The authors found that the learning curve for robotassisted radical cystectomy is constantly evolving to improve oncologic outcomes.
Keywords: Cystectomy, Robot, Bladder cancer, Minimally invasive surgery, Learning curve
Abstract
Objective:
Robot-assisted radical cystectomy has the potential to cure patients from bladder cancer while offering the benefits of minimally invasive surgery. We sought to evaluate the learning curve for this technically demanding procedure.
Materials and Methods:
Robot-assisted radical cystectomy was attempted in 100 consecutive patients. An IRB-approved review of our robot-assisted radical cystectomy database was conducted. Total operative (OR) time, cystectomy time, pelvic lymph node dissection (PLND) time, estimated blood loss (EBL), margin positivity, complications, and length of hospital stay were compared among patients divided into 4 cohorts of increasing surgical experience. Scattergrams and continuous curves were plotted to develop a robotic cystectomy learning curve.
Results:
Overall OR time decreased from 375 minutes in cohort 1 to 352 minutes in cohort 4, with less than 1% change in OR time after case 16. Time from incision to bladder extirpation decreased from 187 minutes in cohort one to 165 minutes in cohort 4. Time for PLND increased from 44 minutes in cohort 1 to 77 minutes in cohort 4. Lymph node yield increased from 14 nodes in cohort 1 to 23 nodes in cohort 4. Positive surgical margins decreased from 4 patients in cohort 1 to 0 patient in cohort 4. The complication rate had no change from 9 patients in cohort 1 to 9 patients in cohort 4.
Conclusion:
Operative results and oncologic outcomes for robot-assisted radical cystectomy constantly improve as the technique evolves.
INTRODUCTION
Minimally invasive surgery is being incorporated more frequently into urologic practice and appears to be replacing many open procedures. Laparoscopic radical nephrectomy has become the standard of care in most institutions for the treatment of renal tumors <8 cm.1
Laparoscopic surgery initially gained its popularity in urology with the performance of pelvic lymph node dissection.2 Laparoscopy in urology has progressed from less demanding procedures to more technically challenging ones. In 1991, Clayman3 performed the first laparoscopic nephrectomy. Laparoscopic nephrectomy eventually became the standard of care for the treatment of most renal cancers. Although laparoscopic prostatectomy was introduced in 1991, only 9 cases were reported in the literature up until 1996, because the surgery was difficult with a steep learning curve and long operative times. Minimally invasive radical prostatectomy gained in popularity with the advent of the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA).
Radical cystectomy remains one of the most challenging procedures performed by urologists and is associated with high morbidity. An initial experience was used to determine learning curves for several performance variables associated with robot-assisted radical cystectomy.
MATERIALS AND METHODS
From October 2005 to July 2008, 100 consecutive patients were offered robot-assisted radical cystectomies at our institution using the 4-arm da Vinci surgical system. A robotic surgeon, a right-sided bedside assistant, and nursing staff, who were all experienced in robot-assisted laparoscopic prostatectomy, were part of the cystectomy team. The procedure was offered to all patients who were candidates for an open radical cystectomy.
Surgical Technique
The technique at Roswell Park Cancer Institute has evolved over the 100 case series. Initially, after pneumoperitoneum was established, the ports were placed as described by Menon et al4 for robot-assisted laparoscopic prostatectomy. The ureters were identified and dissected towards the ureterovesical junction. The distal ureters were clipped and divided. We learned over the course of our experience that dividing the operation into a consecutive development of spaces was a simpler approach and that delayed transection of the ureters helped the surgeon to orient and avoid closer dissection to the bladder.
Development of Spaces
The incision of the posterior peritoneum over the peristalsing ureter is carried out with separation of the visceral fascia and identification of the ureter in the loose areolar tissue. The periureteral space is opened with mobilization and dissection of the ureter distally up to the ureterovesical junction. Proximal mobilization of the ureters is carried out lateral to the aortic bifurcation. One of the caveats in this technique is to avoid early clipping of the ureter during initial dissection. The intact distal ureters act as a landmark in identifying the lateral pedicles and ensure free surgical margins. After completion of the periureteral space, incision of the posterior peritoneum is carried parallel and lateral to the umbilical ligaments onto the anterior abdominal wall above the superior pubis ramus, which helps one in developing the second space, the lateral pelvic space. The avascular areolar space is opened following the medial curve of the rami of the pubic bone. The vas deferens is divided as it is seen traversing across the space. The bladder is still left attached to the anterior abdominal wall and provides natural anterior retraction.
Once the avascular bilateral pelvic spaces are developed, one is able to identify the levator ani muscle on the lateral pelvic sidewall and medially the lateral and posterior aspect of the bladder. Once dissection of periureteral and lateral pelvic spaces is complete, one should be able to identify the distal ureter up to the ureterovesical junction at the medial edge of the dissection and the vascular pedicle arising from the anterior division of the internal iliac (hypogastric) artery. The external iliac vessels as well as the obturator nerve and vessels are recognized on the lateral aspect of the pelvic space. Once the periureteric and lateral avascular spaces are defined bilaterally, the anterior rectal space is developed. The 2 lateral incisions of the posterior peritoneum are joined together at the peritoneal reflection of the pouch of Douglas. The dissection of this space is carried distally as far as the apex of the prostate. The plane between the anterior sheath of Denonvilliers fascia and the rectum is easily accessible. Blunt dissection following the anterior rectal wall is continued caudally. Using a zero-degree lens, the anterior rectal fibers adherent to the apex of the prostate can be clearly seen. Careful blunt and sharp dissection using a cold round-tip scissors is preferred to separate the rectum from the prostatic apex. The bladder is left suspended from the anterior abdominal wall while posterior dissection is completed. Anterior and lateral traction on the bladder using the fourth arm with a cobra grasper would help expose the lateral vascular pedicles of the bladder. The distal UV junction is identified, and the ureters are ligated with 2 Weck Hem-o-lok clips.
An endovascular stapling device or Hemo-locking clips can be used to secure the lateral pedicles in an expeditious fashion. After controlling the inferior vesical vessels, the endopelvic fascia is opened bilaterally. Incision of the median and medial umbilical ligaments to release the bladder from the anterior abdominal wall is carried out once the posterior dissection is complete. The bladder will drop posteriorly. Suture ligation of the deep venous complex is performed; further release of the prostate is accomplished once the deep dorsal complex is incised. Once the proximal membranous urethra is skeletonized, the urethral catheter is removed. A Hem-o-lock clip is applied just distal to the apex to prevent urine spillage.
Extended Lymph Node Dissection
After the specimen is freed, extended pelvic lymph node dissection is performed following the template described by Stein.5 We also have started developing the space of Marcille, which has initially increased our operative time as well as the overall lymph node yield. Once the nodal package is dissected off the common and external iliac artery, attention is paid to identify the iliac vein that typically appears flat due to pneumoperitoneum. The nodal package including the obturator is mobilized and rolled medially as each vascular structure is identified and visualized. The nodal package is gently mobilized to clear the nodal package off the pubic bone after which the obturator nerve is identified and skeletonized. After the dissection up to the aortic bifurcation, one should be able to identify the underlying common iliac vessels, especially the vein. Once this is completed, attention is paid to define the triangle of Marcille. The investing fascia is dissected off the psoas fascia to mobilize the iliac vessels medially. The fascia is dissected distally for easy mobilization. The arterial branch of the common iliac artery needs to be controlled as it enters the psoas muscle. Once this vessel is controlled, attention is paid to the collapsed hidden iliac vein from which the nodal package has been removed without injuring the obturator nerve. At completion of the definition of the space of Marcille once, the obturator nerve should be clearly revealed exiting the psoas muscle underneath the external iliac vessels.
Finally, the left ureter is tunneled under the sigmoid colon and extracorporeal urinary diversion is performed through a 4-cm to 6-cm midline incision.
In women, after the ureters are dissected, the ovarian vessels are clipped and ligated. The round ligaments are incised next. Then, the broad ligaments are divided with an endovascular stapler. After the lateral bladder pedicles are controlled, the vagina is incised. The anterior vaginal wall is removed en bloc with the bladder, urethra, ovaries, and uterus. A moist laparotomy sponge is packed within the vagina to maintain pneumoperitoneum during vaginal dissection. Once the specimen is freed, vaginal reconstruction is performed with absorbable suture in a clam-shell fashion.6
An IRB-approved retrospective review of our prospective bladder cancer database was conducted. Variables examined included overall operative time, cystectomy time, time for PLND, EBL, positive margin rate, lymph node yield, length of hospital stay, and complications.
Scattergrams and continuous curves were plotted for each variable to note the plateau effect. The patient number where a <1% change occurred within a variable gave the minimum of cases needed to reach the learning curve for each variable.
To examine changes in operative parameters as subsequent surgeries are performed, the data were also split into 4 groups based on the order in which the patient was operated on. Descriptive statistics, such as the mean in the case of numeric variables and the relative frequency in the case of categorical variables, were computed by group. Note that due to the dependence between observations, calculation of P-values based on analysis of variance and the Pearson chi-square tests are not valid here, and such inferences are not provided. To further examine the learning curve associated with selected continuous endpoints as the number of patients operated on increases, a negative exponential model was fit via least squares estimation. This model was selected because associated with it is a parameter that represents the estimated plateau. A logistic regression model was used to examine the change in the probability corresponding to binary endpoints. All statistical analyses were performed using SAS version 9.1 statistical software (Cary, NC).
RESULTS
Robot-assisted radical cystectomy was attempted in 100 patients. Two patients required open conversion: one patient had bulky tumor with rectal invasion and the second patient could not tolerate a steep Trendelenburg position although more than 80% of the cystectomy was completed with robotic assistance in this case. Mean patient age was 68 years. Eight-one patients were male and 19 were female. Ninety-three patients had an ileal conduit urinary diversion, 6 had continent neobladder, and one had continent cutaneous diversion. An extended lymph node dissection was performed in 92% of patients (Table 1).
Table 1.
Demographics
| Mean Age | 68 years |
|---|---|
| Sex (M:F) | 81:19 |
| Mean ASA Score | 2 |
| Mean Body Mass Index | 29 |
| Pathologic Stage | 9 pT0; 5 CIS; 1 pTa; 12 pT1; 17 pT2; 38 pT3; 17 pT4 |
| Diversion Type | 93 ileal conduits |
| 6 neobladders |
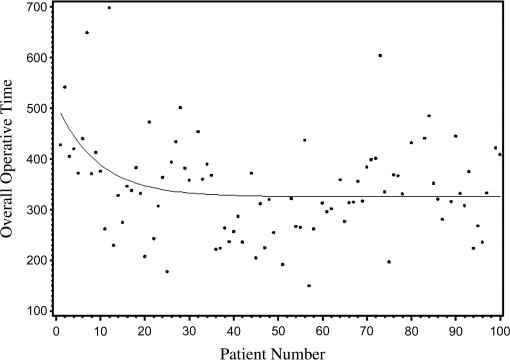
Mean total operative time was 343 minutes. The average time needed from incision to docking the robot was <15 minutes. Operative time did not significantly decrease but is attributed to the change of technique and development of the “spaces” procedure. The first 25 cases averaged 375 minutes with overall OR time decreasing to 352 minutes in the last 25 cases. Maximum OR time was 698 minutes, while the quickest case took 150 minutes. The plateau on the curve for total OR time occurred at the 16th patient with a <1% change in OR time in subsequent cases (Figure 1). The time for bladder extirpation decreased from 187 minutes in cohort 1 to 165 minutes in cohort 4 (Table 2).
Figure 1.
Overall operative time during first 100 robot-assisted radical cystectomy cases.
Table 2.
Results by Cohort
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | |
|---|---|---|---|---|
| Total OR Time (Min) | 375 | 321 | 321 | 352 |
| Time for Cystectomy (Min) | 187 | 176 | 165 | 165 |
| Time for PLND (Min) | 44 | 43 | 71 | 77 |
| Estimated Blood Loss (cc) | 536 | 591 | 573 | 695 |
| Lymph Node Yield (Nodes) | 14 | 21 | 26 | 23 |
| Positive Surgical Margins | 4 | 1 | 2 | 0 |
| Length of Stay (Days) | 9 | 10 | 11 | 11 |
| Complications | 9 | 10 | 10 | 9 |
Blood loss was calculated by the anesthesiologist who subtracted the volume in the suction canister at the end of the case from the amount of irrigation utilized. Mean EBL was 598mL. EBL was 536mL in cohort 1 and increased to 695mL in cohort 4. A <1% change in EBL occurred after case 11 was performed. Eighteen patients required a blood transfusion.
All patients with organ-confined (pT0 –2) bladder cancer had negative surgical margins. Seven patients with pT3– 4 transitional cell carcinoma had positive surgical margins. Two surgical margins were present in pT3 patients, and the remaining 5 patients had pT4 disease. Three patients with positive surgical margins also had positive lymph nodes.
The time for PLND increased from 44 minutes in cohort 1 to 77 minutes in cohort 4. Our lymph node yield increased from 14 lymph nodes excised in cohort 1 to 23 nodes in cohort 4 with a <1% change in lymph node yield occurring after the 30th case. Twenty-six patients had positive lymph nodes.
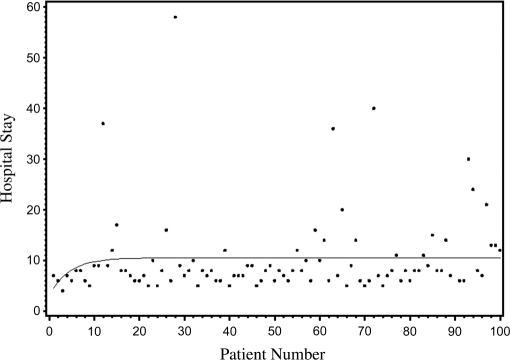
Mean hospital stay was 10 days with a maximum stay of 58 days and a minimum stay of 4 days. Length of stay increased from 9 days in cohort one to 11 days in cohort 4, with a <1% change in LOS occurring after case 12 (Figure 2).
Figure 2.
Length of hospital stay in days after robot-assisted radical cystectomy in first 100 consecutive cases.
Postoperative complications (major and minor) developed in 38 patients, ranging from an unscheduled visit, renal insufficiency, ileus, bowel obstruction, pyelonephritis, wound infection, fascial dehiscence, deep venous thrombosis, clostridium difficile colitis, pneumonia, perforated appendix, to mortality in 2 patients; one patient died from complications related to small bowel obstruction, sepsis, and another one died from alcohol liver disease and its complications. Nine patients in cohort 1 had complications, while 9 patients in cohort 4 had complications. No intraoperative complications occurred in the current series.
DISCUSSION
Minimally invasive surgery has progressed from simpler procedures to more technically demanding surgeries. With the adoption of a new minimally invasive procedure, surgeons must be aware of a learning curve. Attaining the learning curve can be challenging, as well as time consuming. Some surgeons successfully ascend the learning curve and continue to master the newly learned procedure, while others will fail to attain the learning curve and stop performing the surgery. Knowing the learning curve in advance will give surgeons an idea of what they should expect and can ease their frustrations while they adopt a new surgical procedure.
Learning curves have been examined for other urologic procedures. Unfortunately, no accepted definition or measurement of a learning curve exists. Thresholds to define a learning curve tend to vary from study to study. Herrell et al7 examined the confidence and comfort levels of the operating surgeon while assessing the learning curve of robot-assisted laparoscopic prostatectomy. Ahlering et al8 noted a 12-patient learning curve to reach 4-hour proficiency with robotic prostatectomy. Atug and colleagues9 stated that the robot-assisted laparoscopic prostatectomy learning curve was approximately 30 cases after examining positive surgical margin rates with increasing surgical experience. Baumert et al10 looked at positive margin length and rate of multiple positive margins while assessing the learning curve of laparoscopic radical prostatectomy. Gaston et al11 examined OR times and self-assessed difficulty scores when studying the learning curve of laparoscopic hand-assisted nephrectomy. Vickers and colleagues12 assessed the prostate cancer recurrence rate after open radical prostatectomy with increasing surgeon volume.
To our knowledge, this is the first series to evaluate multiple variables and examine the robot-assisted cystectomy learning curve. While most studies examining a learning curve tend to focus on a single variable, we examined multiple variables including total OR time, cystectomy time, PLND time, lymph node yield, EBL, positive margin rate, and complications. Similar to other learning curve studies, we also divided our patients into cohorts of increasing surgical experience and examined the affects of increasing surgical experience on each variable. Overall OR time and cystectomy time did not decrease significantly with increasing surgical experience due to a change of technique. Although this evolution of technique resulted in marked improvement in margin rate and lymph node yield with surgical experience, developing anatomic spaces and delaying the ureteral transection resulted in avoiding close approximation to the specimen because tactile feedback is lacking.
A unique aspect of the current series is the development of a continuous curve to detect a plateau where a <1% change occurred within each variable. The plateau of the curve more accurately pinpoints where the learning curve lies. The plateau on the curve for total OR time occurred after case 16. The plateau on the EBL curve occurred after case 11, while the plateau on the LOS curve occurred at case 12. The mean length of stay in our series was 10 days. Meanwhile, the plateau on the lymph node yield curve occurred after case 30. Performing an extended lymph node dissection is the most challenging part of the procedure while providing important staging information and possible curative advantage. Our lymph node dissection time and yield increased as we defined the space of Marcille. Although operating times for these portions of the operation increased, satisfactory oncologic outcomes that should be critical for this operation were accomplished with these changes.
Few robot-assisted radical cystectomy series have been published.13–17 Our mean OR time of 343 minutes was less than the mean OR time of 370 minutes reported by Pruthi et al16 and 638 minutes reported by Rhee and colleagues.17 The mean EBL of 598cc was higher than the 479cc EBL seen in the University of Virginia experience.16 The mean lymph node yield of node dissection reported by the University of North Carolina group was 19 nodes, which was similar to our mean lymph node yield of 21 nodes. Pruthi and colleagues16 did not have any positive margins, but 78% of their patients had organ-confined bladder cancer (pT0 –2). In the current series, 7% of patients had a positive surgical margin, and 55% of patients had locally advanced disease (pT3– 4).
One limitation of the current study is that the surgeons at Roswell Park Cancer Institute had extensive experience with robot-assisted laparoscopic prostatectomy and a formal robotic fellowship. The learning curve developed in this study pertains only to surgeons experienced with robot-assisted laparoscopic prostatectomy and a robotic surgery fellowship. The learning curve for robot-assisted radical cystectomy will be shifted to the right for robot-naïve surgeons. We highly recommend mastering robot-assisted radical prostatectomy before attempting robot-assisted radical cystectomy. With wide acceptance and advance of robot-assisted surgery, techniques for all pelvic operations are now established, and learning of robot-assisted radical cystectomy is done at the residency level in high-volume robotic centers. Grantcharov et al18 revealed that different learning curves existed for surgeons with various levels of experience. Low-volume surgeons will take longer to learn the procedure than high-volume surgeons will, because increased frequency of repetition hastens the learning process. Birkmeyer and colleagues19 discovered a higher surgical mortality rate at low-volume institutions compared with a lower surgical mortality rate at high-volume institutions. As seen in our series, operating time should not be considered the only critical aspect of learning. Optimizing oncologic outcomes outweighs all other results desired for this operation to find a place in urologic oncology.
Another limitation of the current series is our small patient size. Only 100 patients underwent robot-assisted radical cystectomy. Therefore, broad application of our results is not appropriate. The current series should lay the ground for future robot-assisted radical cystectomy studies. Larger series with long-term follow-up are needed before robotic cystectomy is considered a viable alternative to open radical cystectomy.
CONCLUSION
Robot-assisted radical cystectomy is a challenging procedure. Operative results and oncologic outcomes appear to improve as the technique evolves. The variation in different parameters shows how with experience one can improve surgical outcomes and continue to meet standards set by over a century of evolution with open radical cystectomy.
Contributor Information
Khurshid A. Guru, Department of Urologic Oncology, Roswell Park Cancer Institute, Buffalo, New York, USA. Department of Urology, State University of New York at Buffalo, New York.
Adam E. Perlmutter, Department of Urologic Oncology, Roswell Park Cancer Institute, Buffalo, New York, USA.
Zubair M. Butt, Department of Urologic Oncology, Roswell Park Cancer Institute, Buffalo, New York, USA.
Pamela Piacente, Department of Urologic Oncology, Roswell Park Cancer Institute, Buffalo, New York, USA..
Gregory E. Wilding, Department of Biostatistics, Roswell Park Cancer Institute, Buffalo, New York, USA. Department of Biostatistics, State University of New York at Buffalo, New York, USA.
Wei Tan, Department of Biostatistics, Roswell Park Cancer Institute, Buffalo, New York, USA..
Hyung L Kim, Department of Urologic Oncology, Roswell Park Cancer Institute, Buffalo, New York, USA.; Department of Urology, State University of New York at Buffalo, New York.
James L. Mohler, Department of Urologic Oncology, Roswell Park Cancer Institute, Buffalo, New York, USA. Department of Urology, State University of New York at Buffalo, New York.
References:
- 1. Portis AJ, Yan Y, Landman J, et al. Long-term follow-up after laparoscopic radical nephrectomy. J Urol. 2002;167:1257–1262 [PubMed] [Google Scholar]
- 2. Schuessler WW, Vancaillie TG, Reich H, et al. Transperito-neal endosurgical lymphadenectomy in patients with localized prostate cancer. J Urol. 1991;145:988–991 [DOI] [PubMed] [Google Scholar]
- 3. Clayman RV, Kavoussi LR, Soper NJ, et al. Laparoscopic nephrectomy: initial case report. J Urol. 1991;146:278–282 [DOI] [PubMed] [Google Scholar]
- 4. Menon M, Hemal AK, Tewari A, et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int. 2003;92:232–236 [DOI] [PubMed] [Google Scholar]
- 5. Stein JP. Lymphadenectomy in bladder cancer: how high is “high enough”? Urol Oncol. 2006;24:349–355 [DOI] [PubMed] [Google Scholar]
- 6. Guru KA, Nogueira M, Piacente P, et al. Rapid communication: robot-assisted anterior exenteration: technique and initial series. J Endourol. 2007;21:633–639 [DOI] [PubMed] [Google Scholar]
- 7. Herrell SD, Smith JA., Jr Robotic-assisted laparoscopic pros-tatectomy: what is the learning curve? Urology. 2005;66:105–107 [DOI] [PubMed] [Google Scholar]
- 8. Ahlering TE, Skarecky D, Lee D, et al. Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: initial experience with laparoscopic radical pros-tatectomy. J Urol. 2003;170:1738–1741 [DOI] [PubMed] [Google Scholar]
- 9. Atug F, Castle EP, Srivastav SK, et al. Positive surgical margins in robotic-assisted radical prostatectomy: impact of learning curve on oncologic outcomes. Eur Urol. 2006;49:866–871; discussion 871–872. Epub 2006 Mar 10 [DOI] [PubMed] [Google Scholar]
- 10. Baumert H, Fromont G, Adorno Rosa J, et al. Impact of learning curve in laparoscopic radical prostatectomy on margin status: prospective study of first 100 procedures performed by one surgeon. J Endourol. 2004;18:173–176 [DOI] [PubMed] [Google Scholar]
- 11. Gaston KE, Moore DT, Pruthi RS. Hand-assisted laparoscopic nephrectomy: prospective evaluation of the learning curve. J Urol. 2004;171:63–70 [DOI] [PubMed] [Google Scholar]
- 12. Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;99:1171–1170 Epub 2007 Jul 24 [DOI] [PubMed] [Google Scholar]
- 13. Hemal AK, Abol-Enein H, Tewari A, et al. Robotic radical cystectomy and urinary diversion in the management of bladder cancer. Urol Clin North Am. 2004;31:719–729 viii Review [DOI] [PubMed] [Google Scholar]
- 14. Beecken WD, Wolfram M, Engl T, et al. Robotic-assisted laparoscopic radical cystectomy and intra-abdominal formation of an orthotopic ileal neobladder. Eur Urol. 2003;44:337–339 [DOI] [PubMed] [Google Scholar]
- 15. Sala LG, Matsunaga GS, Corica FA, et al. Robot-assisted laparoscopic radical cystoprostatectomy and totally intracorpo-real ileal neobladder. J Endourol. 20:233–235, 2006; discussion 236 [DOI] [PubMed] [Google Scholar]
- 16. Rhee JJ, Lebeau S, Smolkin M, et al. Radical cystectomy with ileal conduit diversion: early prospective evaluation of the impact of robotic assistance. BJU Int. 2006;98:1059–1063 [DOI] [PubMed] [Google Scholar]
- 17. Pruthi RS, Wallen EM. Robotic-assisted laparoscopic radical cystoprostatectomy: operative and pathological outcomes. J Urol. 2007;178:814–818 Epub 2007 Jul 16 [DOI] [PubMed] [Google Scholar]
- 18. Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Learning curves and impact of previous operative experience on performance on a virtual reality simulator to test laparoscopic surgical skills. Am J Surg. 185:146 149, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137 [DOI] [PubMed] [Google Scholar]