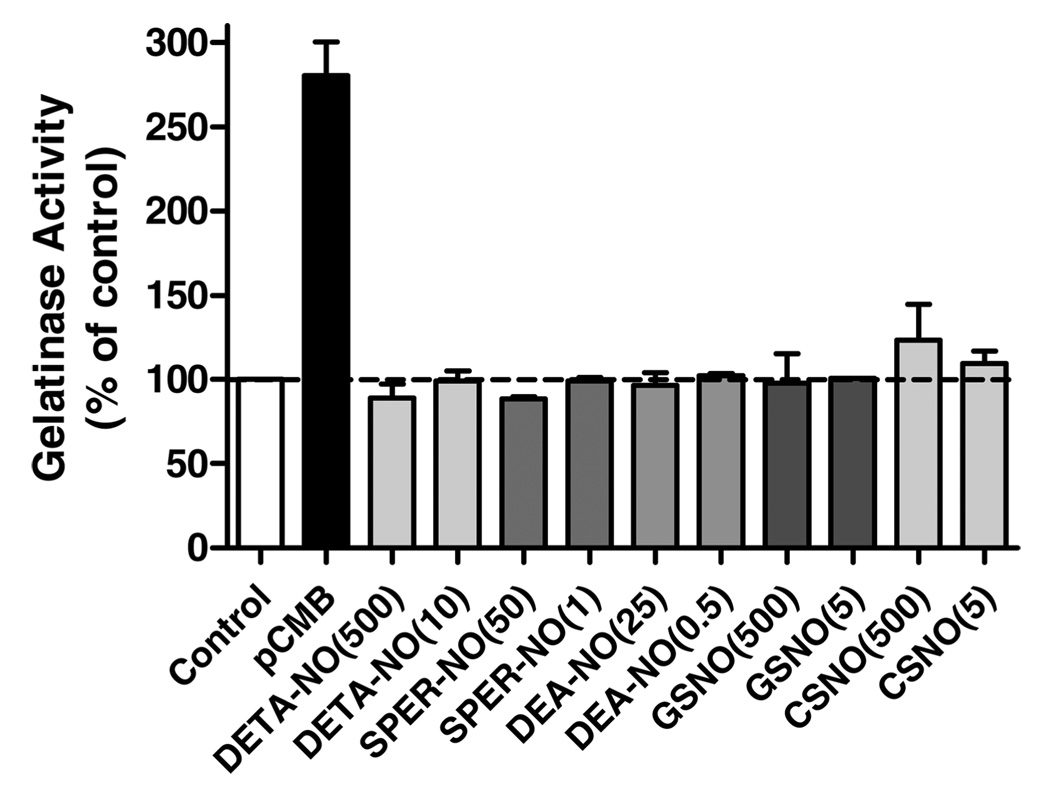
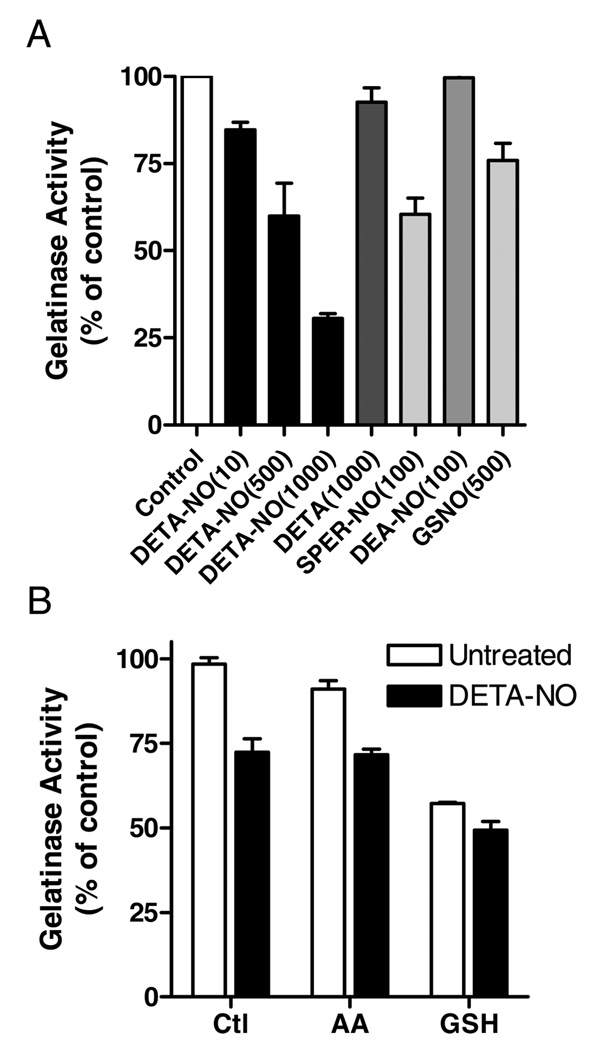
We first tested the ability of various NONOates and S-nitrosothiols to activate purified proMMP-9, in comparison with its activation by the known mercury-based activator pCMB (18). Two concentrations for each NO donor were used, and concentrations of the various NONOates were chosen on the basis of their respective half-lives such that relatively equivalent maximum concentration of NO was achieved for each NONOate (21, 22). As shown in Figure 1, marked (2.5–3-fold) proMMP-9 activation was observed in response to pCMB. Consistent with previous observations (3, 4), high concentrations of CSNO (500 µM) also resulted in modest activation of proMMP-9, about 22% compared to activation by 200 µM pCMB. However, neither GSNO (up to 500 µM) nor any of the NONOates caused significant activation of proMMP-9, and high concentrations of DETANO or SPER-NO in fact appeared to inhibit gelatinase activity compared to untreated controls. While these results appear to contradict those of Gu et al. (4), they are consistent with earlier observations suggesting that biologically relevant concentrations of NO or S-nitrosothiols are not capable of significantly activating proMMPs (3, 9).
FIGURE 1.
Effects of NONOates and S-nitrosothiols on proMMP-9 activation. Purified proMMP9 (final concentration 0.2 µg/mL) was exposed to the indicated concentrations (µM) of NONOates or S-nitrosothiols, and gelatinase activity was monitored using DQ-Gelatin by fluorescence (485 Ex/530 Em). The rate of fluorescence increase was expressed relative to untreated controls (mean ± S.E.M; n = 4). For comparison, proMMP-9 activation by pCMB (200 µM) served as a positive control.
Analysis of Cys Modifications within the MMP-9 Pro-Domain
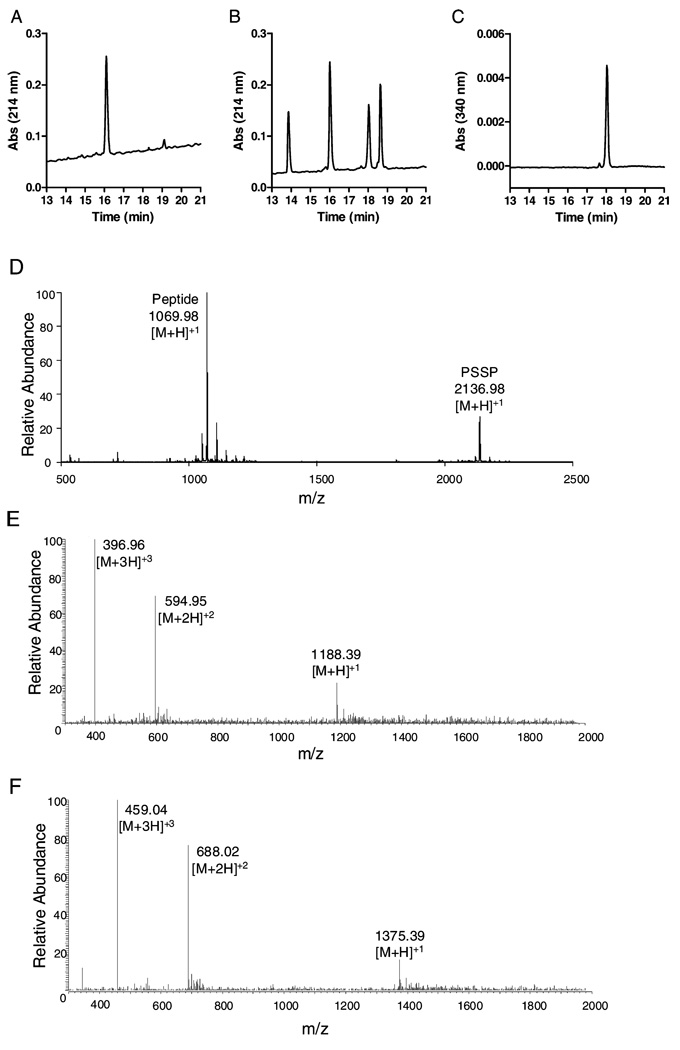
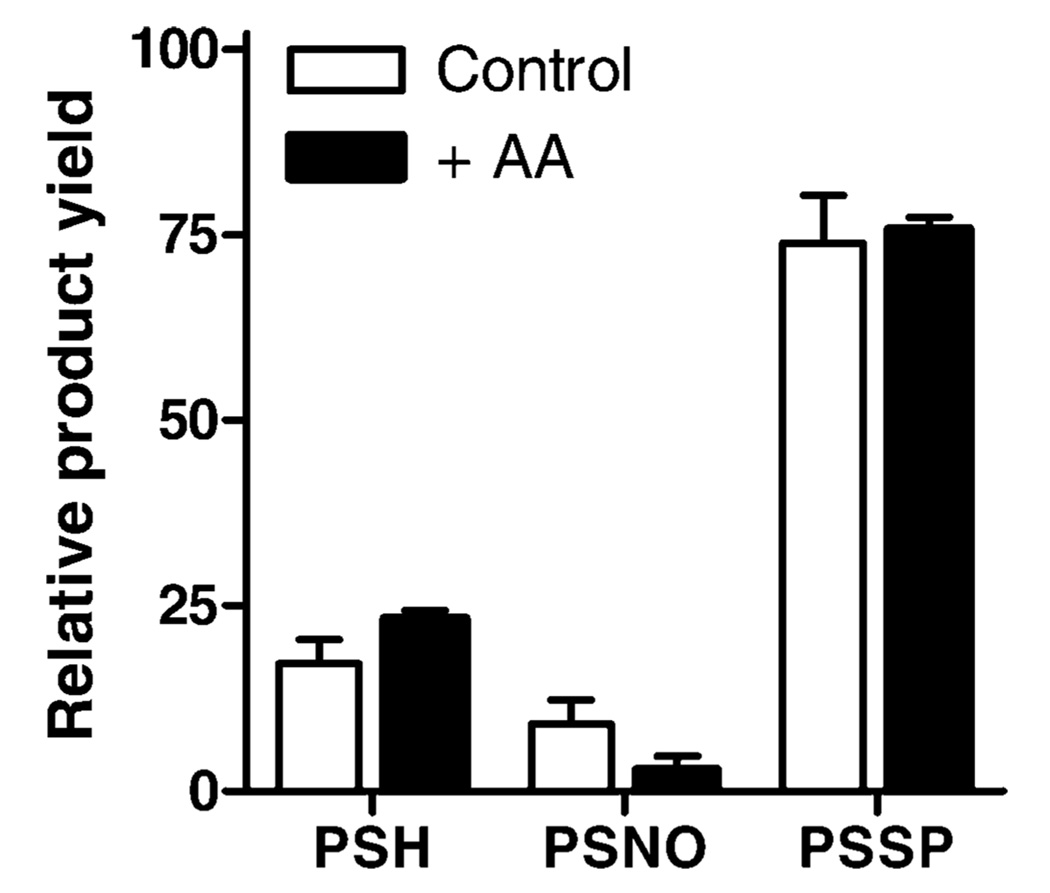
To evaluate whether the relatively selective ability of SNOC to activate proMMP-9 was associated with unique modifications of the Cys switch, we analyzed modifications in a synthetic deca-peptide that represents the Cys-containing pro-domain region of proMMP-9, under identical reaction conditions. Figure 2A–C illustrates typical HPLC chromatograms of the synthetic peptide PRCGVPDLGR and reaction mixtures after reaction of PRCGVPDLGR with CSNO. Reaction of PRCGVPDLGR with CSNO (500 µM) caused the rapid disappearance of the peptide (PSH; eluting at 16 min) and formation of three main products, eluting at 14, 18, and 19 min (Figure 2B), with absorbance at 214 nm. To determine the potential formation of an S-nitrosylated peptide (PSNO), reaction mixtures were also monitored at 340 nm (20), revealing one major peak at 18 min (Figure 2C), suggesting that this product is PSNO. Product peaks were collected for further identification by MALDI-TOF MS, which revealed that the product at 19 min represents a peptide dimer, presumably through intermolecular disulfide formation (PSSP; Figure 2D). Accordingly, reduction of reaction mixtures with DTT caused the disappearance of this product (data not shown). Since other products could not be conclusively identified by MALDI-MS, we performed additional analysis of product mixtures by LC-ESI/MS. This revealed that the product at 14 min contained a molecular mass that was increased by 119 mass units, corresponding to the peptide + Cys (PSSC; Figure 2E). Similarly, reaction products of PRCGVPDLGR incubation with GSNO contained a unique product with a mass increase of 306, representing a mixed disulfide with GSH (PSSG; Figure 2F). Although we were unsuccessful in confirming NO-addition to the peptide by MS, presumably due to the instability of the product, the characteristic absorbance at 340 nm is consistent with the formation of an S-nitrosylated peptide. Identification of this product as PSNO was confirmed by the fact that the peak at 18 min largely disappeared after 1 h of incubation with 1 mM ascorbate, which is known to decompose S-nitrosothiols (23). As illustrated in Figure 3, quantification of peak areas at 214 nm to determine relative product yields revealed that the addition of 1 mM ascorbatic acid (AA), 1 h after initiating the reaction between PRCGVP-DLGR and DEA-NO, which rapidly releases NO with t1/2 = 2 min at neutral pH, dramatically reduced PSNO product yield with a corresponding increase in unmodified peptide and a minimal effect on PSSP yield.
FIGURE 2.
HPLC and MS characterization of MMP-9 propeptide modifications by NO donors. Representative HPLC chromatograms at 214 nm of the synthetic peptide PRCGVPDLGR (M.W. 1068.6) before (A) or after (B) treatment with CSNO. Analysis of the reaction mixture at 340 nm revealed a major peak at 18 min, representing an S-nitrosated peptide (PSNO) (C). (D) MALDI-MS analysis of collected HPLC fraction at 19 min in panel B, indicating the formation of the peptide–disulfide dimer (PSSP), as well as the unmodified peptide (PSH), presumably generated during isolation/ionization. (E) ESI/MS analysis of peak at 14 min indicated the formation of the peptide–cysteine adduct (M + 118; PSSC). (F) ESI/MS analysis after the analogous reaction of PRCGVPDLGR with GSNO revealed a molecular peptide M + 305, corresponding to a mixed disulfide with GSH (PSSG).
FIGURE 3.
Effects of ascorbic acid on NO-mediated MMP-9 propeptide modifications. The peptide–peptide PRCGVPDLGR (PSH; 1 mM) was incubated with 200 µMDEA NONOate for 2 h, and subsequently in the absence or presence of 1 mM ascorbic acid (AA) for an additional 2 h before product analysis by HPLC. Remaining peptide (PSH) or products (PSNO and PSSP) were quantitated based on peak areas at 214 nm and expressed as a percentage of total peak areas of all products. Mean ± SEM (n = 3).
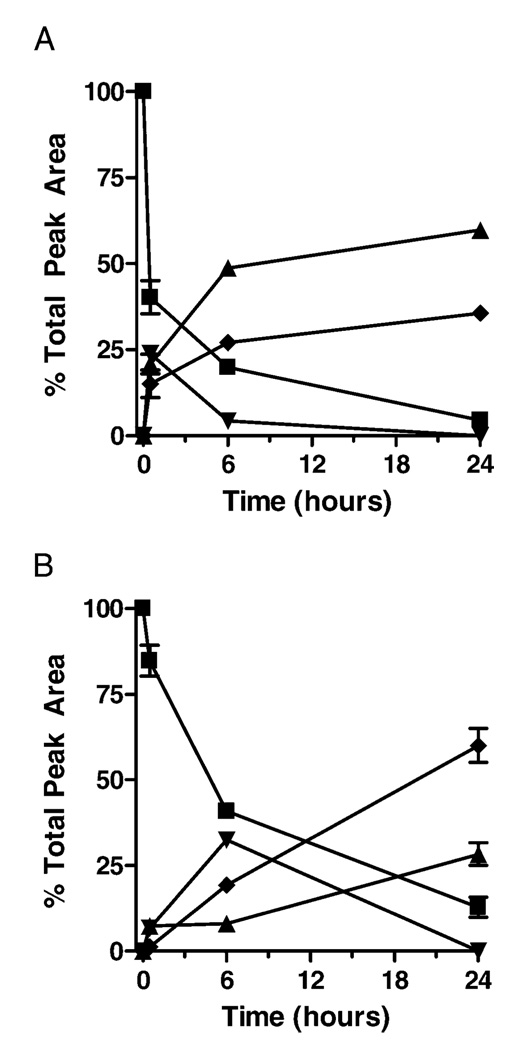
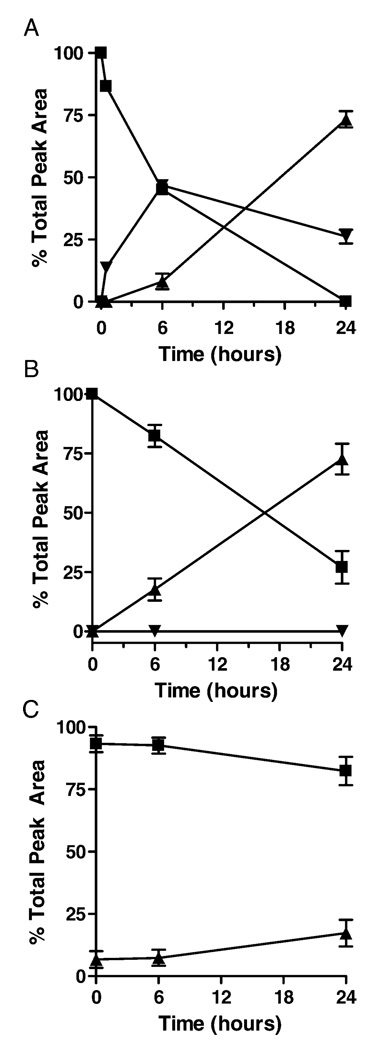
To evaluate product profiles over time, we quantified peak areas at 214 nm to determine relative product yields over time in the various reactions, assuming that the molar absorptivity of each of the peptide products at 214 nm is similar. As illustrated in Figure 4, reaction of PRCGVP-DLGR with either CSNO (A) or GSNO (B) initially yielded S-nitrosylated peptide products (PSNO), which disappeared over time with accumulation of more stable disulfide products (PSSP and PSSC, or PSSG, respectively). Similarly, the peptide reaction with DEA-NO revealed the initial formation of the S-nitrosylated product (PSNO), which was converted over time to molecular disulfide PSSP (Figure 5A). Analysis of the peptide reaction with DETA-NO, which slowly releases NO with t1/2 = 20 h at neutral pH, also resulted in gradual peptide oxidation to the disulfide, but without the detectable formation of S-nitrosylated intermediates at 340 nm (Figure 5B). Incubation of the peptide in the absence NONOates yielded modest autoxidation to the disulfide, as shown in Figure 5C. To confirm whether PSSP was formed via intermediate formation of PSNO, reactions were also performed in the presence of 1 mM AA, which markedly reduced PSNO formation and slowed the accumulation of PSSP over time (data not shown).
FIGURE 4.
Quantitative analysis of product formation after the reaction of PRCGVPDLGR (1 mM) with CSNO (500 µM; panel A) or GSNO (500 µM; panel B). Data (mean ± S.E.M.; n = 3) are expressed as a percentage of total peak area (detected at 214 nm) of all peptide products. ■ = PSH; ▴ = PSSP; ▾ = PSNO; ♦= PSSR (R = Cys or GSH).
FIGURE 5.
Quantitative analysis of product formation after incubation of PRCGVPDLGR (1 mM) in the presence of DEA-NO (100 µM; panel A) and DETA-NO (500 µM; B), or in their absence (C). Data (mean ± S.E.M.; n = 3) are expressed as percent of total peak area of all peptides. ■ = PSH; ▴ = PSSP, ▾ = PSNO.
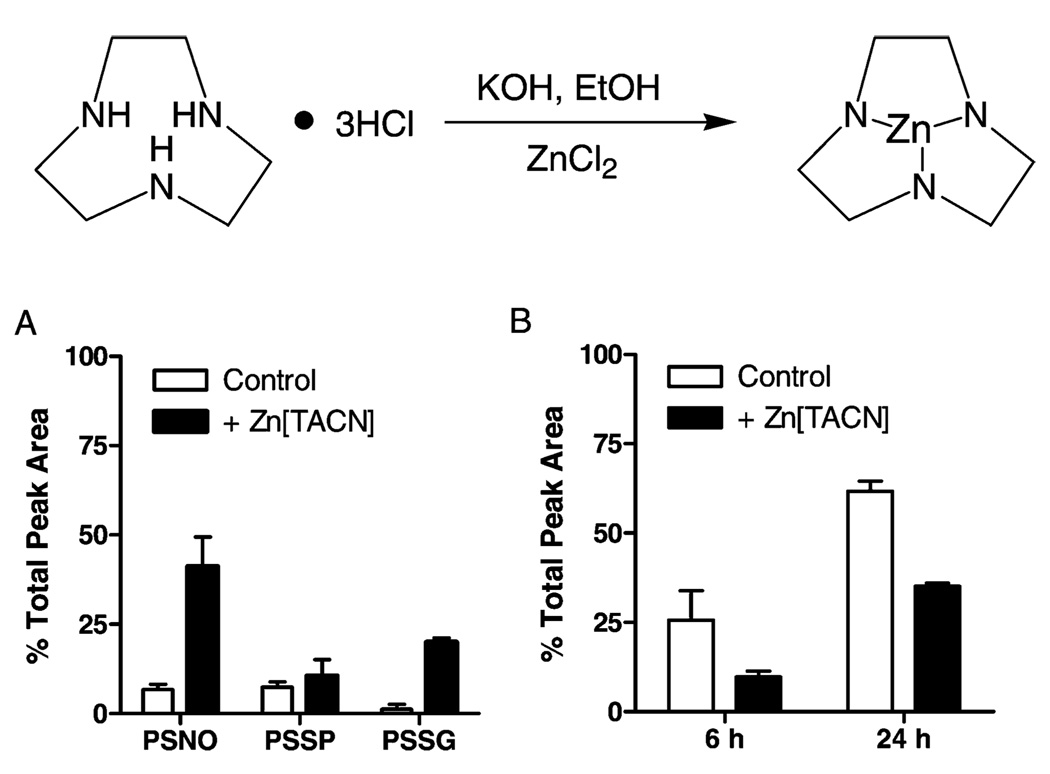
Because solutions of the Cys-containing peptide do not accurately represent the pro-domain Cys within MMP-9 and its interaction with the active site Zn2+, which could affect its nucleophilic character and reactivity toward NO (24, 25), we performed a similar analysis of peptide modifications after initial complexation with Zn[TACN]. The Zn2+ ion is ligated with three N-atoms in TACN (to represent the 3 His residues within MMP; Figure 6), allowing the fourth coordination site to interact with the peptide Cys, to form a Zn-thiolate–peptide complex. After preincubation of the peptide with Zn[TACN], the mixture was reacted with either GSNO or DETA-NO, and reaction profiles were compared to those in the absence of Zn[TACN]. Analysis of initial reaction products after the reaction of peptide or peptide/Zn[TACN] with GSNO indicated increased peptide nitrosylation and oxidation of peptide/Zn[TACN] compared to the peptide alone (Figure 6A), suggesting increased nucleophilic character of the peptide Cys by complexation with Zn[TACN] and increased sensitivity to transnitrosylation by GSNO. In contrast, the rate of PSSP accumulation by DETA-NO was markedly reduced in the presence of Zn[TACN] (Figure 6B), suggesting that Zn coordination reduces the susceptibility of Cys for oxidation by NO.
FIGURE 6.
Effect of peptide complexation with Zn[TACN] on oxidation and nitrosylation by NO-donors. Top: Schematic of Zn[TACN] synthesis. Bottom: Quantification of HPLC analysis after peptide treatment with 500 µM GSNO for 30 min (A) or PSSP accumulation by 500 µM DETA NONOate (B) in the absence (open bars) or presence (filled bars) of Zn[TACN]. Data (mean ± S.E.M.; n = 3) are expressed as a percentage of total peak area of all peptides.
High Concentrations of NO Inhibit MMP-9 Activity
Our initial studies indicated that high concentrations of NONOates appeared to suppress proMMP-9 activation (Figure 1). To explore this possibility further, we performed experiments with active MMP-9 more directly to determine proteolytic activity in the presence of NO. The MMP-9 used was activated using a mercurial compound (Calbiochem) and because of autoproteolytic processing no longer contains the Cys-containing prodomain (18). As shown in Figure 7A, MMP-9 activity was markedly decreased by DETA-NO, in a dose-dependent manner. Control experiments with DETA indicate that this inhibitory effect was due to NO release and not to the polyamine byproduct. Similar results were also obtained when an alternative fluorescence MMP-9 substrate (MMP-2/MMP-9 substrate I; Calbiochem) was used instead of DQ Gelatin (data not shown). Some inhibition of MMP-9 activity was also observed using SPER-NO, but the short-lived NO donor DEA-NO (which generates NO with a half-life of 2 min) was ineffective (Figure 7A), suggesting that MMP-9 inhibition requires the prolonged presence of NO. Similarly, GSNO (500 µM) did not significantly inhibit MMP-9 activity (Figure 7A), suggesting that MMP-9 inhibition by NO was not due to S-nitrosylation of the protein. To address the potential involvement of oxidative or nitrosative mechanisms of MMP-9 inhibition by NO, we performed similar experiments in the presence of the antioxidants AA or glutathione (GSH), which would be expected to prevent or reverse oxidative/nitrosative modifications by NO. As shown in Figure 7B, addition of AA (1 mM) did not prevent inhibition of MMP-9 activity by DETA-NO. Similarly, although the addition of GSH (1 mM) markedly inhibits MMP-9 activity, as previously reported (26), MMP-9 activity was reduced further in the presence of DETA-NO (Figure 6B). Overall, these findings suggest that NO-mediated inhibition of MMP-9 activity is due to prolonged exposure to NO itself and appears to be unrelated to S-nitrosylation or other oxidative modifications within the enzyme.
FIGURE 7.
Effects of NO donors on gelatinase activity of MMP-9. (A) Activated MMP-9 (0.2 µg/mL) was incubated with gelatin-DQ in the absence or presence of DETA-NO, SPER-NO, DEA-NO, or GSNO at indicated concentrations (µM), and gelatinase activity was monitored by fluorescence as in Figure 1. (B) Inhibition of MMP-9 gelatinase activity by 500 µM DETA-NO in the absence or presence of 1 mM ascorbic acid (AA) or 1 mM GSH. Mean ± SEM (n = 3).
DISCUSSION
Since inflammatory diseases that are commonly associated with increased production of NO and reactive nitrogen species (RNS) are also generally characterized by increased expression and activation of various MMPs, including MMP-9 (2, 3, 27), a number of investigators have explored the possibility that NO/RNS can directly regulate MMP expression and/or activation. This latter possibility was fueled by several observations that MMPs can be activated by oxidative disruption of the cysteine switch (5–10). Accordingly, several recent studies have claimed that NO is capable of directly activating proMMP-9 or related metalloproteinases, such as tumor necrosis factor Α-converting enzyme (TACE; ADAM17), by S-nitrosylation of the cysteine switch (4, 12). However, this latter notion was only supported by indirect experimental evidence. For example, analysis of NO-mediated MMP-9 activation only revealed the formation of sulfinic/sulfonic acid within the pro-domain cysteine, without direct evidence for intermediate S-nitrosylation (4). Moreover, the ability of NO to activate TACE was inferred from studies using an inhibitor peptide, corresponding to the TACE pro-peptide domain, and observations that inhibitory actions were prevented by NO that can cause S-nitrosylation of the peptide (12). However, this does not infer that NO directly activates proTACE by S-nitrosylation. Adding to this controversy, a number of studies showed that NO or S-nitrosothiols such as GSNO are incapable of directly activating purified proMMPs (8, 9). Moreover, recent studies by us and others have suggested that NO-mediated increases in MMP-9 activation in airway epithelial cells or cardiac myocytes are largely due to increased MMP-9 expression without direct evidence for NO-mediated MMP-9 activation (3, 15).
The main objective of the current study was to address this apparent controversy and to explore the potential of NO or S-nitrosothiols to directly activate proMMP-9 and to characterize the responsible chemical modifications of the pro-domain Cys residue. The main important conclusion that can be drawn from our studies is that NO or S-nitrosothiols at biologically relevant concentrations are incapable of directly activating proMMP-9, which is in contrast to several earlier suggestions (4, 11). Curiously, a recent report described activation of MMP-9 by selected concentrations of SPER-NO, which appears to counter our observations, although the mechanism of activation was not addressed (28). First, only high concentrations of an exceptionally reactive S-nitrosothiol, CSNO (29), were found to weakly activate proMMP9, consistent with earlier findings (4), but the biological significance of this is questionable because biological concentrations of SNO are in the nanomolar range (30). Second, although several diverse NO donors and S-nitro-sothiols are similarly capable of inducing S-nitrosylation within a peptide that represents the Cys-containing prodomain region of proMMP-9, the fact that most of these agents cannot activate proMMP-9 would suggest that S-nitrosylation may not be responsible for MMP-9 activation by CSNO or by other RNS (4, 9). In fact, our analysis of peptide modifications demonstrated that major final products in each case are disulfide peptide dimers or mixed disulfides, which is facilitated by the diffusible nature of small peptides compared to the pro-domain within intact MMPs. This presumably contributed to the fact that we did not detect significant formation of sulfinic/sulfonic acids because intermediate S-nitrosylated or oxidized peptides react readily with excess thiols in the reaction mixture to form disulfides. Although the formation of an intermolecular disulfide between MMP pro-domains under oxidative or nitrosative conditions in vivo is much less likely, formation of mixed disulfides during proMMP oxidation with GSH or other low-molecular weight thiols, which are ubiquitously present in vivo, may be favored over irreversible oxidation. Therefore, it appears unlikely that intermediate Cys nitrosylation in vivo would result in substantial oxidation to sulfinic/sulfonic acid, as was suggested by Gu et al. (4).
While analyses of peptide modifications by various NO donors can yield valuable mechanistic information, our studies also reveal important limitations of such peptidebased studies in addressing activation mechanisms within intact proMMPs. For example, each NO donor was readily capable of modifying the Cys-containing peptide, although they were not able to activate intact proMMP-9, presumably because of the fact that the pro-domain Cys within proMMPs is less accessible or less reactive because of its coordination with the active site Zn2+ ion. We attempted to address the latter possibility by synthesizing a zinc complex, Zn[TACN], in an attempt to mimic the structural and chemical characteristics of Zn2+ within the active site of proMMP-9. The Zn[TACN] complex is known to adopt a tetrahedral geometry (19), the reported geometry of zinc as determined in MMP-9 crystallographically (31). Additionally, the three nitrogen vertices mimic histidine coordination of Zn2+ in the active site of MMP-9. By preincubation of our synthetic peptide with the Zn[TACN] to generate Zn[TACN]-S-peptide complex, we were able to address the impact of Zn2+-S coordination on the reactivity of the peptide Cys toward NO donors. Indeed, our results indicated that Zn2+ coordination reduced the ability of NONOates to oxidize/nitrosylate the peptide Cys residue, consistent with the fact that these NONOates are unable to activate proMMP-9. Curiously, the Zn[TACN]-S-peptide complex appeared to be more susceptible to transnitrosylation by GSNO compared to the peptide alone, perhaps indicating increased nucleophilic character of the peptide Cys residue upon coordination with Zn2+. However, the fact that GSNO is incapable of activating proMMP-9 (Figure 1) or other MMPs (9) indicates that other factors, most likely related to steric hindrance, prevent GSNO from reacting with the Cys switch within intact proMMPs.
Results from our peptide studies also have important implications for studies in which inhibitor peptides for TACE or other metalloproteases are used. For example, several authors have interpreted the ability of NO or H2O2 to oxidize or nitrosate a peptide inhibitor of TACE, reducing its ability to block active TACE, as evidence that NO and H2O2 can directly activate proTACE by similar oxidative or nitrosative mechanisms (12, 32, 33). However, such a conclusion is premature and most likely incorrect. Our results (Figure 4) and previous studies (6) indicate that Cys-containing peptides are readily oxidized by NO or H2O2 to primarily form peptide dimers; hence, it is not surprising that this prevents the ability of such peptides to inhibit active TACE or other MMPs. However, formation of intermolecular disulfides is most likely irrelevant to intact proMMPs, and direct analysis of oxidative modifications within proMMPs will therefore be needed to definitively link chemical Cys modifications to oxidative MMP activation under various experimental conditions.
Although our studies did not confirm proMMP-9 activation by biologically relevant concentrations of NO or S-nitrosothiols, perhaps the most interesting and unexpected observation from our studies is the fact that high NO concentrations, which may be present during conditions of ongoing inflammation, can in fact inhibit MMP-9 activity. Recently, a similar inhibition of MMP-9 activity by high concentrations of SPER-NO was reported, but a mechanism for inhibition was not investigated or suggested (28). Although MMP-9 inhibition was observed using relatively high concentrations of DETA-NO (500–1000 µM) or SPER-NO (100 µM), these concentrations of NO donors produce sub-micromolar steady state NO levels that are reflective of NO production by, for example, activated macrophages (22, 28), and may thus be relevant to inflammatory conditions. Since MMPs are subject to inactivation by oxidative mechanisms (7, 9, 34), it may not be surprising that high concentrations of NO or RNS may similarly inactivate MMPs. However, MMP-9 inhibition by NONOates was not related to their ability to induce S-nitrosylation, and antioxidants such as AA or GSH failed to prevent MMP-9 inhibition by DETA-NO, thus suggesting that NO-mediated MMP-9 inhibition was not due to oxidative/ nitrosative mechanisms. Since NO is capable of inducing the release of zinc from Zn-thiolate centers or Zn-sulfur clusters by S-nitrosylation (25), it is possible that NO may similarly induce zinc release from MMPs by N-nitrosylation of the histidines that coordinate Zn2+. Because N-nitrosamines are relatively resistant to ascorbate (35), this would be consistent with the inability of ascorbate to prevent NO-mediated inhibition of MMP-9 activity. However, the fact that significant inhibition of gelatinase activity was observed only using relatively stable NONOates, which cause prolonged NO release throughout the experiment, suggests that this inhibition requires the persistent presence of sufficient concentrations of NO, rather than S- or N-nitrosation chemistry, which would be more prominent with high NO concentrations arising from short-lived NONOates such as DEA-NO. Alternatively, NO might inhibit MMP-9 activity by direct interaction with the active site Zn2+, thus competitively inhibiting substrate hydrolysis.
In summary, our studies demonstrate that biologically relevant concentrations of NO or S-nitrosothiols are incapable of directly activating proMMP-9, indicating that NO-mediated increases in MMP-9 activity in biological systems are not due to direct activation of proMMP-9 by S-nitrosylation of the cysteine switch. Rather, high concentrations of NO that may be generated during inflammation may in fact inhibit the protease activity of MMP-9 and perhaps other MMPs because of potential interactions with Zn2+ or release of Zn2+ from the active site. Since elevated NO production during inflammation may also inhibit MMP-9 expression (3,28), the pathophysiological significance of direct NO-mediated inhibition of MMP-9 activity for overall MMP regulation is unclear but may represent an alternative mode of MMP regulation under inflammatory conditions.
ACKNOWLEDGMENT
We thank John Heim and Brent W. Osborne for technical assistance.
Footnotes
This work was supported by NIH research grants R01 HL074295 and R01 HL068865 (to A.vdV.), T32 ES07122 (training fellowship to S.M.M.), and P20 RR16462 (to the Vermont Genetics Network).
REFERENCES
- 1.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat. Rev. Mol. Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 2.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 3.Bove PF, Wesley UV, Greul AK, Hristova M, Dostmann WR, van der Vliet A. Nitric oxide promotes airway epithelial wound repair through enhanced activation of MMP-9. Am. J. Respir. Cell Mol. Biol. 2007;36:138–146. doi: 10.1165/rcmb.2006-0253SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 5.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. U.S.A. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of promatrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J. Biol. Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 7.Michaelis J, Vissers MC, Winterbourn CC. Different effects of hypochlorous acid on human neutrophil metalloproteinases: activation of collagenase and inactivation of collagenase and gelatinase. Arch. Biochem. Biophys. 1992;292:555–562. doi: 10.1016/0003-9861(92)90030-z. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto T, Akaike T, Nagano T, Miyajima S, Suga M, Ando M, Ichimori K, Maeda H. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch. Biochem. Biophys. 1997;342:261–274. doi: 10.1006/abbi.1997.0127. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J. Biol. Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 10.Peppin GJ, Weiss SJ. Activation of the endogenous metalloproteinase, gelatinase, by triggered human neutrophils. Proc. Natl. Acad. Sci. U.S.A. 1986;83:4322–4326. doi: 10.1073/pnas.83.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manabe S, Gu Z, Lipton SA. Activation of matrix metalloproteinase-9 via neuronal nitric oxide synthase contributes to NMDA-induced retinal ganglion cell death. Invest. Ophthalmol. Visual Sci. 2005;46:4747–4753. doi: 10.1167/iovs.05-0128. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Kolls JK, Oliver P, Good D, Schwarzenberger PO, Joshi MS, Ponthier JL, Lancaster JR., Jr Activation of tumor necrosis factor-alpha-converting enzyme-mediated ectodomain shedding by nitric oxide. J. Biol. Chem. 2000;275:15839–15844. doi: 10.1074/jbc.M000604200. [DOI] [PubMed] [Google Scholar]
- 13.Berendji D, Kolb-Bachofen V, Meyer KL, Grapenthin O, Weber H, Wahn V, Kroncke KD. Nitric oxide mediates intracytoplasmic and intranuclear zinc release. FEBS Lett. 1997;405:37–41. doi: 10.1016/s0014-5793(97)00150-6. [DOI] [PubMed] [Google Scholar]
- 14.St Croix CM, Wasserloos KJ, Dineley KE, Reynolds IJ, Levitan ES, Pitt BR. Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2002;282:185–192. doi: 10.1152/ajplung.00267.2001. [DOI] [PubMed] [Google Scholar]
- 15.Cuenca J, Martin-Sanz P, Alvarez-Barrientos AM, Bosca L, Goren N. Infiltration of inflammatory cells plays an important role in matrix metalloproteinase expression and activation in the heart during sepsis. Am. J. Pathol. 2006;169:1567–1576. doi: 10.2353/ajpath.2006.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura F, Nakagawa R, Akuta T, Okamoto S, Hamada S, Maeda H, Kawabata S, Akaike T. Proapoptotic effect of proteolytic activation of matrix metalloproteinases by Streptococcus pyogenes thiol proteinase (Streptococcus pyrogenic exotoxin B) Infect. Immun. 2004;72:4836–4847. doi: 10.1128/IAI.72.8.4836-4847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7891–7896. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaser J, Knauper V, Osthues A, Reinke H, Tschesche H. Mercurial activation of human polymorphonuclear leucocyte procollagenase. Eur. J. Biochem. 1991;202:1223–1230. doi: 10.1111/j.1432-1033.1991.tb16494.x. [DOI] [PubMed] [Google Scholar]
- 19.Yang R, Zompa LJ. Metal complexes of cyclic triamines. 1. Complexes of 1,4,7-triazacyclononane ([9]aneN3) with nickel(II), copper(II), and zinc(II) Inorg. Chem. 1976;15:1499–1502. [Google Scholar]
- 20.van der Vliet A, Hoen PA, Wong PS, Bast A, Cross CE. Formation of S-nitrosothiols via direct nucleophilic nitrosation of thiols by peroxynitrite with elimination of hydrogen peroxide. J. Biol. Chem. 1998;273:30255–30262. doi: 10.1074/jbc.273.46.30255. [DOI] [PubMed] [Google Scholar]
- 21.Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, Bove AA, Isaac L, Hrabie JA, Keefer LK. Complexes of •NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J. Med. Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 22.Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, Wink DA. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8894–8899. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forrester MT, Foster MW, Stamler JS. Assessment and application of the biotin switch technique for examining protein S-nitrosylation under conditions of pharmacologically induced oxidative stress. J. Biol. Chem. 2007;282:13977–13983. doi: 10.1074/jbc.M609684200. [DOI] [PubMed] [Google Scholar]
- 24.Krezel A, Hao Q, Maret W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch. Biochem. Biophys. 2007;463:188–200. doi: 10.1016/j.abb.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Maret W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid. Redox. Signaling. 2006;8:1419–1441. doi: 10.1089/ars.2006.8.1419. [DOI] [PubMed] [Google Scholar]
- 26.Upadhya GA, Strasberg SM. Glutathione, lactobionate, and histidine: cryptic inhibitors of matrix metalloproteinases contained in University of Wisconsin and histidine/tryptophan/ ketoglutarate liver preservation solutions. Hepatology. 2000;31:1115–1122. doi: 10.1053/he.2000.6780. [DOI] [PubMed] [Google Scholar]
- 27.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radical Biol. Med. 2003;35:213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 28.Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP, Roberts DD, Wink DA. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16898–16903. doi: 10.1073/pnas.0702761104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson LA, Wagener T, Sies H, Stahl W. Decomposition of S-nitrosocysteine via S- to N-transnitrosation. Chem. Res. Toxicol. 2007;20:721–723. doi: 10.1021/tx700095u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gow A, Doctor A, Mannick J, Gaston B. S-Nitrosothiol measurements in biological systems. J. Chromatogr. B. 2007;851:140–151. doi: 10.1016/j.jchromb.2007.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowsell S, Hawtin P, Minshull CA, Jepson H, Brockbank SM, Barratt DG, Slater AM, McPheat WL, Waterson D, Henney AM, Pauptit RA. Crystal structure of human MMP9 in complex with a reverse hydroxamate inhibitor. J. Mol. Biol. 2002;319:173–181. doi: 10.1016/S0022-2836(02)00262-0. [DOI] [PubMed] [Google Scholar]
- 32.Shao MX, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 2005;102:767–772. doi: 10.1073/pnas.0408932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Oliver P, Lancaster JJ, Schwarzenberger PO, Joshi MS, Cork J, Kolls JK. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. FASEB J. 2001;15:303–305. doi: 10.1096/fj.00-0371fje. [DOI] [PubMed] [Google Scholar]
- 34.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin): an oxidative mechanism for restraining proteolytic activity during inflammation. J. Biol. Chem. 2003;278:28403–28409. doi: 10.1074/jbc.M304739200. [DOI] [PubMed] [Google Scholar]
- 35.Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc. Natl. Acad. Sci. U.S.A. 2004;101 doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]