Preliminary data support the feasibility of laparoscopic management of early endometrial and cervical cancer in Greece with outcomes similar to those reported in the international literature.
Keywords: Laparoscopy, Radical hysterectomy, Endometrial cancer, Cervical cancer
Abstract
Background:
The role of laparoscopy in the management of early stage endometrial and cervical cancer is continuously validated by many reports throughout the world. Interestingly, such data are still unavailable in many European countries, as it is in Greece. In this prospective study, we report on initial feasibility, safety, and cost outcomes of laparoscopic management of early stage endometrial and cervical cancer, recently introduced in our country.
Materials and Methods:
This was a prospective pilot study comprising a case series. Patients referred to a tertiary referral medical center with a recent diagnosis of endometrial or cervical cancer were evaluated, and those meeting inclusion criteria were offered laparoscopic surgical staging.
Results:
Out of 64 patients evaluated, 17 with early clinical stage endometrial cancer and 8 with early clinical stage cervical cancer underwent successful laparoscopic staging. Mean patient age was 61.6 and 39.2 years, mean BMI was 32.3 and 24.1kg/m2, mean operative time was 243 and 284 minutes, mean estimated blood loss was 190mL and 270mL, mean lymph node count was 27.2 and 29.1, and mean hospital stay was 2 and 3 days for endometrial and cervical cancer cases, respectively. The overall costs for the procedures performed were not greater than their laparotomy counterpart. One intraoperative complication was managed laparoscopically, and 2 cases occurred of postoperative lymphocyst formation.
Conclusion:
To our knowledge, this is the first study of laparoscopic management of early endometrial and cervical cancer in Greece. Our preliminary data support the feasibility, safety, and cost effectiveness of laparoscopic management of early endometrial and cervical cancer in our country and are in accordance with series reported in the international literature.
INTRODUCTION
Since the first case reports of laparoscopic management of early endometrial and cervical cancer in the late 80s and early 90s,1,2 many case-series have demonstrated the feasibility, safety, and advantages of laparoscopic staging of gynecologic malignancies.3–7 Recent studies have also shown equivalent long-term survival outcomes of laparoscopic staging compared with those of the abdominal staging procedures.8,9
Although laparoscopic surgery has been routinely performed in our country for benign gynecologic conditions for nearly 2 decades, its adoption in the management of gynecologic malignancies is still at a preliminary stage. A total laparoscopic radical hysterectomy (type III) with pelvic and paraaortic lymphadenectomy for cervical cancer was first described in late 2004, published a year later10 and marked the opening of our pilot study. Thereafter, we report our preliminary data on the feasibility, safety, and cost of laparoscopic management of early stage endometrial and cervical cancer in Greece. We discuss our experience, and a review of the literature is provided.
MATERIALS AND METHODS
In November 2004, a database was created at our institution, commencing a prospective pilot study on the feasibility safety and costs of laparoscopic management of early endometrial and cervical cancers. Eligible candidates were offered total laparoscopic radical or total laparoscopic extrafascial hysterectomy, as well as systematic pelvic lymphadenectomy. If high-risk features were perioperatively identified, such as grossly involved pelvic nodes, suspected stage IC or greater endometrial cancer as well as bulky stage IB1 or greater cervical cancer, additional paraaortic lymph node dissection was instituted.
A total of 64 new cases were evaluated. Eligibility criteria for a laparoscopic approach were defined as Eastern Cooperative Oncology Group (ECOG) or Zubrod performance status ≤2, primary disease, early stage endometrial cancer (IA to IIB), and early clinical stage cervical cancer (IA2 to IIA). Exclusion criteria were poor performance status, recurrent or suspected advanced stage disease, a history of incompletely staged prior laparotomy or laparoscopy, or those diagnosed based on a posthysterectomy pathology report, who had been referred for complete surgical staging, were also excluded.
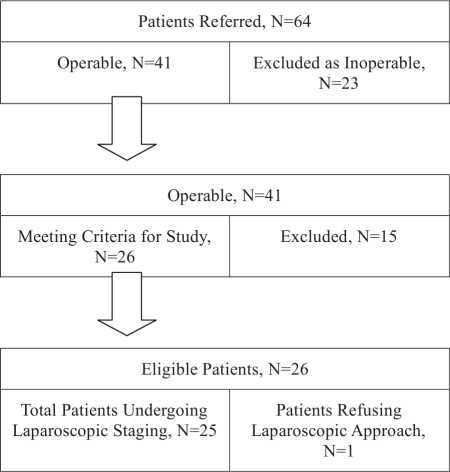
After thorough evaluation, 41 patients were deemed operable. The remainder (n=23) were excluded due to poor performance status (n=8) or advanced stage disease (n=15). Of the operable patients (n=41), 15 (n=15) were excluded due to recent a history of an incompletely staged procedure (n=7), diagnosis provided on a posthysterectomy specimen (n=3), or recurrent disease (n=5). Those cases were managed independently. Twenty-six patients met eligibility criteria for our study. One patient refused the laparoscopic approach despite eligibility (Figure 1). All patients with endometrial cancer had a preoperative dilatation and fractional curettage or hysteroscopy D&C. Patients with microscopic preclinical cervical cancer had a cold knife cone biopsy. All patients underwent MRI, according to our institution's protocol, evaluation of the abdomen and pelvis, and a chest x-ray as part of the preoperative evaluation. The night before, a mechanical and antibiotic bowel preparation was administered along with a prophylactic dose of low-molecular weight heparin 12 hours before the procedure.11 Ureteral stents were placed in 2 patients who had a history of previous abdominal surgery for diverticular colon disease and endometriosis, respectively.
Figure 1.
Patient selection diagram.
All cases were managed by the same surgeon and operating room personnel. All portions of the procedures were performed entirely laparoscopically.
Lymph nodes were retrieved in a laparoscopic bag and removed through the suprapubic port in all cases. Preoperative clinical stage, operative time, lymph nodes retrieved, estimated blood loss, and hospital stay were recorded. All intraoperative complications and complications that occurred within the first 30 postoperative days were also included in the analysis.
RESULTS
From November 2004 until September 2008, 25 patients met inclusion criteria and were laparoscopically managed, 17 patients with early stage endometrial cancer and 8 with early stage cervical cancer. A total laparoscopic hysterectomy and bilateral salpingo-oophorectomy (BSO) was performed for endometrial cancer cases, and a total laparoscopic radical hysterectomy (type II or III) with or without BSO (depending on risk factors or age) was performed in cervical cancer patients. Mean patient age was 61.6 years and 39.2 years and mean BMI was 32.3 kg/m2 and 24.1 kg/m2 in endometrial and cervical cancer patients respectively. Patient characteristics and demographics are summarized in Table 1.
Table 1.
Patients Characteristics and Demographics
| Total Laparoscopic Hysterectomy | Total Radical Hysterectomy | |
|---|---|---|
| Number (n) | 17 | 8 |
| Mean patient age (years) | 61.6 (45–89) | 39 (33–47) |
| BMI (kg/m2) | 32.3 (19.7–43.8) | 24.1 (21.9–27.4) |
| Clinical Stage | ||
| Endometrial Cancer | ||
| IA | 8 | – |
| IB | 7 | – |
| IC | 1 | – |
| IIA | 1 | – |
| Endometrial Grade | ||
| G1 | 6 | – |
| G2 | 9 | – |
| G3 | 2 | – |
| Endometrial Histology | ||
| Endometroid | 16 | – |
| Clear cell | 1 | – |
| Clinical Stage | ||
| Cervical Cancer: | ||
| IA2 | – | 4 |
| IB1 | – | 2 |
| IB2 | – | 1 |
| IIA | – | 1 |
| Cervical Histology: | ||
| Squamous | – | 5 |
| Adenocarcinoma | – | 3 |
| Type II/III Radical Ratio | – | 6/2 |
A complete pelvic lymphadenectomy was carried out in all endometrial and cervical cancer patients. Of the endometrial cancer patients (n=18), 3 had suspected or known high-risk features and underwent additional paraaortic lymphadenectomy. The first had a grade 2 endometrioid endometrial cancer with macroscopic evidence of tumor invasion of more than one-half of the myometrium upon inspection of the open specimen on the operating table (clinical stage IC), the second one was a patient with grade 2 to 3 endometrioid endometrial cancer and macroscopic evidence of tumor, extending below the isthmus into the cervix (clinical stage IIA), and the third had stage IA clear cell endometrial carcinoma. Finally, there was one case of poorly differentiated stage IIA barrel-shaped squamous cervical cancer, in which an extended paraaortic lymphadenectomy was performed as well.
For endometrial cancer and cervical cancer cases, mean operative time was 243 and 296 minutes, mean estimated blood loss was 190 mL and 270 mL, mean lymph node count was 31.6 and 28.1, and mean hospital stay was 2 days and 3 days, respectively. The results, complications, and adjuvant treatment of the study patients are summarized in Table 2.
Table 2.
Study Results, Complications and Postoperative Adjuvant Treatment
| Total Laparoscopic Hysterectomy | Total Laparoscopic Hysterectomy | Total Radical Hysterectomy |
|---|---|---|
| Number (n) | 17 | 8 |
| Mean Operative Time (min | 243 (120–300) | 284 (210–480) |
| Mean Estimated Blood Loss (ml) | 190 (50–250) | 270 (250–300) |
| Mean Lymph Nodes Retrieved (n) | 27.2 (10–39) | 29.1 (14–42) |
| Mean Hospital Stay (days) | 2 (1–3) | 3 (1–8) |
| Conversion to Laparotomy (n) | 0 | 0 |
| Major Complications (%) | 1/25 (4%) | |
| Major and Minor Complications (%) | 1/17 (6%) | 2/8 (25%) |
| Total Complications (%) | 3/25 (12%) | |
| Intraoperative | 0 | 1 |
| Postoperative | ||
| Early (<7 days) | 0 | 0 |
| Late (<30 days) | 1 | 1 |
| Lymphocyst formation | 1 | 1 |
| Patients Upstaged Due to (+) Lymph Nodes (%) | 17.5% (3/17) | 25% (2/8) |
| Adjuvant Treatment (total n) | 7 | 3 |
| EBRT* plus Chemotherapy | 3 | – |
| Vaginal Brachytherapy | 4 | – |
| Pelvic EBRT | – | 1 |
| Chemoradiation† | – | 1 |
| Chemoradiation† plus Vaginal Brachytherapy | – | 1 |
EBRT b external beam radiation therapy.
Platinum based.
Three of the 17 patients (17.5%) with endometrial cancer had node metastasis. The first had pelvic and paraaortic involvement (clinical stage IA, clear cell carcinoma), and the other 2 microscopic pelvic node metastases (both with clinical stage IB grade 2 endometrioid cancer). All 3 received adjuvant chemotherapy with carboplatin/paclitaxel and tailored external beam radiation. Four patients with endometrioid endometrial cancer, stage IB grade 2 with negative nodes but risk factors such as positive lymphovascular involvement (n=1) or lower uterine segment involvement (n=2) or both (n=1), received adjuvant vaginal brachytherapy.
Nodal involvement was also present in 25% (2 of 8) of cervical cancer patients. They both received adjuvant platinum-based chemoradiation, and one of them with a large-volume stage IIA tumor and vaginal extension, additional vaginal brachytherapy. Last, one patient with cervical adenocarcinoma stage IB2 disease and negative nodes received adjuvant pelvic radiation therapy due to significant deep stromal invasion. The remaining 5 patients with cervical cancer had no additional risk factors after surgery and are being observed by a standard surveillance protocol.
The first patient suffered a left external iliac vein laceration. However, it was promptly repaired intraoperatively with placement of vascular clips parallel to the vessel. In the same patient, a blood transfusion was required with 2 units of packed red blood cells. The postoperative course was uneventful. However, to minimize the risk of thromboembolic events, the patient was placed on a therapeutic dose of unfractionated heparin, as per deep venous thrombosis protocol. She was discharged on day 8 on oral Coumadin. Two patients, one with endometrial cancer and one with cervical cancer, developed asymptomatic lymphocysts, with no further sequelae. The remaining 22 patients had no intraoperative, early or late postoperative complications or infectious morbidity. An elderly patient with clear cell endometrial cancer died of a noncancer-related cause. At the time of this report, all patients remain clinically disease-free.
At the conclusion of this study, a cost-analysis was implemented by assessing the hospital's accounting administration computer database. When almost only reusable instruments were utilized, the average standard hospital cost for the laparoscopic procedures was decreased compared with the cost of abdominal procedures. Abdominal procedures derived from cases performed by the authors during the same study period in endometrial or cervical cancer cases that either did not meet inclusion criteria for the present study or were performed prior to the opening of the study in the same and other medical centers in Athens. However, when disposable instruments were extensively utilized (trocars, shears, electrocoagulation devices, uterine manipulators, and others), the average adjusted hospital cost for the laparoscopic procedures was significantly increased. Furthermore, the cost-analysis revealed that in those cases where reusable instruments were utilized along with the use of several disposable instruments as deemed necessary, such as the laparoscopic endobag and laparoscopic vascular clip applicator, the average calculated hospital cost of the laparoscopic procedures was comparable to that of the abdominal procedures, without affecting operative time, lymph node yield, or safety (Table 3).
Table 3.
Comparison of Calculated Costs (in Euros) Between Abdominal and Laparoscopic Approaches
| Type of Procedure | TAH-BSO, PLND (+/− PALND)* | TLH-BSO, PLND (+/− PALND)* | ARH-BSO PLND (+/− PALND)* | TLRH-BSO PLND (+/− PALND)* |
|---|---|---|---|---|
| Average Standard Cost† | 4,000 | 3,000 | 4,500 | 4,000 |
| Average Adjusted Hospital Cost‡ | 4,750 | 4,900 | 5,000 | 5,500 |
| Average Calculated Cost§ | 4,250 | 4,400 | 4,750 | 5,000 |
TAH = total abdominal hysterectomy; ARH = abdominal radical hysterectomy; TLH = total laparoscopic Hysterectomy; TLRH = total laparoscopic radical hysterectomy; PLND = pelvic lymph node dissection; PALND = paraaortic lymph node dissection.
In standard 4-bed patient room, assuming a 5–7 day stay in open and a 2–3 day stay in laparoscopic procedures utilizing mainly reusable instruments.
Utilizing mainly disposable surgical instruments.
Utilizing reusable and up to two on average disposable instruments in selected circumstances.
DISCUSSION
Laparoscopic staging for early stage endometrial and cervical cancer had not been universally accepted until recently. An increasing number of reports in the international literature have assessed feasibility and safety data as well as survival data, in both endometrial12,13 and cervical cancers.14–16 To the best of our knowledge, this is the first series of laparoscopic management of early stage endometrial and cervical cancers in Greece.
The recent Gynecologic Oncology Group prospective randomized study (LAP-2) of surgical staging in uterine cancer, confirmed that the laparoscopic approach was comparable to open surgery with equivalent surgical outcomes and complications and that no difference exists in long-term quality of life resulting from these techniques; however, the laparoscopic approach was associated with a decreased hospital length of stay, improved short-term quality of life, and earlier return to baseline functioning.17
Similar results validating the role of laparoscopic management of early cervical cancer are being published as well. For example, a recent study from China with a 6-year follow-up reported on 295 prospectively studied patients with stage IA2-IIB cervical cancer. Operative time was comparable to that of abdominal radical hysterectomy, whereas estimated blood loss, hospital stay, and overall complication rates were better in the laparoscopic group compared with the laparotomy group. Follow-up at 72 months showed survival in 95.2%, 96.2%, and 84.5% of patients with stage IA, IB, and IIA, respectively.18
Our goal in this study was to report on feasibility, safety, and cost outcomes not previously described in our country. Surprisingly, the minority of European countries has contributed to the existing pool of data, regarding feasibility, safety, and cost of laparoscopic management of endometrial and cervical cancers.
Operative time for both radical hysterectomy as well as pelvic/paraaortic lymphadenectomy was longer for the first 10 cases but has improved thereafter reflecting the adjustment of our newly assembled surgical team. Lymph nodes retrieved and hospital stays are comparable to those of other published series.3–7 Our complication rate in this series compares favorably to that of other published series.15 Specifically, our overall major complication rate reached an acceptable 4%. Nonetheless, such figures will await confirmation from larger series.
All of our patients underwent routine pelvic and, in select high-risk patients, additional paraaortic lymphadenectomy. However, the role and extent of lymphadenectomy in early endometrial cancer is still controversial and a subject of ongoing debate in the gynecologic oncologic community.19 Guidelines by many expert panels recommend systematic lymphadenectomy in early endometrial cancer, even in patients with a priori low-risk features.20–22 Others believe that lymphadenectomy, especially in low-risk endometrial cancer patients, may only increase morbidity without an actual benefit for the patient.23 Adding to the existing controversy, surgical staging requirements, such as sampling versus systemic lymphadenectomy or extent of lymphadenectomy, have not been detailed by the 1998 FIGO cancer committee.24
The role of lymphadenectomy in early endometrial cancer, must await large well-designed randomized controlled trials. Meanwhile our practice is to perform a systemic lymphadenectomy in most endometrial cancer patients. On this note, 2 of 6 patients with grade 2 lesions and clinical stage IB had microscopic lymph node metastases, and they both received adjuvant treatment. Similar cases are commonly being managed by a total laparoscopic or abdominal hysterectomy and bilateral salpingooophorectomy by many institutions.
Average standard costs for laparoscopic cases managed using reusable-only instruments proved cheaper in our institution compared with the abdominal ones, which may be attributed mainly to the shorter hospital stay. However, when disposable instruments were extensively used, the adjusted costs tended to be higher. As the surgical team gained more experience with laparoscopic staging, both reusable and disposable—when deemed necessary—instruments were utilized bringing the calculated cost to almost the same as that for the abdominal procedures.
The limitation of our study besides its size is also the lack of a control arm both of which are common in reports of this nature. Nonetheless, data have been prospectively recorded and compare with those from similar published series.3–9 Larger randomized trials from our country are obviously required to validate our results. Our patient database is being continuously populated by new cases with the addition of robotic-assisted laparoscopy as well. In the years to follow, we expect to also report on a larger series along with survival outcomes.
Interestingly, a thorough PubMed/Medline literature search reveals a relative paucity in related studies from Eastern and Southeastern Europe compared with studies from Scandinavian, Central, or Western European countries. However, the cost of such procedures as demonstrated in our pilot study does not represent an actual limiting factor in their application. The few published studies from Eastern and Southeastern Europe probably reflect an insufficient degree of training in minimally invasive surgical techniques by gynecologic oncologists in these countries.
CONCLUSION
The preliminary results from this pilot study support the feasibility, safety, and cost effectiveness of laparoscopic management of early stage endometrial and cervical cancer in our country. Such data may prove useful in stimulating the gynecologic oncologic community in Greece and other European countries, which may have been late in adopting minimally invasive surgery in gynecologic oncology, to engage in larger prospective studies.
Contributor Information
Georgios E. Hilaris, Adjunct Clinical Instructor of Obstetrics and Gynecology, Department of Obstetrics & Gynecology and Center for Special Minimally Invasive and Robotic Surgery, Stanford University School of Medicine, Stanford, California, USA.; Department of Gynecologic Endoscopic Surgery and Gynecologic Oncology, Iaso Women's and Iaso General Hospitals, Athens, Greece.
Thomas Tsoubis, Department of Gynecologic Endoscopic Surgery and Gynecologic Oncology, Iaso Women's and Iaso General Hospitals, Athens, Greece..
Vasilios Konstantopoulos, Department of Anesthesiology, Iaso Women's and Iaso General Hospitals, Athens, Greece..
Kitty Pavlakis, Department of Pathology, Iaso Women's Hospital, Kapodistrian University of Athens, Athens, Greece..
References:
- 1. Dargent D, Salvat J. L' Envahissement Ganglionnaire Pelvien. Paris: McGraw-Hill; 1989 [Google Scholar]
- 2. Nezhat CR, Burrell MO, Nezhat FR, Benigno BB, Welander CE. Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am J Obstet Gynecol. 166(3): 864–865, 1992. March [DOI] [PubMed] [Google Scholar]
- 3. Spirtos NM, Schlaerth JB, Gross GM, et al. Cost and quality-of-life analyses of surgery for early endometrial cancer: laparotomy versus laparoscopy. Am J Obstet Gynecol. 174:1795–1799, 1996; discussion 1799–1800 [DOI] [PubMed] [Google Scholar]
- 4. Gemignani ML, Curtin JP, Zelmanovich J, Patel DA, Venkatraman E, Barakat RR. Laparoscopic-assisted vaginal hysterectomy for endometrial cancer: clinical outcomes and hospital charges. Gynecol Oncol. 73(1): 5–11, 1999. April [DOI] [PubMed] [Google Scholar]
- 5. Malur S, Possover M, Schneider A. Laparoscopically assisted radical vaginal versus radical abdominal hysterectomy type II in patients with cervical cancer. Surg Endosc. 2001. March; 15(3): 289–292 Epub 2000 Dec 12 [DOI] [PubMed] [Google Scholar]
- 6. Malur S, Possover M, Michels W, Schneider A. Laparoscopic-assisted vaginal versus abdominal surgery in patients with endometrial cancer-a prospective randomized trial. Gynecol Oncol. 2001. February; 80(2) 239–244 [DOI] [PubMed] [Google Scholar]
- 7. Nezhat F, Yadav J, Rahaman J, et al. Laparoscopic lymphadenectomy for gynecologic malignancies using ultrasonically activated shears: analysis of first 100 cases. Gynecol Oncol. 97(3): 813–819, 2005. June [DOI] [PubMed] [Google Scholar]
- 8. Tozzi R, Malur S, Koehler C, et al. Laparoscopy versus laparotomy in endometrial cancer: first analysis of survival of a randomized prospective study. J Minim Invasive Gynecol. 12(2): 130–136, 2005. Mar-Apr [DOI] [PubMed] [Google Scholar]
- 9. Ramirez PT, Slomovitz BM, Soliman PT, et al. Total laparoscopic radical hysterectomy and lymphadenectomy: the M. D. Anderson Cancer Center experience. Gynecol Oncol. 2006. August; 102(2) 2252–255 Epub 2006 Feb 10 [DOI] [PubMed] [Google Scholar]
- 10. Hilaris GE, Tsoubis T, Alargof E, et al. Total laparoscopic radical hysterectomy (type III) in a 44 year old patient with stage IIA barrel-shaped cancer of the cervix. A recently reported case in a Greek tertiary center. Hellenic Journal of Obstetrics and Gynecology. 2005. Oct-Dec; 279–291 [in Greek] [Google Scholar]
- 11. Prevention of deep vein thrombosis and pulmonary embolism. ACOG Practice Bulletin No. 84. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2007;110:429–440 [DOI] [PubMed] [Google Scholar]
- 12. Querleu D, Leblanc E, Cartron G, et al. Audit of preoperative and early complications of laparoscopic lymph node dissection in 1000 gynecologic cancer patients. Am J Obstet Gynecol. 2006. November; 195(5): 1287–1292 Epub 2006 May 3 [DOI] [PubMed] [Google Scholar]
- 13. Magrina JF. Outcomes of laparoscopic treatment for endometrial cancer. Curr Opin Obstet Gynecol. 17(4): 343–346, 2005. August [DOI] [PubMed] [Google Scholar]
- 14. Hertel H, Kohler C, Michels W, et al. Laparoscopic-assisted radical vaginal hysterectomy (LARVH): prospective evaluation of 200 patients with cervical cancer. Gynecol Oncol. 2003;90:505–511 [DOI] [PubMed] [Google Scholar]
- 15. Steed H, Rosen B, Murphy J, et al. A comparison of laparoscopic-assisted radical vaginal hysterectomy and radical abdominal hysterectomy in the treatment of cervical cancer. Gynecol Oncol. 2004;93:588–593 [DOI] [PubMed] [Google Scholar]
- 16. Frumovitz M, dos Reis R, Sun CC, Milam MR, et al. Comparison of total laparoscopic and abdominal radical hysterectomy for patients with early-stage cervical cancer. Obstet Gynecol. 110(1): 96–102, 2007. July [DOI] [PubMed] [Google Scholar]
- 17. Walker JL, Piedmonte M, Spirtos N, et al. Surgical staging of uterine cancer: randomized phase III trial of laparoscopy compared with laparotomy—A Gynecologic Oncology Group Study [GOG]: preliminary results [abstract]. Proceedings of the 2006 Annual Meeting of the American Society of Clinical Oncology, Atlanta, Georgia, June 2-6, 2006 [Google Scholar]
- 18. Chen Y, Xu H, Li Y, et al. The outcome of laparoscopic radical hysterectomy and lymphadenectomy for cervical cancer: a prospective analysis of 295 patients. Ann Surg Oncol. 2008. October; 15(10): 2847–2855 Epub 2008 Jul 23 [DOI] [PubMed] [Google Scholar]
- 19. Aalders JG, Thomas G. Endometrial cancer-Revisiting the importance of pelvic and para aortic lymph nodes. Gynecol Oncol. 2007;104(1): 222–231 Epub 2006 Nov 28 [DOI] [PubMed] [Google Scholar]
- 20. Management of Endometrial Cancer ACOG Practice Bulletin. Obstet Gynecol. 2005;106:413–425 [DOI] [PubMed] [Google Scholar]
- 21. 2007. National Comprehensive Cancer Network (NCCN) Practice Guidelines.
- 22. Society of Gynecologic Oncologists (SGO) Management of Endometrial Cancer. Available online at www.sgo.org/publications/EndoGuidelines.doc Accessed 2008
- 23. ASTEC study group. Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658): 125–136 Epub 2008 Dec 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uterine cancer. In: Berek and Hacker Practical Gynecologic Oncology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005; 397–434 [Google Scholar]