Abstract
Purpose of review
This review focuses on studies from the past year that have greatly advanced our understanding of molecular and cellular regulation of pancreatic acinar cell function.
Recent findings
Recent advances focus on signals dictating pancreatic development, acinar cell fate, pancreatic growth, and secretion. Regeneration of acinar cells after pancreatitis depends on expression of embryonic signals in mature acinar cells. In this setting, acinar cells can also transdifferentiate into adipose cells. With the forced induction of certain early and endocrine-driving transcription factors, acinar cells can also transdifferentiate into β-cells. There has also been an increased understanding of acinar-to-ductal metaplasia and the subsequent formation of pancreatic intraepithelial neoplasia lesions. Multiple proteins involved in secretion have been characterized, including small guanosine triphosphate-binding proteins, soluble N-ethylmaleimide-sensitive factor attachment proteins, and ion channels.
Summary
These findings demonstrate the regenerative potential of the acinar cell to mitigate injurious states such as pancreatitis. The ability of acinar cells to transdifferentiate into β-cells could potentially provide a treatment for diabetes. Finally, the results might be helpful in preventing malignant transformation events arising from the acinar cell. Developments in proteomics and computer modeling could expand our view of proteins mediating acinar cell function.
Keywords: calcium signaling, growth, regeneration, secretion, transdifferentiation
Introduction
The pancreatic acinar cell is a highly specialized structure developed for synthesis, storage, and secretion of digestive enzymes. The acinar cell arises from the same pancreatic progenitor as duct and islet cells and is tightly polarized. Its apical pole is densely packed with zymogen granules that secrete digestive enzymes by exocytosis. This review of the past year’s literature is divided into two sections. The first summarizes studies of ontogeny, expansion, and differentiation of acinar cells and pathways permissive to adaptive growth, regeneration, transdifferentiation, and malignant transformation (Fig. 1). The second section describes studies that examine the molecules that form the membrane of zymogen granules and their function (Fig. 2) and the roles of K+ and Ca2+ channels.
Figure 1. Developmental and differentiation patterns relating to the pancreatic acinar cell.
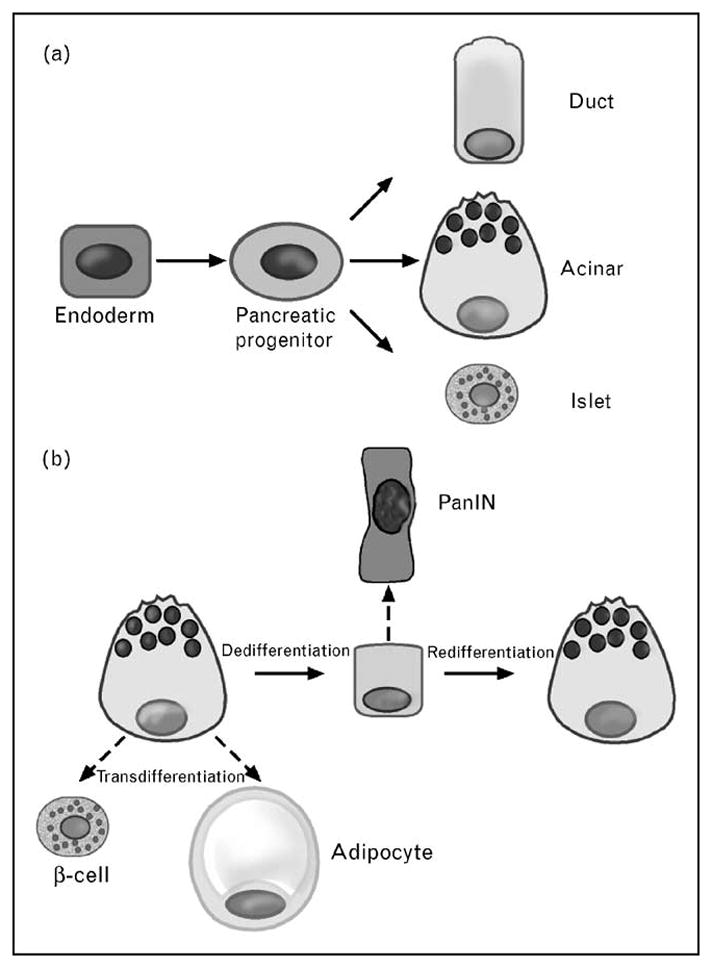
(a) Foregut endoderm gives rise to pancreatic progenitors that then differentiate into acinar, duct, or islet cells. (b) In response to pancreatic injury, acinar cells can regenerate by dedifferentiating to a ductal epithelium and then redifferentiating to mature acinar cells (solid arrows). They can transdifferentiate to adipocytes or β-cells, depending on genetic and environmental cues (dashed arrows). However, the dedifferentiated state is prone to neoplastic transformation, such as to a PanIN lesion. PanIN, pancreatic intraepithelial neoplasia.
Figure 2. Zymogen granule proteins.
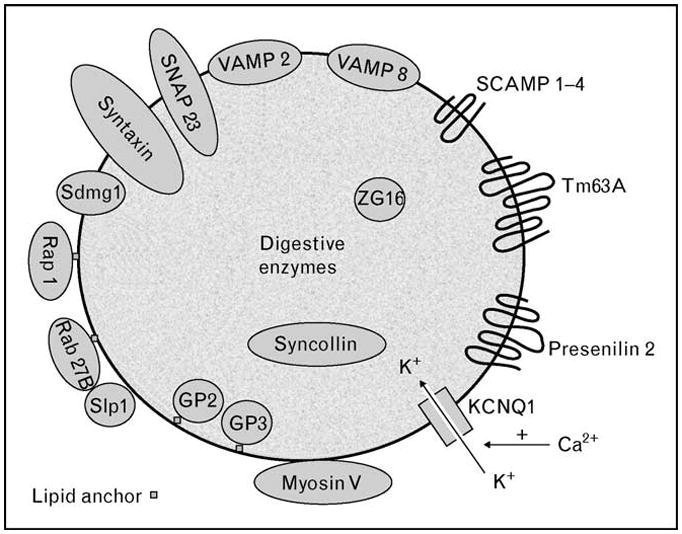
A quantitative proteomics approach has detected previously known zymogen granule proteins (myosin V, VAMPs, syntaxins, Rab proteins and SCAMP) as well as identified new ones (Tm63A and presenilin 2). Luminal proteins include digestive enzymes as well as matrix proteins (GP2, GP3, syncollin, and ZG16). Recent advances describing the functions of some of these proteins are discussed in the text. KCNQ1, potassium voltage-gated channel, KQT-like subfamily, member 1; SCAMP, secretory carrier membrane protein; SNAP, synapse-associated protein; VAMP, vesicle-associated membrane protein.
Factors regulating the ontogeny of the pancreatic primordium and acinar cell
The pancreas develops from two epithelial buds in the foregut endoderm [1•]. It expresses critical transcription factors that, first, drive the expansion of pancreatic progenitors and, second, their differentiation into acinar, duct, and endocrine cells (Fig. 1a). One of the earliest markers of pancreatic development is activation of the homeobox gene pancreatic and duodenal homeobox 1 (Pdx1). Gao et al. [2] showed that the winged-helix transcription factors forkhead box A1 (Foxa1) and fork-head box A2 (Foxa2) co-occupy multiple regulatory domains on the Pdx1 gene and are essential in controlling the expansion and differentiation of the pancreatic primordium. Mice with a compound, conditional ablation of both Foxa1 and Foxa2 resulted in loss of Pdx1 expression, severely disrupted acinar and islet development, and neonatal death.
Pancreas-specific transcription factor, 1a (PTF1a) is another factor expressed by pancreatic progenitor cells, which, similar to Pdx1, contributes to all three lineages. However, its expression becomes restricted to acinar progenitor cells in the mouse by embryonic day 13.5 (or E13.5) [3]. Recent work by Masui et al. [4] elucidates the mechanisms for this temporal shift. During embryonic development, a 2.3-kb autoregulatory enhancer region initiates expression of PTF1a in the early precursor epithelium and then superinduces expression in nascent acinar cells. In mature acinar cells, the enhancer, along with an active trimeric form of PTF1a, establishes an autoregulatory loop that reinforces and maintains PTF1a.
Determining acinar cell fate
In the mouse, at E13.4–E14.5, 4–6 days after the onset of pancreatic development (E8.5–E9.0), intensive pancreatic epithelial cell proliferation and differentiation ensues in what has been termed the ‘secondary transition.’ By E16.5, acinar cells separate from the central ducts. Two recent reports demonstrate that the Wnt/β-catenin-dependent gene target Myc plays a key role in acinar cell expansion. Pancreatic tissue-specific deletion of Myc in mice using Pdx1 [5••] or PTF1a-driven [6] expression resulted in extensive acinar cell hypoplasia but only a minor disturbance in endocrine development. These data highlight an important role for Wnt/β-catenin signaling in acinar precursors via Myc.
Jia et al. [7] reported that the basic helix-loop-helix (bHLH) transcription factor Mist1 constitutes another key regulator in the terminal differentiation of acinar cells. Mist1 maintains acinar cell lineage in the developing pancreas by limiting its proliferation. Acinar cells from mice with a gene deletion in Mist1 exhibit a higher proliferative index, and this phenotype could be rescued by ectopic expression of Mist1 in acinar cells using an elastase promoter. Mist1 induced the expression of the cyclin-dependent kinase inhibitor p21CIP1/WAF1. The results imply that Mist1 promotes differentiation of the acinar cell by affecting its cell cycle.
The modification of chromatin by either loosening or compacting its structure via histone acetylation or deacetylation, respectively, constitutes an important mode of epigenetic regulation. Haumaitre et al. [8] demonstrated that both expression and activity of histone deacetylase (HDAC) are reduced over embryonic life into adulthood in the rat. Using an in-vitro embryonic culture method of the pancreas, they reported that pharmacologic inhibition of HDAC classes I and II abolished acinar cell differentiation but enhanced ductal and β-cell pools. The results indicate a role for histone modification in determining pancreatic cell fate.
Acinar cell growth and regeneration
In the adult pancreas, acinar cell growth is influenced by hormonal stimulation, notably by the gut hormone cholecystokinin (CCK). Gurda et al. [9] reported that CCK induces adaptive acinar cell growth by causing nuclear translocation of nuclear factor of activated T-cells (NFAT) via the Ca2+/calmodulin-dependent phosphatase calcineurin. In response to injury, the pancreas activates regenerative processes to maintain tissue homeostasis. The prevailing notion is that after injury, acinar cells might dedifferentiate into a ductal epithelium that expresses early developmental factors. These ‘facultative progenitor cells’ would then redifferentiate into mature acinar cells. Two recent reports highlight the importance of the expression of embryonic factors by acinar cells in guiding the regenerative process. Siveke et al. [10•] showed that reactivation of the Notch signaling pathway during injury from caerulein-induced pancreatitis is required for acinar cell regeneration. Fendrich et al. [11•] found that embryonic signaling by Hedgehog was upregulated in acinar cells after caerulein-induced pancreatitis, and that its blockade either pharmacologically or genetically, using Pdx1 or elastase-Cre recombinase, allowed the formation of a ductal epithelium from acinar cells, but it did not permit the redifferentiation into acini. Intriguingly, the authors suggest that the ‘redifferentiation arrest’ might provide a link between pancreatitis injury and subsequent neoplasia. The results also underscore the capacity of the acinar cell to revert to an earlier progenitor state in response to injury.
Transdifferentiation of acinar cells
Recent work highlighted the potential of the acinar cell to transdifferentiate directly into a different mature cell type (Fig. 1b). Bonal et al. [5••] reported that the adult pancreas of mice with conditionally inactivated c-Myc underwent loss of acinar cells along with adipocyte accumulation. Using genetic cell lineage analysis, they showed that the adipocytes were directly derived from transdifferentiating acinar cells. Remarkably, this phenomenon of ‘epithelial-to-mesenchymal’ transdifferentiation was also observed during caerulein-induced injury as well as in human pancreas from middle-aged donors.
Further evidence demonstrating acinar cell capacity for transdifferentiation was provided by Zhou et al. [12••]. Adenoviral vectors expressing early progenitor and islet transcription factors Pdx1, neurogenin3, and Mafa were directly injected into the tail of the pancreas in vivo. They primarily infected acinar cells and within days greater than 20% of former acinar cells, evidenced by genetic lineage tracing, began to express insulin and assumed β-cell morphology. The results confirm the potential of the acinar cell to undergo forced transdifferentiation (Fig. 1). They also offer the prospect of an endogenous pancreatic source of β-cells to treat diabetes.
Malignant transformation of acinar cells
The change of acinar cells to a less differentiated cell type may predispose to malignant transformation. Mounting evidence suggests that pancreatic ductal adenocarcinoma (PDAC) and its noninvasive precursor lesion known as pancreatic intraepithelial neoplasia (PanIN) are the result of acinar cell metaplasia to a ductal cell form. Habbe et al. [13] reported that targeting of oncogenic Kras mutations to elastase and Mist1-expressing acinar cells of adult mice resulted in the spontaneous induction of PanIN lesions. Similarly, De La et al. [14•] reported that conditional expression of oncogenic Kras along with Notch using Pdx1 Cre recombinase synergistically caused mature acinar cells to convert to PanIN lesions. A downstream target of oncogenic Kras may involve the serine–threonine kinase Akt. Elghazi et al. [15] reported that acinar cell-specific activation of Akt signaling using Cre recombinase under the control of an elastase promoter induced acinar-to-ductal metaplasia. The studies further support the theory that PDAC may form initially from acinar or acinar-like cells.
Thus far, we have focused on acinar cell development, growth, and differentiation. In the next section, we will cover advancements in acinar cell function, from new insight into zymogen granule membrane (ZGM) architecture to acinar cell proteins involved in secretion and ion flow.
Zymogen granule membrane architecture
Understanding the molecular architecture of the ZGM is critical for studying zymogen granule function. The membrane topology of a ZGM protein influences its accessibility to binding partners and enzymatic modification. To provide such information, a new quantitative proteomics approach has been developed that combines a protease protection assay with quantification using isobaric tags [isobaric tag for relative and absolute quantitation (iTRAQ)] [16,17••]. Statistical modeling then yields probabilities for proteins to be cytoplasmic or luminal based on their iTRAQ ratios. In addition to detecting expected cytoplasm-oriented membrane and membrane-associated proteins as well as content proteins (Fig. 2), the study identified unexpected zymogen granule proteins. The latter included Tm63A (no known function) and presenilin 2 (a subunit of the γ–secretase complex). The study could provide a foundation for developing a higher order architecture model of the ZGM and for future functional studies of individual ZGM protein.
Zymogen granule membrane proteins mediating secretion
Once ZGM proteins have been identified, their function has to be assessed. A number of recent articles have explored the functional roles of these proteins, and some of the latest developments are detailed below.
Small guanosine triphosphate-binding proteins
Recent studies [16,17••,18,19] have identified small guanosine triphosphate (GTP)-binding proteins on the external surface of zymogen granules, including Rap1 and Rab27B, and these have been implicated in pancreatic exocytosis. One study [18] not only showed that Rap1 was required for mediation of cyclic AMP (cAMP) signaling via exchange protein directly activated by cAMP (EPAC) but was also necessary for CCK and carbachol-evoked amylase secretion. Another study [19] looked at the interaction of Rab27B with a putative effector protein, Slp1, a protein implicated in membrane transport. Slp1 was found to be in the apical region of the acinar cell, bound to Rab27B in vivo, and both proteins colocalized on the zymogen granule surface. Furthermore, zymogen granules accumulated at the apical pole of the cell in Slp1 knockout mice under fasted conditions. Although Slp1−/− mice exhibited greater absolute levels of stimulated secretion than wild type, the percentage secretion of the mutant and wild type was similar. The findings suggest that Slp1 may influence zymogen granule mass and cellular enzyme content but probably does not directly affect exocytosis.
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins
The soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins are known to regulate exocytosis. During exocytosis, specific vesicle-SNAREs (v-SNAREs) interact with other SNARE proteins, such as synapse-associated proteins (SNAPs) and syntaxins (Syn) on target membranes, to form a SNARE complex. In the pancreatic acinar cell, the v-SNARES, synaptobrevin/vesicle-associated membrane protein isoforms 2 and 8 (VAMP-2 and VAMP-8), are found on ZGM. Syn-2 is found at the apical plasma membrane, whereas Syn-3, 7, and 8 are on the zymogen granule and Syn-4 is at the basolateral membrane. SNAP 23 is found both on the zymogen granule and the plasma membrane. It has been proposed that the interaction of these proteins determines whether zymogen granule exocytosis occurs at the apical membrane (as in physiologic secretion) or basolateral membrane (as in pathologic secretion). In a recent study [20•] using VAMP-8−/− mice, VAMP-8 was found to be the zymogen granule SNARE, which mediated basolateral exocytosis and was also necessary for the homotypic fusion of zymogen granules required for compound exocytosis.
In the past year, additional proteins have been localized to subdomains of the ZGM and may modulate the secretory process. For example, Sdmg1 is a conserved eukaryotic membrane protein of unknown function, which has recently been identified in pancreatic acinar cells [21]. Expression of Sdmg1 was upregulated during pancreatic development when zymogen granules started to appear. Furthermore, Sdmg1 was copurified with zymogen granules during subcellular fractionation of the pancreas and localized to subdomains in the granule membrane, distinct from those for VAMP-2. These data suggested that Sdmg1 plays a role in regulated pancreatic secretion. It has not yet been determined whether Sdmg1 subdomains in the secretory granule membrane overlap with VAMP-8 subdomains or with any subdomains of other SNARE proteins present on the secretory granule membrane. How these domains are arranged and oriented on the ZGM may influence secretory granule exocytosis.
Another potential modulator of secretion is protein kinase D (PKD), a serine/threonine kinase, which undergoes translocation, phosphorylation, and activation after secretagogue stimulation. The predominant isoform in mouse and human pancreatic acinar cells is PKD3. A recent study [22] has shown that adenoviral overexpression of PKD3 enhances CCK-induced amylase secretion in mouse acinar cells. Furthermore, partial localization of PKD3 to VAMP-2-positive structures was observed, although adenoviral overexpression of PKD3 did not seem to promote recruitment of VAMP-2 structures to the apical membrane. As distinct populations of zymogen granules have been associated with either VAMP-2 or VAMP-8, it is possible that PKD3 may target another subset of zymogen granules (e.g. VAMP-8-positive zymogen granules) for exocytosis.
Ion channels
During zymogen granule exocytosis, ion channels in the ZGM are inserted into the apical membrane. The ZGM has at least two cation and two anion conductive pathways, which contribute to K+ and Cl− fluxes into the granules and promote release of digestive enzymes. Emerging evidence points to a Ca2+-sensitive potassium channel [potassium voltage-gated channel, KQT-like subfamily, member 1 (KCNQ1)] as a strong candidate for mediating K+ flux [23]. The presence of KCNQ1 was confirmed by immunoperoxidase labeling of pancreatic tissue, immunogold labeling of zymogen granule, and immunoblotting of ZGM. Furthermore, the single channel characteristics were verified using ZGM fused to planar lipid bilayers. An inhibitor of KCNQ1, 293B, not only inhibited single channel activity but also reduced CCK-induced amylase secretion in permeabilized acini. This study underlines the importance of ion channels in the secretory process.
Thus far, we have detailed some of the proteins in, or associated with, the ZGM, which account for exocytosis and secretion. However, a number of intracellular signaling pathways are required to initiate the events leading up to the exocytotic process. Receptor-mediated processes are beyond the scope of this review, but in the next section, we summarize the latest developments concerning the role of Ca2+ signaling in secretion.
Ca2+-dependent events
Previously, CCK had been thought to act indirectly on human pancreatic acinar cells via vagal nerve stimulation, but new evidence suggests that CCK can directly activate secretion from human pancreatic acinar cells, similar to that seen in rodents [24••]. At physiologic concentrations of CCK-8 and human CCK-58 (1–20 pmol/l), rapid oscillatory increases in cytosolic Ca2+ were seen with an apical to basal progression followed by increases in mitochondrial ATP production and secretion. These Ca2+ responses were inhibited with caffeine, an inhibitor of inositol 1,4,5 trisphosphate-sensitive Ca2+ channels (IP3R), suggesting that they occur through IP3-induced Ca2+ release (IICR).
Studies in rodents have shown that IICR is the primary signal driving enzyme and fluid secretion, and the type 2 and 3 IP3Rs are the predominant isoforms in the acinar cell. Both IP3Rs are positively regulated by cytosolic ATP, but type 2 is 10-fold more sensitive to ATP than type 3. Current evidence shows that, in type 2 IP3R knockout mice, 10-fold higher levels of ATP were required to sensitize IICR in permeabilized acinar cells compared with wild type [25]. This suggests that type 2 IP3R determines the sensitivity of IICR to ATP. Furthermore, type 2 IP3R may protect IICR from the inhibitory effects of ATP depletion during times of metabolic stress.
Finally, Shah et al. [26] have shown that the second messenger, cAMP, can modulate the speed of the Ca2+ wave in acinar cells, and that the likely target of this modulation is a Ca2+-sensitive Ca2+ channel, the ryanodine receptor (RyR). Although this latter study examined pathological conditions, it does underscore the critical role that Ca2+ dynamics play in pancreatic acinar cell regulation. How wave speed might be translated into cellular responses remains unclear.
Conclusion
This review has detailed current progress in understanding the epigenetic signals that dictate pancreatic development, acinar cell fate, and pancreatic growth. Furthermore, advances in proteomics and computer modeling have led to an expanded view of the proteins mediating acinar cell function. Studies focusing on acinar cell processes such as secretion and calcium signaling have yielded new insights into these events at the molecular level. Although the mature acinar cell arises from the same pancreatic progenitor as duct and islet cells, it is specific signals, some outlined in this review, that lead to its development into a highly specialized cell type programmed for synthesis, storage, and secretion of digestive enzymes.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 489–490).
- 1•.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. This excellent review covers generation and regeneration of cells in both pancreas and liver and discusses the therapeutic implications which arise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao N, LeLay J, Vatamaniuk MZ, et al. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawaguchi Y, Cooper B, Gannon M, et al. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 4.Masui T, Swift GH, Hale MA, et al. Transcriptional autoregulation controls pancreatic Ptf1a expression during development and adulthood. Mol Cell Biol. 2008;28:5458–5468. doi: 10.1128/MCB.00549-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Bonal C, Thorel F, Ait-Lounis A, et al. Pancreatic inactivation of c-Myc decreases acinar mass and transdifferentiates acinar cells into adipocytes in mice. Gastroenterology. 2009;136:309.e9–319.e9. doi: 10.1053/j.gastro.2008.10.015. This study shows that activity of the transcription factor c-Myc is required for pancreatic growth and maturation. A second intriguing finding is that pancreatic adipocyte accumulation in these mice is derived from transdifferentiated acinar cells. [DOI] [PubMed] [Google Scholar]
- 6.Nakhai H, Siveke JT, Mendoza-Torres L, Schmid RM. Conditional inactivation of Myc impairs development of the exocrine pancreas. Development. 2008;135:3191–3196. doi: 10.1242/dev.017137. [DOI] [PubMed] [Google Scholar]
- 7.Jia D, Sun Y, Konieczny SF. Mist1 regulates pancreatic acinar cell proliferation through p21 CIP1/WAF1. Gastroenterology. 2008;135:1687–1697. doi: 10.1053/j.gastro.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haumaitre C, Lenoir O, Scharfmann R. Histone deacetylase inhibitors modify pancreatic cell fate determination and amplify endocrine progenitors. Mol Cell Biol. 2008;28:6373–6383. doi: 10.1128/MCB.00413-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurda GT, Guo L, Lee SH, et al. Cholecystokinin activates pancreatic calcineurin-NFAT signaling in vitro and in vivo. Mol Biol Cell. 2008;19:198–206. doi: 10.1091/mbc.E07-05-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Siveke JT, Lubeseder-Martellato C, Lee M, et al. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology. 2008;134:544–555. doi: 10.1053/j.gastro.2007.11.003. The degree to which regeneration of the pancreas occurs after pancreatitis may turn out to be as important in determining the outcome of the disease as the level of inflammation induced by the injury. This study shows that there is reactivation of Notch signaling during pancreatitis, and that inhibition of the Notch pathway impairs acinar cell regeneration. [DOI] [PubMed] [Google Scholar]
- 11•.Fendrich V, Esni F, Garay MV, et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135:621–631. doi: 10.1053/j.gastro.2008.04.011. In this study, a block in the Hedgehog signaling pathway resulted in an arrest of acinar cell redifferentiation after pancreatitis. The results suggest a link between pancreatitis injury and subsequent pancreatic neoplasia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Zhou Q, Brown J, Kanarek A, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. This is a fascinating demonstration that mature acinar cells hold the capacity to transdifferentiate to β-cells. A large number of acinar cells transfected in vivo with the transcription factors Pdx1, neurogenin3, and Mafa began to express insulin. The results suggest a potential breakthrough in providing diabetic patients with a source of β-cells from acinar cell pools. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habbe N, Shi G, Meguid RA, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.De La OJ, Emerson LL, Goodman JL, et al. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci U S A. 2008;105:18907–18912. doi: 10.1073/pnas.0810111105. The presence of mutant Kras alone predisposes only a small minority of cells to PanIN formation. This study shows that coexpression of mutant Kras along with Notch in Pdx1 or elastase-expressing cells increases the induction of PanIN lesions by several folds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elghazi L, Weiss AJ, Barker DJ, et al. Regulation of pancreas plasticity and malignant transformation by Akt signaling. Gastroenterology. 2009;136:1091–1103. doi: 10.1053/j.gastro.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Andrews PC. Purification and proteomics analysis of pancreatic zymogen granule membranes. Methods Mol Biol. 2008;432:275–287. doi: 10.1007/978-1-59745-028-7_19. [DOI] [PubMed] [Google Scholar]
- 17••.Chen X, Ulintz PJ, Simon ES, et al. Global topology analysis of pancreatic zymogen granule membrane proteins. Mol Cell Proteomics. 2008;7:2323–2336. doi: 10.1074/mcp.M700575-MCP200. This study is the first to use an experimentally constrained, comprehensive topology model of identified ZGM proteins. This study may provide a firm foundation for development of a higher order architecture model of the ZGM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabbatini ME, Chen X, Ernst SA, Williams JA. Rap1 activation plays a regulatory role in pancreatic amylase secretion. J Biol Chem. 2008;283:23884–23894. doi: 10.1074/jbc.M800754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saegusa C, Kanno E, Itohara S, Fukuda M. Expression of Rab27B-binding protein Slp1 in pancreatic acinar cells and its involvement in amylase secretion. Arch Biochem Biophys. 2008;475:87–92. doi: 10.1016/j.abb.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 20•.Cosen-Binker LI, Binker MG, Wang CC, et al. VAMP8 is the v-SNARE that mediates basolateral exocytosis in a mouse model of alcoholic pancreatitis. J Clin Invest. 2008;118:2535–2551. doi: 10.1172/JCI34672. This study further characterizes the mechanism of exocytosis through specific v-SNAREs and has implications for both pathological and physiological secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Best D, Adams IR. Sdmg1 is a component of secretory granules in mouse secretory exocrine tissues. Dev Dyn. 2009;238:223–231. doi: 10.1002/dvdy.21827. [DOI] [PubMed] [Google Scholar]
- 22.Chen LA, Li J, Silva SR, et al. PKD3 is the predominant protein kinase D isoform in mouse exocrine pancreas and promotes hormone-induced amylase secretion. J Biol Chem. 2009;284:2459–2471. doi: 10.1074/jbc.M801697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee WK, Torchalski B, Roussa E, Thevenod F. Evidence for KCNQ1 K+ channel expression in rat zymogen granule membranes and involvement in cholecystokinin-induced pancreatic acinar secretion. Am J Physiol Cell Physiol. 2008;294:C879–C892. doi: 10.1152/ajpcell.00490.2007. [DOI] [PubMed] [Google Scholar]
- 24••.Murphy JA, Criddle DN, Sherwood M, et al. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology. 2008;135:632–641. doi: 10.1053/j.gastro.2008.05.026. This elegant study demonstrates, for the first time, a direct effect of the hormone CCK on human pancreatic acinar cells. Prior to this study, CCK was only thought to stimulate human acini indirectly via vagal nerve stimulation. [DOI] [PubMed] [Google Scholar]
- 25.Park HS, Betzenhauser MJ, Won JH, et al. The type 2 inositol (1,4,5)-trisphosphate (InsP3) receptor determines the sensitivity of InsP3-induced Ca2+ release to ATP in pancreatic acinar cells. J Biol Chem. 2008;283:26081–26088. doi: 10.1074/jbc.M804184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah AU, Grant WM, Latif SU, et al. Cyclic-AMP accelerates calcium waves in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1328–G1334. doi: 10.1152/ajpgi.00440.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


