Results of both methods of gastric closure (omental and biodegradable patch) were similar suggesting that a biodegradable patch glued to the outside of the stomach may be a viable alternative for closure of perforations of the digestive tract.
Keywords: Gastric perforation, Laparoscopy, Biodegradable patch
Abstract
Background:
The current treatment of perforated peptic ulcers is primary closure, supported by the application of an omental patch. It is difficult and time consuming to perform this procedure by laparoscopic surgery, largely because of the required suturing. It was our aim to develop and test a new method of closure for gastric perforation that is similar in efficacy and safety to a traditional repair. This technique could have utility in laparoscopic repair, as it does not require sutures or mobilization of the omentum.
Method:
The new method, called the “stamp” method consists of closure of the perforation by gluing a biodegradable patch made of lactide-glycolide-caprolacton (LGC, Polyganics, B.V. Groningen, The Netherlands) on the outside of the stomach. It was compared with the omental patch procedure. Perforations were made in the stomach of 20 rats and closed by either method (10 rats in each group). The rats were followed for 10 weeks.
Results:
No complications were seen in any of the rats. In both groups, histological degradation of the patch by giant cells started at week 2. No signs of inflammation existed in either group. Signs of closure of the mucosa were seen after 2 weeks, and the muscular layer started to regenerate after 8 weeks in both groups.
Conclusion:
Results of both methods were similar, which means that treatment of a gastric perforation through the application of a biodegradable patch to the outside of the stomach is a feasible option and might even be an interesting technique for closure of other perforations in the digestive tract.
INTRODUCTION
The current treatment of perforated peptic ulcer is primary closure, covered by omentoplasty. The classical Graham patch technique, described by Cellan-Jones in 1929 and in 1937 by Graham can be applied.1–3 The idea in closing the perforation not only by sutures but also with an omental plug is the sealing and tamponade effect of the plug. Adding an omental plug also reduces the risk of tearing out sutures, accelerates ulcer healing, and inhibits ulcer recurrence.4,5 Laparoscopic surgery has gained in popularity, because there seems to be a decrease of postoperative complications, pain, and length of hospital stay.6–9 Despite this, laparoscopic correction of a perforated peptic ulcer (PPU) still is not the first treatment of choice for many surgeons. One of the disadvantages of laparoscopic closure of perforated peptic ulcer is that it takes more operating time and requires more operating skills, which makes the procedure more costly and less popular.9,10 The prolonged operating time might be caused by the laparoscopic suturing procedure.6,10 There is a learning curve for laparoscopic intracorporeal or extracorporeal suture techniques, and because of the fragile edges of the peptic ulcer walls, sutures tear out easily.10 An alternative technique for closing the perforation, avoiding the necessity to use stitches, might facilitate the laparoscopic procedure. An alternative to omentoplasty and stitching could be the use of a glued patch of biodegradable material on the outside of the stomach. Besides reducing operating time, another advantage of using a glued patch instead of suturing is that touching of the friable edges is avoided, which lowers the risk of enlarging the perforation. Also the patch method might be the solution for closing larger peptic ulcers. Performing an omentoplasty in these patients is difficult, and alternative techniques have been tried.7,11–13 Previously the “stamp method,” closing a gastric perforation with a biodegradable stamp, was tested in a pilot study in 5 rats, showing that is was a safe procedure.14 In this pilot study, there was no control group and the follow-up period was only 5 weeks. Therefore, a new rat study has been performed in which we compare closing an iatrogenic perforation in rats' stomachs by Graham omentoplasty with the application of a glued biodegradable (lactide-glycolide-caprolacton) patch to the outside of the stomach. The aim of this study was to test the stamp method, which has to be a technique of a similar safety profile as primary closure and omentopexy but could allow the laparoscopic procedure to be done more easily so operating time can be reduced.
METHODS
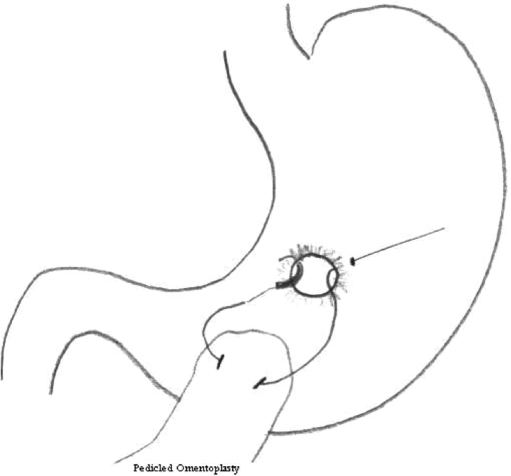
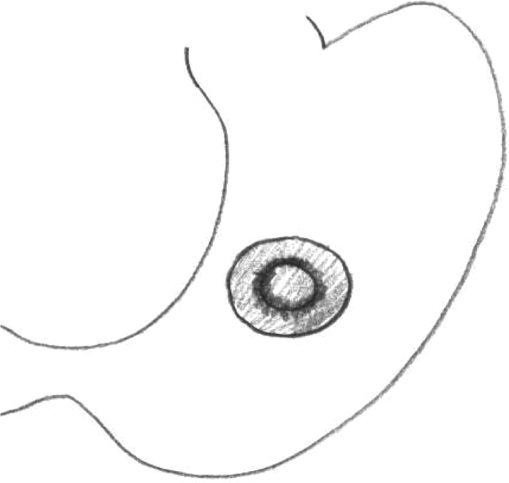
Twenty male Wistar rats, 12 weeks to 13 weeks old (Harlan, The Netherlands) were used in this trial, which was approved by the animal ethics committee of the University Medical Center Groningen. Ten rats were in the omentum group, and 10 were in the stamp group. All procedures were performed with the rats under general anesthesia by using isoflurane gas at 2% with oxygen. In both groups, an upper laparotomy was performed, and a perforation was created on a fixed point on the ventral side of the stomach. Because this was located underneath the liver lobe, careful retraction of the liver lobe was necessary. The perforation was made by cutting a small hole, with a diameter of 0.5 cm in the gastric wall. In the omentum group, the perforation was closed by using a Graham patch. For this, the omentum nearby the perforation site was mobilized in the fixed pedicle into the perforation with mattress stitches. A 6 – 0 Vicryl suture was used (Figure 1). In the stamp group, a circular shaped patch made of lactide-glycolide-caprolacton (LGC, Polyganics, B.V. Groningen, The Netherlands) with a diameter of 1cm was glued on top of the perforation (Figure 2), ensuring an overlap of 0.25 cm around the perforation. The glue used was Glubran 2 made of NBCA (n- butyl 2 cyanoacrylate) OCA (2- octil cyanoacrilate), which has been approved for intracorporeal usage (GEM, Italy). Only a few drops needed to be applied on the dry biodegradable patch, which then was glued onto the gastric wall surrounding the perforation. After repair of the perforation by either one of the above techniques, the abdomen was irrigated with saline 0.9% and closed in 2 layers with Polysorb 4.0. Directly postoperatively, one subcutaneous dosage of 0.1 mL Temgesic (0.3 mg/mL) was given as an analgesic. Rats were fed standard rat chow and received nonacidified tap water. After one week, one rat, from either group was brought under general anesthesia again and underwent relaparotomy. After inspection of the abdomen, the rats were first perfused transcardially with “prerinse” containing 0.9% NaCl and 1% heparin, followed by 200 mL 2% glutaraldehyde buffered with 0.1mol/L phosphate buffer, pH 7.4. A full-thickness biopsy with a diameter of 2cm was then taken from the perforation site and postfixed for several days in the same fixative. The specimens were then dehydrated through a graded concentration of ethanol and embedded in glycol methacrylate. From all specimens, 2-μm thick slices were prepared using a disposable histoknife and a Reichert-Jung “2050 supercut” microtome. The sections were mounted on glass slides and stained with toluidine blue. In addition, some sections were evaluated and photomicrographed using an Olympus BX-50 microscope (Olympus Optical Co, Japan). Every following week, one rat from each group underwent the above-mentioned procedure.
Figure 1.
Drawing of Graham omentoplasty. A suture runs through the gastric wall first then takes a bit of pedicled omentum and runs back to the other site of the perforation.
Figure 2.
The stamp method: the biodegradable patch is glued on the outside of the stomach with a 0.25 cm overlap.
RESULTS
All 20 rats survived the operations without complications; none of the rats died or showed any signs of peritonitis, sepsis, or wound infection. The most important clinical sign of peritonitis due to leakage, caused by insufficient sealing of the perforation, is that the rats do not eat and will not gain or even loose weight. The weight on the day of the first surgery (creating the perforation and closure by the stamp method or omentum patch) was measured. On the day of relaparotomy, the weight was measured again. All rats, except one in each groups, gained weight. The rat in the stamp group that did not gain any weight and the one in the omentum group that lost some weight were rats that already had their second surgery after one and two weeks so had less time to recover from their first surgery.
During relaparotomy in both groups, no signs of leakage or peritonitis were found. Some adhesions of the liver to the stomach were found, mainly in the omen-tum group, but no official scoring system for classifying the number or severity of adhesions was used. If adhesions were present, adhesiolysis needed to be performed to get proper accessibility to the perforation site. This caused slightly more bleeding.
Histology
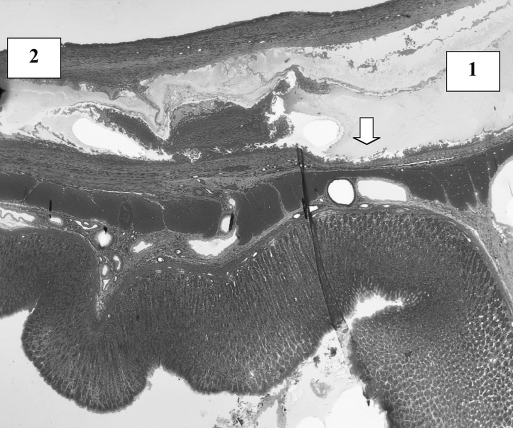
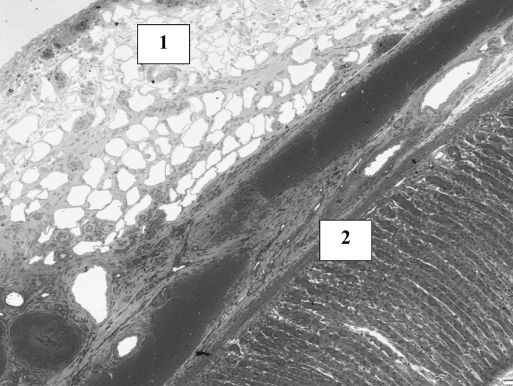
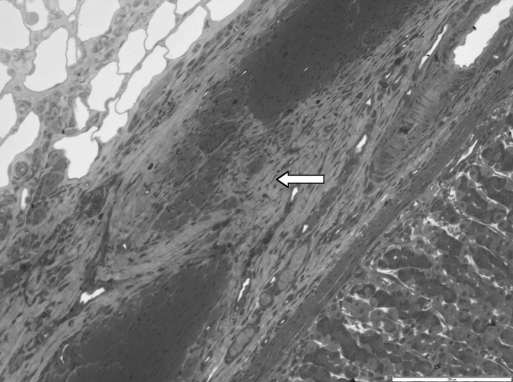
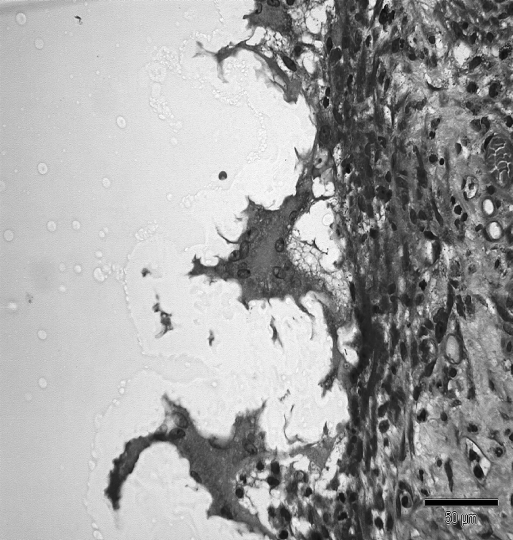
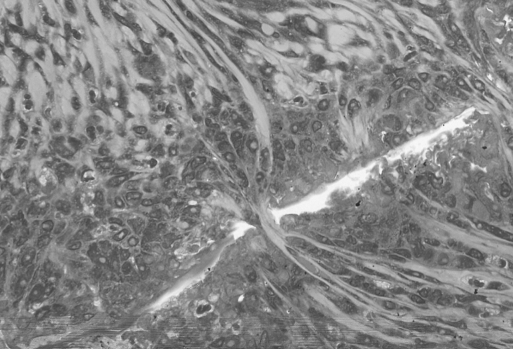
In the first week in both groups, a sign of infiltration of the area by granulocytes was observed. At the start of week 2, invasion of the biodegradable patch by giant cells was observed, indicating that degradation of the patch was started. Also in both groups, closure of the epithelioid layer of the mucosa was seen. In week 3, a fibrotic layer started to form on the outside of the biodegradable patch (Figure 3).Inthe following weeks, this developed into a well-organized, vascularized, and structured layer. This phenomenon was not seen in the rats in which the omental patch was used (Figure 4). In this group, the fat cells of the omentum were covering the perforation site. In week 6, newly formed muscle cells crossing the perforation site were found (Figure 5). In the following weeks, the perforation site was slowly narrowing. The collagen was getting organized, and giant cells (Figure 6) were filled with patch material. These giant cells were only found in the group with the biodegradable patch. The muscle layer seemed to be repaired and continuous after 8 weeks (Figure 7), comparable in both groups. In both groups granulocytes were found during the complete period, slightly more in the patch group, but in none of the groups were there any signs of inflammation or rejection. In the ninth week, the amount of giant cells started to decrease. At week 10, the patch was almost completely absorbed.
Figure 3.
Week 6. Biodegradable patch (1) covering defect. Patch is covered with a well-organized fibrotic layer (2). Giant cells invading patch (arrow).
Figure 4.
Week 8. Omentum covering the perforation site (1). The perforation can still be seen in the noncontinuity of the muscle (2).
Figure 5.
Perforation site at week 6 covered with omentum. New muscle cells start filling up the perforation.
Figure 6.
Three giant cells invading the “stamp.” Giant cells are filled with small particles of patch material.
Figure 7.
Young muscle fibers closing the old perforation site. White line is the remaining operative biodegradable patch at week 8.
DISCUSSION
This new method for closure of a perforated peptic ulcer has previously been tested in a pilot study of 5 rats.10 In the literature, we found no information on the histological phases of recovery of the stomach after perforation, and because of this, there was no guideline in what the follow-up period should have been to observe full healing. In the pilot study, there was only a follow-up of 5 weeks, and the muscle layer still showed signs of perforation after this period. To get more information on the healing process of the gastric wall, a longer follow-up period was necessary. Therefore in the new study, 10 rats were allocated to each group; one rat from each group was terminated weekly, which provided an overview of the healing process of 10 weeks. The histology results from this study have shown that after 1 week in both groups the epithelioid layer of the gastric mucosa already has been repaired. The muscular layer takes longer. After 6 weeks, in-growth of muscle fibers is seen, but the perforation site is still recognizable. After 8 weeks, the muscle layer in both groups was continuous.
There were no signs clinical or histological of inflammation or rejection in either group. The biodegradable patch is absorbed by giant cells, and this process already starts after 1 week. But the giant cells filled with patch material were disappearing after 10 weeks. One of the great advantages of using a stamp to cover the perforation is that the size of the perforation does not seem to matter, because the patch can be cut into any desirable size. Using glue instead of stitches simplifies and speeds up the procedure. The use of a degradable patch replaces the need for omentoplasty. Apparently the necessity for the omental plug considering its sealing and tamponade effect is arguable.4,5 Ates et al15 have already suggested simple laparoscopic repair without an omental patch. Avoiding an omentoplasty might lower the formation of intraperitoneal adhesion, but unfortunately, this was not officially scored and needs further research. Because it is not necessary to mobilize the well-perfused omentum, the risk of peri- and postoperative bleeding will be a lower. An interesting histological feature found in the patch group was the formation of a well-organized and vascularized collagen layer on top of the perforation. Kung12 describes this observation as an outer shield formed by fibrosis on top of a Teflon-felt graft, which was tested in dogs. Whether this has any clinical importance remains unclear, but it might lower the risk of leakage or recurrence of the perforation. No approval from the animal ethics committee was given for a control group in which the spontaneous sealing of a perforation by liver or omentum could have been investigated. It is estimated that about 40% of the perforations in humans, with an average 5mm size, seal by themselves.16 The perforation made in our rat model had a diameter of 5mm, which would be comparable to a giant ulcer in a human stomach. Spontaneous healing of a giant ulcer is less likely to happen and is associated with high morbidity and mortality.17
CONCLUSION
The closure of a perforated peptic ulcer by using a biodegradable patch is feasible. It might even have advantages, such as less adhesion formation, lower recurrence rates, and less hematoma formation. The lactide-glycolide-caprolacton patch has proven to be resistant to gastric acid. Also the biodegradation process did not proceed rapidly; the patch material started disappearing after the perforation was healed and did not persist for a long time. After 10 weeks, the material microscopically was almost completely degraded. This makes the material suitable for other parts of the digestive tract. That is why the stamp method might be an interesting approach to close small bowel perforations. Realizing that the stamp method has not been tested on a real perforated peptic ulcer with associated peritonitis and also needs to be tested laparoscopically, further research will be interesting and necessary.
Contributor Information
Marietta J. O. E. Bertleff, Department of Plastic and Reconstructive Surgery, Academic Hospital Maastricht, Maastricht, The Netherlands..
Toon Stegmann, Department of Molecular Virology, University Medical Center Groningen, Groningen, The Netherlands..
Robert S. B. Liem, Department of Cell Biology, Section Electron Microscopy, University Medical Center Groningen, Groningen, The Netherlands..
Geert Kors, Department of Cell Biology, Section Electron Microscopy, University Medical Center Groningen, Groningen, The Netherlands..
Peter H. Robinson, Department of Plastic and Reconstructive Surgery, University Medical Center Groningen, The Netherlands..
Jean Philippe Nicolai, Department of Plastic and Reconstructive Surgery, University Medical Center Groningen, The Netherlands..
Johan F. Lange, Department of Surgery, Erasmus Medical Center, Rotterdam, The Netherlands..
References:
- 1. Böhm B, Ablassmaier B, Müller JM. Laparoskopische chirurgie am oberen gastrointestinaltrakt. Chirurg. 2001( 72): 349–361 [DOI] [PubMed] [Google Scholar]
- 2. Cellan-Jones CJ. A rapid method of treatment in perforated duodenal ulcer. BMJ. 1929( 36): 1076–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Graham RR. The treatment of perforated duodenal ulcers. Surg Gynecol Obstet. 1937( 64): 235–238 [Google Scholar]
- 4. Baker RJ. Chapter 81: Operation for acute perforated duodenal ulcer. Mastery of Surgery. 3th ed. Little, Brown and Company; 1997 [Google Scholar]
- 5. Matoba Y, Katayama H, Ohami H. Evaluation of omental implantation for perforated gastric ulcer therapy: findings in a rat model. J Gastroenterol. 1996;31(6): 777–784 [DOI] [PubMed] [Google Scholar]
- 6. Lam PW, Lam MC, Hui EK, Sun YW, Mok FP. Laparoscopic repair of perforated duodenal ulcers: the “three-stitch” Graham patch technique. Surg Endosc. 2005;19(12): 1627–1630 [DOI] [PubMed] [Google Scholar]
- 7. Lau WY, Leung KL, Zhu XL, Lam YH, Chung SC, Li AK. Laparoscopic repair of perforated peptic ulcer. Br J Surg. 1995;82(6): 814–816 [DOI] [PubMed] [Google Scholar]
- 8. So JB, Kum CK, Fernandes ML, Goh P. Comparison between laparoscopic and conventional omental patch repair for perfo-rated duodenal ulcer. Surg Endosc. 1996;10(11): 1060–1063 [DOI] [PubMed] [Google Scholar]
- 9. Bertleff MJ, Halm JA, Bemelman WA, et al. Randomized clinical trial of laparoscopic versus open repair of the perforated peptic ulcer: The LAMA Trial. World J Surg. 2009;33(7): 1368–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsuda M, Nishiyama M, Hanai T, Saeki S, Watanabe T. Laparoscopic omental patch repair for perforated peptic ulcer. Ann Surg 1995;221(3): 236–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta S, Kaushik R, Sharma R, Attri A. The management of large perforations of duodenal ulcers. BMC Surg 2005;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kung SP. Teflon-felt grafting of giant gastroduodenal perfo-ration in a canine model. Surg Today 2004;34(2): 145–149 [DOI] [PubMed] [Google Scholar]
- 13. Sharma D, Saxena A, Rahman H, Raina VK, Kapoor JP. ‘Free omental plug’: a nostalgic look at an old and dependable technique for giant peptic perforations. Dig Surg 2000;17(3): 216–218 [DOI] [PubMed] [Google Scholar]
- 14. Bertleff MJ, Liem RS, Bartels HL, et al. The “stamp method”: a new treatment for perforated peptic ulcer? Surg Endosc. 2006;20(5): 791–793 [DOI] [PubMed] [Google Scholar]
- 15. Ates M, Sevil S, Bakircioglu E, Colak C. Laparoscopic repair of peptic ulcer perforation without omental patch versus conventional open repair. J Laparoendosc Adv Surg Tech A. 2007;17(5): 615–619 [DOI] [PubMed] [Google Scholar]
- 16. Donovan AJ, Berne TV, Donovan JA. Perforated duodenal ulcer: an alternative therapeutic plan. Arch Surg. 1998;133(11): 1166–1171 [DOI] [PubMed] [Google Scholar]
- 17. Shyu JF, Chen TH, Shyr YM, Su CH, Wu CW, Lui WY. Gastric body partition for giant perforated peptic ulcer in critically ill elderly patients. World J Surg. 2006;30(12): 2204–2209 [DOI] [PubMed] [Google Scholar]