An early evaluation of the feasibility of training fellows in robotic surgery suggests that it is feasible to incorporate a systematic approach to robotic-assisted laparoscopic training at the onset of incorporating this technology into current practice.
Keywords: Robotics, Laparoscopic surgery, Education, Gynecology
Abstract
Background and Objective:
The robotic surgical platform is an alternative technique to traditional laparoscopy and requires the development of new surgical skills for both the experienced surgeon and trainee. Our goal was to perform an early evaluation of the feasibility of training fellows in robotic-assisted gynecologic procedures at the outset of our incorporation of this technology into clinical practice.
Methods:
A systematic approach to fellow training included (1) didactic and hands-on training with the robotic system, (2) instructional videos, (3) assistance at the operating table, and (4) performance of segments of gynecologic procedures in tandem with the attending physician. Time to complete the entire procedure, individual segments, rate of conversion to laparotomy, and complications were recorded.
Results:
Twenty-one robotic-assisted gynecologic procedures were performed from April 2006 to January 2007. Fellows participated as the console surgeon in 14/21 cases. Thirteen patients (62%) had prior abdominal surgery. Median values with ranges were age 51 years (range, 33 to 90); BMI 28 (range, 19.4 to 43.8); EBL 25 mL (range, 25 to 250); and hospital stay 1 day (range, 1 to 4). No significant difference existed between fellow and attending mean total operative and individual segment times. One conversion to laparotomy was necessary. No major surgical complications occurred.
Conclusion:
These data suggest that it is feasible to incorporate a systematic approach to robotic-assisted laparoscopic training for trainees at the outset of incorporation of this technology into current practice.
INTRODUCTION
Robotic surgery received FDA approval in the United States for gynecologic laparoscopic procedures on March 15, 2005. Current applications of robotic surgery in gynecology include tubal reanastomosis, myomectomy, Burch cystourethropexy, sacrocolpopexy, hysterectomy, salpingo-oophorectomy, and cancer staging procedures.1–3 Surgical robotics provides the operator an improved 3-dimensional view,4 magnified visualization, enhanced dexterity,5 instrumental precision, and advanced ergonomics that allow one to perform exceedingly complex procedures in a minimally invasive manner compared with conventional laparoscopy. Several authors have reported on the feasibility of incorporating robotic surgery into a gynecologic practice and found fewer complications, less blood loss, shorter hospital stay, and comparable or improved number of lymph nodes harvested.6–11 It is important to note that current evidence on potential benefits from robotic surgery is not from randomized control trials. Furthermore, a significant concern is how to incorporate robotic surgery into a training program without compromising teaching or patient safety and to determine the ideal methodology for educating trainees to utilize this innovative technology. Structured robotic surgical education has been described in general surgery and urology residency programs12–14 but has not yet been reported in gynecologic or gynecologic oncology training programs.
A recent consensus statement on robotic surgery was released by the Society of American Gastrointestinal and Endoscopic Surgeons and Minimally Invasive Robotic Association emphasizing guidelines for training and credentialing.15 Guidelines for training included expert instruction, didactic experience, live case observation, and hands-on experience including simulation and clinical experience. The panel recommended that formal assessment of competency in specific procedures should be documented and an adequate number of cases be performed to allow proficiency under appropriate mentoring by an expert.
Our robotic surgical program was initiated in April of 2006 with a special emphasis on providing fellow participation as a console surgeon at the inception of the program. Given the prior experience in urology and general surgery,13,14 we determined that a systematic approach to fellow training included (1) didactic and hands-on instruction with the robotic system in conjunction with the attending surgeons, (2) review of instructional videos, (3) patient-side first assistance, and (4) performance of segments of gynecologic procedures in tandem with the senior surgeon. Our goal was to perform an early evaluation of the feasibility of training fellows in robotic-assisted gynecologic procedures at the outset of our incorporation of this technology into our clinical practice.
MATERIALS AND METHODS
Routine surgical informed consent was obtained from patients undergoing gynecologic procedures on the gynecologic oncology service at a university medical center. With Institutional Review Board approval, data were collected prospectively from April 2006 to January 2007 on all consecutive robotic-assisted procedures.
Two clinical fellows received didactic teaching regarding operation and safety features of the robotic system and training with inanimate models. One of the fellows received additional training in a porcine laboratory at a designated training center. Goals of training with inanimate models included gaining familiarity and practice with the foot-controlled clutch system and suturing. Fellows reviewed videos of robotic endometrial staging and radical hysterectomy procedures both with attendings and independently. Fellows initially served as first assistants at bedside with an attending physician at the operating console. After demonstrating proficiency as assistant and understanding of the procedure, fellows performed segments of robotic-assisted laparoscopic procedures as the console surgeon in tandem with the attending physician. As proficiency was achieved with simpler procedures, fellows began to perform pelvic lymph node and parametrial dissections. The attending surgeon determined proficiency of the fellows’ performance on individual segments. Parameters examined by the attending surgeon were ease with the clutch system to maneuver the camera and robotic instruments, tissue handling, recognition of the anatomy under magnified field of vision, and time to complete each segment.
Robotic-assisted procedures included in the current study are (1) bilateral salpingo-oophorectomy (BSO); (2) total laparoscopic hysterectomy with BSO; (3) endometrial cancer staging including total laparoscopic hysterectomy, bilateral slapingo-oophorectomy, and pelvic lymphadenectomy (LND); and (4) laparoscopic radical hysterectomy with LND. Performance of adhesiolysis was recorded. Total times for the procedure and individual segments were recorded. Total operative time was measured from skin incision to completion of skin closure. Procedures were divided into the following segments: (1) dissection of the pararectal and paravesical spaces with ligation of the ovarian and uterine vessels, (2) creation of bladder flap, (3) colpotomy, (4) vaginal cuff closure, (5) unilateral pelvic lymph node dissection, and (6) parametrial dissection with uterine artery ligation, ureterolysis, and vesicouterine ligament division to the level of the ureterovesical junction. Abstracted data also included history of prior abdominal surgery, age, body mass index (BMI), estimated blood loss (EBL), length of hospital stay, complications, and conversion to laparotomy. Fellows performed operative segments of increasing difficulty as they demonstrated proficiency with the robotic system. Descriptive statistical analysis and the Student t test for pairwise comparisons were performed using SAS software version 9.1 (Cary, NC). Statistical significance was set at P≤0.05 for all comparisons.
RESULTS
Twenty-one robotic-assisted procedures were performed during the 9-month period (Table 1). Of the 21 cases, adhesiolysis was performed in 7. Fellows participated in all consecutive cases and were console surgeons in 14/21 cases. Sixty-two percent (13/21) of patients had prior abdominal surgery. Median values for age, body mass index (BMI), estimated blood loss (EBL), and length of hospital stay are presented in Table 2. There was no significant difference in the mean total operative time for the 14 cases between fellows (223±120 minutes; range, 109 to 517) as console surgeon and attendings (174±65 minutes; range, 127 to 316). Mean times for individual operative segments are listed in Table 3. No significant difference existed between fellow and attending mean times for individual segments.
Table 1.
Gynecologic Procedures With Mean Operative Times
| Procedure (N = 21) | N | Mean ± SD (minutes) | Range (minutes) |
|---|---|---|---|
| BSO* | 2 | 247 ± 170 | 127–368† |
| Hysterectomy and BSO* | 10 | 149 ± 18 | 118–186 |
| Endometrial staging | 7 | 213 ± 84 | 109–316 |
| Radical hysterectomy | 2 | 428 ± 125 | 340–517 |
BSO = Bilateral salpingo-oophorectomy.
Case conversion to open laparotomy due to ovarian malignancy.
Table 2.
Demographic Data
| Demographic | Median (range) |
|---|---|
| Age (years) | 51 (33–90) |
| Body mass index | 28 (19.4–43.8) |
| Estimated blood loss (mL) | 25 (25–250) |
| Length of hospital stay (days) | 1 (1–4) |
Table 3.
Mean Operative Times for Individual Segments of Operative Procedures
| Segment of Procedure | Fellows | Attendings | P Value | |||
|---|---|---|---|---|---|---|
| N | Minutes (SD) | N | Minutes (SD) | |||
| Unilateral sidewall dissection | 12 | 17 (7) | 11 | 14 (6) | 0.22 | |
| Bladder flap | 3 | 6 (4) | 8 | 10 (7) | 0.44 | |
| Colpotomy | 10 | 12 (5) | 3 | 9 (1) | 0.26 | |
| Vaginal cuff closure | 10 | 18 (7) | 3 | 24 (16) | 0.34 | |
| Unilateral pelvic LND* | 4 | 24 (9) | 8 | 36 (13) | 0.13 | |
| Unilateral parametrial dissection | 1 | 95 (NA) | 3 | 56 (19) | 0.21 | |
| Total operative time | 14 | 223 (120) | 7 | 174 (65) | 0.34 | |
LND = lymph node dissection; NA = not applicable.
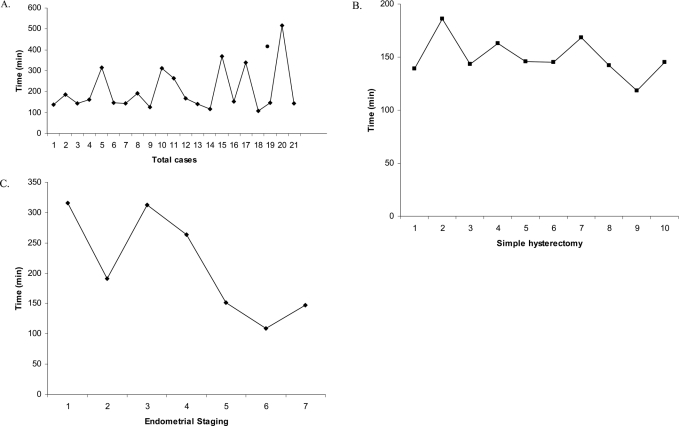
Total operative times for all robotic procedures, simple hysterectomy, and endometrial staging cases throughout this series are presented in Figure 1. When comparing the first 10 with the final 11 cases, there was no significant difference in total operative time, either comparing all procedures or simple hysterectomies only. However, over the study period, there was a significant improvement in total operative time when endometrial cancer staging procedures were performed (P=0.04).
Figure 1.
Total operative times for all cases (A), simple hysterectomy (B), endometrial cancer staging (C).
One conversion to laparotomy occurred due to an unanticipated diagnosis of ovarian cancer in addition to the known endometrial cancer. The conversion was necessary in order to perform high para-aortic lymph node dissection to complete ovarian cancer staging. At that time, we were using the first generation da Vinci surgical system (Intuitive Surgical Inc, Sunnyvale, CA); given the limitation of the range of motion, we were not able to perform a high para-aortic nodal dissection that we may have been able to perform with the second-generation model. One urinary tract infection diagnosed on postoperative day 6 was treated with antibiotics. One patient was kept intubated overnight due to upper airway edema and was extubated the following morning. This was a combined case with the urogynecologist who performed uterosacral suspension after endometrial staging was completed. Total operative time was 316 minutes, and the patient was discharged on postoperative day 2.
DISCUSSION
Adoption of new technology into current practice carries challenges for both experienced gynecologic oncology surgeons and their trainees in academic institutions. Although several institutions have published their experience in incorporating robotic surgery into their gynecologic practices,8,9,16–20 there have been no published data regarding instruction of trainees at the inception of a robotics program. Our data demonstrate that operative times of specific procedure segments were comparable between fellows and attending surgeons. Operative times reflect the learning curve phase on the robotic platform for both the fellows and attendings; significant improvement in operative times was observed for fellows performing sidewall dissection. No perioperative adverse events specific to the robot occurred.
Of utmost importance is development of an established methodology to educate and train fellows and residents in robotic techniques. General surgeons and urologists have described organized surgical training approaches for both residents and fellows with robotic surgery.13,14 Emphasis has been placed on developing an effective curriculum21 and increasing robotic responsibilities when proficiency has been established. Ali et al14 described a gradual approach to their robotic curriculum that focused on completion of 3 discrete tasks of increasing difficulty in performing Roux-en-Y gastric bypass. Fellows were required to perform 10 cases of one operative segment before performing the next task. Rashid et al13 required urology residents to assist in 12 cases before starting console training. Residents proceeded to the next step in performing a prostectomy only after showing proficiency on 3 separate occasions. In contrast, our fellows were console surgeons within the first 10 robotic cases performed at our institution. With the exception of laparoscopic radical hysterectomy, fellows were undergoing concurrent instruction in advanced conventional laparoscopic and open techniques.
Previously described models for robotic surgical training include the use of small and large animals,22 virtual simulators,23 and repetitive drill performance on the robotic system.24–26 Animal training laboratories provide an effective live surrogate for robotic operative training,27 however, may be too costly for educational programs. As shown in our experience, animate model training was not necessary to gain proficiency.
Our current strategy with the use of a didactic program, instructional videos, repetitive drills on inanimate models followed by systematic surgical integration with patient bedside assistance and tandem performance of progressively more difficult procedures has been successful in our fellowship training program. Furthermore, telestration using the interactive monitor on the daVinci S system (Intuitive Surgical Inc, Sunnyvale, CA) facilitated the attending physician’s ability to instruct the trainee in the surgical field in the latter cases when the newer system became available. The newer system has the ability to telestrate from the bedside monitor. The attending physician can mark the 2-dimensional screen to direct the fellow where to continue with the dissection, and the fellow can see these markings through the 3-dimensional view in the console.
The advantage of our training paradigm was the rapid incorporation of our fellows as console surgeons; we believe this strengthened their surgical abilities in minimally invasive surgery. The robotic platform may even allow faster acquisition of laparoscopic skills compared with traditional laparoscopy.28 In this initial experience, we noted that the learning curve for fellows with robotic surgery is far more rapid than that associated with conventional laparoscopy for more complex gynecologic oncology procedures. A perceived disadvantage to our approach is that the attending surgeon may need to perform more cases to become proficient in robotic surgery if the trainee is performing part of every operative procedure. In our experience, the transition from conventional laparoscopy to robotic hysterectomy and pelvic lymph node dissection was not problematic. In contrast, radical hysterectomy was a more challenging procedure because we did not perform traditional laparoscopic radical hysterectomy at our institution prior to performing this procedure robotically.
Prolonged total operative times recorded in our current study may be considered a disadvantage to using the robotic platform. However, the cases presented are from our initial experience with the robotic platform, thus reflecting the learning curve of the attending surgeons, operative team, anesthesiologists, and nursing staff. Included in the learning curve is the operative time for assembly and disassembly of the robot that have been described previously.29 Furthermore, a contributing factor to the increased operative time was the lack of a dedicated robotic surgical team. This is important as members of the surgical team who are knowledgeable about the new technology can assist and troubleshoot more efficiently.
Our study is limited by the small number of cases presented. Because of this, the minimum number of cases required to achieve proficiency with each procedure with the robotic platform was not achieved. However, there was a significant improvement in total operative time for endometrial staging procedures after the initial 4 cases. Other authors have reported that 20 cases to 50 cases for benign gynecologic procedures30,31 and 20 cases for endometrial cancer staging procedures32 are the minimum number of cases required to gain proficiency with the robotic technology. It should be noted that the side of dissection was not allocated before beginning each case. The decision was made after intraoperative assessment, and the attending often completed the more difficult side. This may have contributed to the finding of no significant difference between attending and fellow operative times. Theoretically, early involvement of the fellows as console surgeon could be associated with greater complications. However, in this initial experience, we did not find either an increase in adverse events or compromised patient safety. Further investigation is necessary to ensure patient safety with teaching of physician trainees of various surgical experience levels in residency programs.
CONCLUSION
Once a robotic surgical team has been established, we believe the success of the robotic program includes early integration and participation of fellows at the inception of the adoption of this new modality. We have shown the feasibility of incorporating a systematic introduction to robotic-assisted surgery for trainees. With the adoption of new technologies into our surgical armamentarium, a structured curriculum and formal assessment of competency will need to be defined for trainees at various levels of surgical skills. Valid concerns exist on the proper use and allocation of health care resources with new technologies. A comprehensive critique of the role of robotic surgery in gynecology is beyond the scope of this current discussion. However, further investigation is warranted, regarding the cost effectiveness of performing robotic-assisted gynecologic procedures.
Contributor Information
Paula S. Lee, Division of Gynecologic Oncology, Duke University Medical Center, Durham, North Carolina, USA..
Amy Bland, Division of Gynecologic Oncology, Duke University Medical Center, Durham, North Carolina, USA..
Fidel A. Valea, Division of Gynecologic Oncology, Duke University Medical Center, Durham, North Carolina, USA..
Laura J. Havrilesky, Division of Gynecologic Oncology, Duke University Medical Center, Durham, North Carolina, USA..
Andrew Berchuck, Division of Gynecologic Oncology, Duke University Medical Center, Durham, North Carolina, USA..
Angeles Alvarez Secord, Division of Gynecologic Oncology, Duke University Medical Center, Durham, North Carolina, USA..
References:
- 1. Dharia SP, Falcone T. Robotics in reproductive medicine. Fertil Steril. 2005;84:1–11 [DOI] [PubMed] [Google Scholar]
- 2. Visco AG, Advincula AP. Robotic gynecologic surgery. Obstet Gynecol. 2008;112:1369–1384 [DOI] [PubMed] [Google Scholar]
- 3. Sroga J, Patel SD, Falcone T. Robotics in reproductive medicine. Front Biosci. 2008;13:1308–1317 [DOI] [PubMed] [Google Scholar]
- 4. Blavier A, Gaudissart Q, Cadiere GB, et al. Comparison of learning curves and skill transfer between classical and robotic laparoscopy according to the viewing conditions: implications for training. Am J Surg. 2007;194:115–121 [DOI] [PubMed] [Google Scholar]
- 5. Guru KA, Kuvshinoff BW, Pavlov-Shapiro S, et al. Impact of robotics and laparoscopy on surgical skills: A comparative study. J Am Coll Surg. 2007;204:96–101 [DOI] [PubMed] [Google Scholar]
- 6. Boggess JF, Gehrig PA, Cantrell L, et al. A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: robotic assistance, laparoscopy, laparotomy. Am J Obstet Gynecol. 2008;199:360 e1–9 [DOI] [PubMed] [Google Scholar]
- 7. Boggess JF, Gehrig PA, Cantrell L, et al. A case-control study of robot-assisted type III radical hysterectomy with pelvic lymph node dissection compared with open radical hysterectomy. Am J Obstet Gynecol. 2008;199:357 e1–7 [DOI] [PubMed] [Google Scholar]
- 8. Ko EM, Muto MG, Berkowitz RS, et al. Robotic versus open radical hysterectomy: a comparative study at a single institution. Gynecol Oncol. 2008;111:425–430 [DOI] [PubMed] [Google Scholar]
- 9. Magrina JF, Kho RM, Weaver AL, et al. Robotic radical hysterectomy: comparison with laparoscopy and laparotomy. Gynecol Oncol. 2008;109:86–91 [DOI] [PubMed] [Google Scholar]
- 10. Veljovich DS, Paley PJ, Drescher CW, et al. Robotic surgery in gynecologic oncology: program initiation and outcomes after the first year with comparison with laparotomy for endometrial cancer staging. Am J Obstet Gynecol. 2008;198:679 e1–9; discussion e9–10 [DOI] [PubMed] [Google Scholar]
- 11. Nezhat FR, Datta MS, Liu C, et al. Robotic radical hysterectomy versus total laparoscopic radical hysterectomy with pelvic lymphadenectomy for treatment of early cervical cancer. JSLS. 2008;12:227–237 [PMC free article] [PubMed] [Google Scholar]
- 12. De Ugarte DA, Etzioni DA, Gracia C, et al. Robotic surgery and resident training. Surg Endosc. 2003;17:960–963 [DOI] [PubMed] [Google Scholar]
- 13. Rashid HH, Leung YY, Rashid MJ, et al. Robotic surgical education: a systematic approach to training urology residents to perform robotic-assisted laparoscopic radical prostatectomy. Urology. 2006;68:75–79 [DOI] [PubMed] [Google Scholar]
- 14. Ali MR, Rasmussen J, BhaskerRao B. Teaching robotic surgery: a stepwise approach. Surg Endosc. 2007;21:912–915 [DOI] [PubMed] [Google Scholar]
- 15. Herron DM, Marohn M. A consensus document on robotic surgery. Surg Endosc. 22:313–325, 2008; discussion 1–2 [DOI] [PubMed] [Google Scholar]
- 16. Shafer A, Boggess JF. Robotic-assisted endometrial cancer staging and radical hysterectomy with the da Vinci surgical system. Gynecol Oncol. 2008;111:S18–23 [DOI] [PubMed] [Google Scholar]
- 17. Reynolds RK, Advincula AP. Robot-assisted laparoscopic hysterectomy: technique and initial experience. Am J Surg. 2006;191:555–560 [DOI] [PubMed] [Google Scholar]
- 18. Seamon LG, Cohn DE, Henretta MS, et al. Minimally invasive comprehensive surgical staging for endometrial cancer: Robotics or laparoscopy? Gynecol Oncol. 2009;113:36–41 [DOI] [PubMed] [Google Scholar]
- 19. Beste TM, Nelson KH, Daucher JA. Total laparoscopic hysterectomy utilizing a robotic surgical system. JSLS. 2005;9:13–15 [PMC free article] [PubMed] [Google Scholar]
- 20. Ferguson JL, Beste TM, Nelson KH, et al. Making the transition from standard gynecologic laparoscopy to robotic laparoscopy. JSLS. 2004;8:326–328 [PMC free article] [PubMed] [Google Scholar]
- 21. Chitwood WR, Jr., Nifong LW, Chapman WH, et al. Robotic surgical training in an academic institution. Ann Surg. 234:475–484, 2001; discussion 84–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehrabi A, Yetimoglu CL, Nickkholgh A, et al. Development and evaluation of a training module for the clinical introduction of the da Vinci robotic system in visceral and vascular surgery. Surg Endosc. 2006;20:1376–1382 [DOI] [PubMed] [Google Scholar]
- 23. Halvorsen FH, Elle OJ, Dalinin VV, et al. Virtual reality simulator training equals mechanical robotic training in improving robot-assisted basic suturing skills. Surg Endosc. 2006;20:1565–1569 [DOI] [PubMed] [Google Scholar]
- 24. Ro CY, Toumpoulis IK, Ashton RC, Jr., et al. A novel drill set for the enhancement and assessment of robotic surgical performance. Stud Health Technol Inform. 2005;111:418–421 [PubMed] [Google Scholar]
- 25. Di Lorenzo N, Coscarella G, Faraci L, et al. Robotic systems and surgical education. JSLS. 2005;9:3–12 [PMC free article] [PubMed] [Google Scholar]
- 26. Narazaki K, Oleynikov D, Stergiou N. Robotic surgery training and performance: identifying objective variables for quantifying the extent of proficiency. Surg Endosc. 2006;20:96–103 [DOI] [PubMed] [Google Scholar]
- 27. Hanly EJ, Marohn MR, Bachman SL, et al. Multiservice laparoscopic surgical training using the daVinci surgical system. Am J Surg. 2004;187:309–315 [DOI] [PubMed] [Google Scholar]
- 28. Sarle R, Tewari A, Shrivastava A, et al. Surgical robotics and laparoscopic training drills. J Endourol. 18:63–66, 2004; discussion 6–7 [DOI] [PubMed] [Google Scholar]
- 29. Nezhat C, Saberi NS, Shahmohamady B, et al. Robotic-assisted laparoscopy in gynecological surgery. JSLS. 2006;10:317–320 [PMC free article] [PubMed] [Google Scholar]
- 30. Lenihan JP, Jr., Kovanda C, Seshadri-Kreaden U. What is the learning curve for robotic assisted gynecologic surgery? J Minim Invasive Gynecol. 2008;15:589–594 [DOI] [PubMed] [Google Scholar]
- 31. Pitter MC, Anderson P, Blissett A, et al. Robotic-assisted gynaecological surgery-establishing training criteria; minimizing operative time and blood loss. Int J Med Robot. 2008;4:114–120 [DOI] [PubMed] [Google Scholar]
- 32. Seamon LG, Cohn DE, Richardson DL, et al. Robotic hysterectomy and pelvic-aortic lymphadenectomy for endometrial cancer. Obstet Gynecol. 2008;112:1207–1213 [DOI] [PubMed] [Google Scholar]