Abstract
Stress fibres and associated focal adhesions in cells constitute a contractile apparatus that regulates cell motility and contraction. Rho-kinase, an effector molecule of small GTPases, regulates non-muscle cell motility and contractility. Rho-kinase mediates the contraction of stress fibres in a Ca2+-independent manner, and is responsible for slower and more finely tuned contraction of stress fibres than that regulated by myosin light chain kinase activity in living cells. The specific inhibition of the Rho-kinase activity causes cells to not only lose their stress fibres and focal adhesions, but also to appear to lose their cytoplasmic tension. Activated Rho-kinase is also involved in the organization of newly formed stress fibres and focal adhesions in living cells.
Keywords: actin, myosin, focal adhesion, Rho-kinase, stress fibre, ROCK
1. Introduction
Actin–myosin-based contractile systems play important roles in regard to the contractile activity of both in situ and in vitro cells, including the contraction of striated and non-striated cells, movement of cells, cleavage activity of dividing cells, etc. Bundles of actin-containing microfilaments in animal cells are prominently found in many types of cells. Bundles of actin filaments and myosin filaments (myosin II) contain motor proteins in the contractile apparatus that enable actin bundles to contract. Myosin filament-containing actin bundles play an important role in motor activity that enables such bundles to contract. There are three major contractile structures in the tissues: striated muscles in skeletal muscles, non-striated muscles in smooth muscles and non-muscle contractile structures called stress fibres. Smooth muscle cells contain typical contractile structures within tissues, localized in the digestive organs, uterus, blood vessels, etc. They do not have the usual periodical striation pattern observed in typical striated muscles. Smooth muscle cells require slow and sustained contraction of the cells in comparison with the skeletal muscle cells. Although the morphological structure of stress fibres significantly differs from that of smooth muscle cells, the basic macromolecular architecture of the smooth muscle is found in the stress fibres, indicating that the stress fibre is really a contractile apparatus within the cell [1]. Moreover, the fundamental structure of a non-striated muscle cell is partially similar to that of the stress fibre structure [1]. Although the contractile force and function as contractile machinery of stress fibres seem to be slightly immature in comparison with those of striated and non-striated muscle cells, the stress fibre seems to be the prototype of the skeletal and smooth muscle cells. The stress fibre is a highly effective contractile mechanism in non-muscle cells, a specifically characterized structure adapted for sustained and fine-tuned contraction within the cell.
Stress fibres are prominent bundles of actin and myosin filaments seen in many cell types, and are generally accepted to be major components of the actin–myosin-based contractile system in cells. The presence of stress fibres has been demonstrated in cultured cell systems, such as fibroblastic cells, endothelial cells and smooth muscle cells. However, stress fibres have also been demonstrated in vivo in scleroblasts of fish scales [2], endothelia cells of blood vessels [3], mesothelial cells of the peritoneal cavity [4] and epithelial cells of the proximal tubules of kidney [5]. All of the stress fibres observed both in vitro and in vivo have actin–myosin-based contractile proteins including tropomyosin, alpha-actinin, filamin and some other contraction and adhesion-related proteins, and represent contractile activity in the cell. The presence of myosin filaments is responsible for the contractile nature of the stress fibres in fibroblastic cells [1,6,7]. Stress fibres terminate directly on focal adhesions where several adhesion-related proteins that connect the cell membrane to the underlying substrate are accumulated [8]. Stress fibres isolated by detergent extraction from cultured fibroblasts contract in response to Mg2+-ATP with Ca2+ [1,9]. The contraction of isolated stress fibres following extraction with Triton X-100 detergent is caused by the phosphorylation of the myosin light chain (MLC) via a calmodulin/myosin light chain kinase (MLCK) system in a calcium-dependent manner, which is the same mechanism as that for smooth muscle contraction.
Ras homology (Rho) proteins are small GTPases involved in signal transduction in the cell. Rho-associated kinase (called Rho-kinase, ROCK2 and Rok-alpha) [10–13] is an effector of Rho small GTPases. The activation of Rho-kinase, which is downstream of Rho, modulates the organization of stress fibres and focal adhesions [14–17]. Moreover, Rho-kinase phosphorylates the MLC either directly or indirectly in a Ca2+-independent manner in vitro [15,16]. Rho-kinase is a serine/threonine kinase that regulates phosphorylation of the 130 kDa myosin-binding subunit (MBS) of myosin phosphatase (myosin phosphatase target subunit 1; MPYPT1). Rho-kinase is activated by the RhoA, which thus results in the phosphorylation of MBS on threonine residue 697 and serine residue 854. The phosphorylation of threonine residue 697 is implicated in the inhibition of myosin phosphatase [18,19]. Rho-kinase-dependent phosphorylation of MLC is by inhibition of the myosin phosphatase activity via phosphorylation on threonine residue 696 of MBS [18–20]. The phosphorylation of MBS on threonine residue 697 inhibits the myosin phosphatase activity and leads to Rho-kinase-associated phosphorylation of MLC [19,21–24]. Both Rho-kinase and MBS are effectors of RhoA. The RhoA/Rho-kinase complex is localized on both smooth muscle fibres and stress fibres via myosin phosphatase–RhoA interacting protein (M-RIP) [25]. Rho-kinase and MBS are involved in many cellular processes downstream of RhoA, including stress fibre and focal adhesion organization, smooth muscle contraction and neurite retraction (for reviews, see [26–28]). Rho-kinase-mediated phosphorylation of MLC via MBS is really essential for stress fibre formation in fibroblasts [29,30].
Previous studies have suggested that the actin–myosin contraction system is regulated by at least two independent systems, i.e. the Ca2+-dependent calmodulin/MLCK system and the Ca2+-independent Rho-kinase system [29–33]. The actions of the actomyosin-based cytoskeleton are functionally diverse, depending on Rho-kinase-mediated regulation and the MLCK-mediated regulation [33]. Treatment of living cells with both MLCK and Rho-kinase inhibitors induces motility, and treatment with MLCK inhibitor reduces the activation of Rac, a typical GTP-binding protein, while Rho-kinase inhibitor treatment does not.
A recent report suggested that both Rho-kinase and MLCK together play highly important roles in cellular behaviour, such as the regulation of cadherin-based adhesion strength [34], the regulation of morphological and permeability changes of endothelial cells [35] and phagocytosis in the central nervous system [36]. Some other reports have demonstrated the presence of differential regulatory effects in Rho-kinase and MLCK. The receptor-type tyrosine-protein phosphatase (RPTPalpha)-mediated increase in the number and size of focal adhesion and stress fibres is dependent on the MLCK activity, but not on the activity of Rho-kinase [37]. On the other hand, extracellular stiffness influences both the expression and the activity level of RhoA/Rho-kinase-mediated contraction-associated proteins [38]. The tissue stiffness of tumour cells could drive transformation by Rho-kinase-dependent cell tension, but not as a result of MLCK-dependent effects [39]. An elevated level of Rho-kinase activity seems to accelerate the rigidity and stiffness of tumour cells by the contraction of cytoskeletal components, such as stress fibres and focal adhesions.
Cell tension organized by stress fibres is essential for many types of cell activities, such as cell movement [40–42], anchoring of cells to the extracellular matrix [8,43–45] and mechanosignal transduction into the cell [8].
The regulation of actin–myosin-based contraction in cells is unclear, despite recent progress in the understanding of the regulation of cytoskeletal organization in non-muscle cells. In addition, the organization of stress fibres and associated focal adhesions in the cell also remains to be elucidated. This review article will discuss the Rho-kinase-dependent contractile activity generated by stress fibres and their organization within the cell, together with the Rho-kinase-dependent organization of focal adhesions.
2. Contraction of stress fibres can be regulated with MLCK in a Ca2+-dependent manner
Phosphorylation of the MLC is the primary event in the regulation of the contractile mechanism in smooth muscle and stress fibres. Myosin light chain phosphorylation is effective at low Ca2+ levels, thus reflecting an increase of Ca2+ sensitivity in the actin–myosin contractile structure. Ca2+ sensitivity is thought to occur via the regulation of myosin phosphatase.
Several groups have reported evidence of a contractile mechanism of stress fibres in living cells [6,7,29–31]. It is possible to isolate stress fibres from cells without any loss of contractility [1,9]. Stress fibres were isolated from cultured fibroblasts using an extraction solution containing Triton X-100, providing a model of the non-muscle actin–myosin contractile system. The regulation of their contraction can be observed in vitro and demonstrates that stress fibres themselves actually have a contractile structure [1].
The contractility of the extracted cells and actin–myosin-containing structures, including the contractile apparatus of non-muscle cells, has been reported previously. The non-muscle contractile apparatus of the circumferential microfilament bundles in retinal pigmented epithelial cells represents 40 per cent of the original length [46], while that of the contractile ring of cells represents about 25–35% of the original length [47,48], that of kidney proximal tubules in rats represents 80 per cent of the original length [5], and brush borders of the intestinal epithelium show a slight diameter change in association with contraction [49]. Several groups have reported that stress fibres are really contractile, and that they can indeed regulate isomeric tension within the cell [50]. The stress fibres with Mg2+-ATP contracted to 60 per cent of the original length in a microdissection model [10], 80 per cent in glycerol-extracted cells [51] and 75–80% in a digitonin-extracted cell model [51]. Stress fibres isolated with detergent treatment contract in response to Mg2+-ATP [1]. The major and essential factor responsible for the contraction of stress fibres is the phosphorylation of MLC. Myosin light chain phosphorylation occurs when isolated stress fibres are treated with Ca2+ and Mg2+-ATP in a Triton X-100-based extraction solution. In this case, phosphorylation of MLC is inhibited by the specific MLCK protein kinase inhibitor ML-7. The contraction of isolated stress fibres is completely inhibited by ML-7, thus indicating that MLC is phosphorylated by MLCK in a Ca2+-dependent manner.
Many simply extracted fibroblast models show contractility limited to only 70–80% of the original length. By contrast, completely isolated single stress fibres contracted to 23 per cent of the original length. The maximum speed of isolated stress fibre contraction is 2.4 µm s−1 [1]. The average speed of tail recoil of living fibroblasts is 1.5 µm s−1 with a maximum of 3.5 µm s−1 [52]. The extent of contraction of isolated stress fibres in fibroblasts is more substantial than other types of cell contractility, and stress fibres represent the fastest actin–myosin-based contractile system found in non-muscle cells.
3. Ca2+-independent Rho-kinase-mediated contraction in stress fibres
RhoA is a Rho-protein expressed in most cell types. Living fibroblasts show reorganization and contraction of stress fibres following treatment with lysophosphatidic acid (LPA) or bombesin [17,53]. These drugs are RhoA activators; thus, this response appears to be mediated by RhoA [17,53], which is an upstream regulator of Rho-kinase. An immunofluorescence analysis and immunoelectron microscopy demonstrated that RhoA and Rho-kinase also bind to stress fibres [29,54]. Moreover, biochemical studies indicate that Rho-kinase binds to myosin II and is thus localized on the stress fibres [54]. These findings strongly suggest that Rho-kinase and myosin II act synergistically to control the contractile activity of the stress fibres. Several biochemical studies have also demonstrated that Rho-kinase phosphorylates the MLC of isolated smooth muscle fibres [16,55] and extracted fibroblasts [56,57]. In vitro experiments have indicated that Rho-kinase phosphorylates MLC directly, and Rho-kinase was also shown to phosphorylate MLC indirectly by inhibition of myosin phosphatase activity [15,16]. The inactivation of myosin phosphatase by the phosphorylation of MBS of myosin phosphatase (threonine residue 697) with active Rho-kinase reduces Ca2+ sensitization and results in the increased phosphorylation of MLC, thus leading to stress fibre contraction [16,18,19,29]. Stress fibres, isolated by detergent treatment, that lack Rho-kinase contracted when constitutively active Rho-kinase was added, and an increased level of MLC phosphorylation was observed [29]. Activation of Rho-kinase in living cells strongly induces stress fibre and focal adhesion organization in cultured fibroblasts [29,31] [58]. These results indicated that the phosphorylation of MLC in non-muscle is attributed to an inhibition of the myosin phosphatase catalytic activity by Rho-kinase.
In addition, stress fibres can be partially isolated using a glycerol-based extraction solution without Triton X-100, and these isolated stress fibres contract upon addition of Mg2+-ATP without Ca2+. The calmodulin/MLCK contractile system requires Ca2+ ions, and therefore the Ca2+-independent contraction is due to Rho-kinase activity. The contraction of glycerol-isolated stress fibres is not inhibited by MLCK inhibitors [29]. Biochemical and immunofluorescence analyses indicate that glycerol-isolated stress fibres contain bound RhoA and Rho-kinase. However, immunoblotting and immunofluorescence analyses indicate that Triton X-100-isolated stress fibres (over 5 min extraction) do not carry RhoA or Rho-kinase. These observations strongly suggest that Ca2+-independent glycerol-isolated stress fibre contraction is owing to the activity of Rho-kinase. Glycerol-isolated stress fibre contraction is inhibited by HA1077 and Y-27632, both of which are specific inhibitors of Rho-kinase. The above results indicate that stress fibre contraction is regulated in two independent ways, i.e. a Ca2+-dependent calmodulin/MLCK system and a Ca2+-independent Rho-kinase system [29].
4. Comparison between the regulation of stress fibre and focal adhesion function by Rho-kinase and myosin light chain kinase
Stress fibre contraction is dually regulated by a Ca2+-dependent calmodulin/MLCK system and a Ca2+-independent Rho-kinase system. Kinetic analyses of isolated stress fibre contraction suggest that the Ca2+-independent Rho-kinase system causes slower contraction than the Ca2+-dependent MLCK system in fibroblasts [29,31]. Both the speed and extent of contraction in the Rho-kinase system are less than those mediated by the typical MLCK/calmodulin-contraction system. Stress fibres show extensive and more rapid contraction when constitutively active Rho-kinase and Ca2+ ions are added to isolated stress fibres to induce both MLCK and Rho-kinase activity. The stress fibres possess two independent regulation systems, which seem to regulate different types of contraction; the calmodulin/MLCK contractile system supports transient and rapid contraction, while the Rho-kinase contractile system supports continuous and slow contraction.
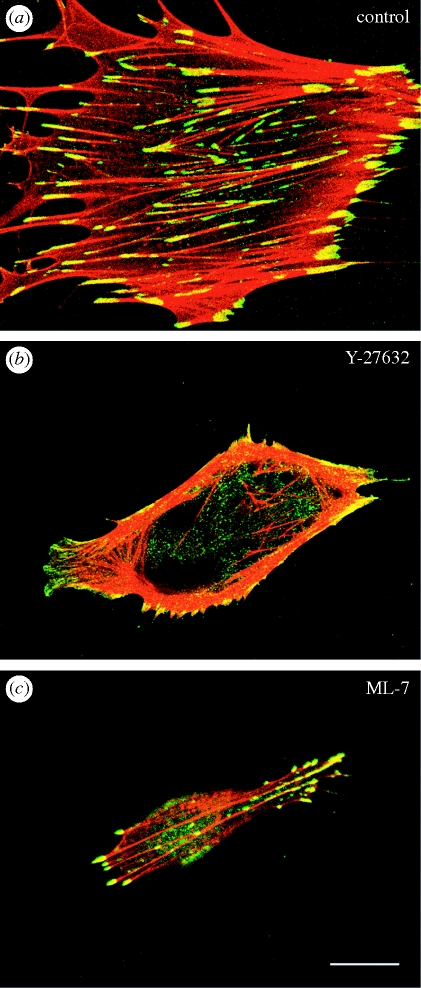
Several studies have demonstrated the roles of MLCK and Rho-kinase in the organization of stress fibres and focal adhesions [29–33,58,59]. The Rho-kinase inhibitors, HA1077 or Y-27632, cause the disassembly of the stress fibres and focal adhesions located in the central portion of the cell within 1 h. However, the stress fibres and focal adhesions located at the cell periphery are less affected by such Rho-kinase inhibitors [29,32]. Rho-kinase inhibitors specifically affected both stress fibres and focal adhesions mainly in the central portion of the cell. In addition, electron microscopic analyses indicate that these two types of fibre are typical stress fibres, although they differ in both their location and thickness [58]. Multiple studies examined whether the two types of stress fibre and their associated focal adhesions differ in the properties of their contraction mechanisms in addition to their morphological features, and also whether they respond differently to various inhibitors that affect MLC phosphorylation [30–33,59]. The treatment of living cells with the Rho-kinase inhibitor Y-27632 results in the specific disassembly of the stress fibres located at the central portion of the cell. The peripherally located stress fibres show only a slight reduction in thickness, but are otherwise hardly affected by the Rho-kinase inhibitor [31,58] (figure 1). The arched region at the leading edge of the cell protrudes gradually after Rho-kinase inhibitor treatment, and thus forms arches that are less distinct. The local movement of the plasma membrane at the cell periphery can be explained by the relaxation and loss of contractile tension within the cell. This observation indicates that the relaxation of the cytoplasm and the cell cortex may be owing to inhibition of Rho-kinase activity. When the inhibitor-treated cells are washed with fresh medium to remove the inhibitor, some of the arches at the cell periphery gradually contract again to form distinct arches [29,58]. These observations, therefore, support the hypothesis that Rho-kinase generates overall cell tension within the cell.
Figure 1.
Effects of Rho-kinase (Y-27632) and MLCK (ML-7) inhibitors on peripheral and central stress fibres of human foreskin fibroblasts. The cells in (a–c) are doubly stained with rhodamine-labelled phalloidin for F-actin staining (red) and anti-vinculum for focal adhesion staining (green). Yellow staining indicates the overlap of the two colours, where F-actin and vinculin co-localize. (a) Untreated control; (b) cell treated with Y-27632 (10 µM) for 1 h; (c) cell treated with ML-7 (25 µM) for 1 h. (b) Cell treated with Y-27632 showed a reduced number of central stress fibres; however, peripheral stress fibres remained unchanged. (c) Peripheral stress fibres were significantly reduced after the treatment with ML-7, but not central stress fibres. All photographs are taken under confocal laser-scanning microscopy. Scale bar, 20 µm.
On the other hand, peripheral stress fibres and focal adhesions are markedly affected by the typical MLCK inhibitor ML-7 [1,29]. The stress fibres located at the cell periphery appear to be disrupted by inhibition of MLCK, while the central stress fibres remain unaffected (figure 1). A loss of peripheral stress fibres causes cell rounding, and the motility of cells is significantly inhibited. The two types of stress fibres have different contractile properties. Contraction of the peripheral stress fibres starts before the central stress fibre begins to contract [31]. The difference in the reactivation of peripheral and central stress fibres indicates that the two stress fibre contraction systems are regulated differently within a cell. The central stress fibre is more sensitive to Rho-kinase inhibitors than to MLCK inhibitors [31]. Rho-kinase activity is necessary for maintaining the organization of the stress fibres and focal adhesions in the central portion of the cell. On the other hand, MLCK activity is necessary for maintaining their organization in the peripheral portion of the cell. The reason for the two different kinase regulation systems within a cell is unclear; however, the difference reflects the functional differences in these two kinetic regulatory systems of stress fibre contraction.
5. Control of Rho-kinase-dependent contraction of stress fibres and organization of focal adhesions
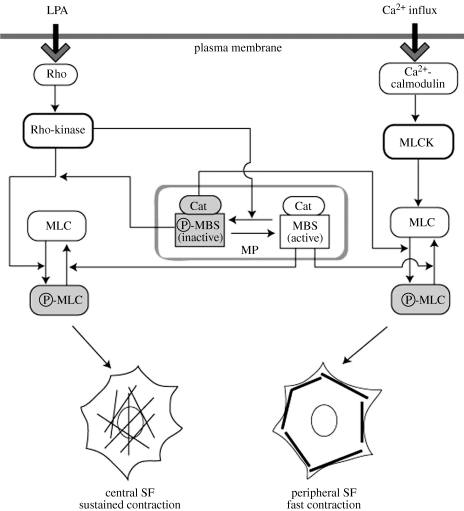
Two systems are needed to generate different types of contractility in living cells. The first type of rapid and short-lived contraction, which can generate strong contractions, is induced by the Ca2+/calmodulin-mediated MLCK system. Ca2+ release within the cell is thought to be a rapid and transient event that occurs in localized areas, and therefore this type of contraction seems to be triggered rapidly by Ca2+ in highly localized regions of the cell. The other type of cell contraction, the sustained type, is apparently not generated by Ca2+ influx. A sustained type of contraction may be induced by Rho-kinase without Ca2+ by flexible control of the level of myosin phosphatase activity. The activation level of myosin phosphatase can be easily fine-tuned by Rho-kinase, because it acts in a Ca2+-independent manner [29,32]. The results of the kinetic analyses also indicated that the Ca2+-dependent MLCK system induces more rapid contraction of stress fibres, while the Ca2+- independent Rho-kinase system causes slower contraction. Schematic illustrations of the proposed model of the two independent regulatory systems of stress fibre organization are shown in figure 2.
Figure 2.
Schematic of the two regulatory actin–myosin contractile systems in non-muscle cells. P-MLC induced cell contraction. P-MLC, phosphorylated MLC; MP, myosin phosphatase; Cat, catalytic subunit of myosin phosphatase; MBS, myosin-binding subunit of myosin phosphatase; P-MBS, phosphorylated MBS.
The mechanism underlying the regulation of Rho-kinase-dependent organization of stress fibres and focal adhesions remains to be elucidated. The Rho-kinase-dependent reorganization of stress fibres occurs along small focal adhesion-like structures located at the centre of adherent fibroblasts. Accumulation of actin filaments and bundling occurs initially along a small focal adhesion-like structure [58]. The organization of the focal adhesion-like structure precedes the organization of the stress fibres. Moreover, treatment with (S)-(−)-blebbistatin, a specific inhibitor of myosin II, prevents the reorganization of stress fibres, but it does not affect the reorganization of focal adhesions [58]. These observations strongly suggest that Rho-kinase either directly or indirectly influences not only the organization of stress fibres but also the organization of focal adhesions.
The Ca2+-independent stress fibre contraction by Rho-kinase-dependent systems may be involved in the maintenance of general and sustained tension over the whole cell, while the Ca2+-dependent MLCK system may be used to induce rapid and transient contraction of the stress fibres. The selective mechanisms of action of Rho-kinase and MLCK in stress fibre and focal adhesion organization have not been determined. Further studies are, therefore, required to analyse the position-specific effects of Rho-kinase-dependent organization of stress fibres and focal adhesions.
6. Conclusion
Stress fibres are contractile cytoskeletal structures present in many cell types. The contraction of stress fibres is regulated by the phosphorylation level of MLC in two independent ways within the cell, i.e. a Ca2+-dependent calmodulin/MLCK system and a Ca2+-independent Rho-kinase system. The Ca2+-dependent contractile system controls the rapid contraction of stress fibres, and thereby regulates the organization of thick peripheral stress fibres. On the other hand, the Rho-kinase-dependent contractile system controls both slow and fine-tuned contraction, while also regulating the organization of the fine central stress fibres and focal adhesions in the cell.
Acknowledgements
The work reported here was supported by Grants-in-Aid for Scientific Research from the Promotion and the Mutual Aid Corporation for Private Schools of Japan.
References
- 1.Katoh K., Kano Y., Masuda M., Onishi H., Fujiwara K. 1998. Isolation and contraction of the stress fiber. Mol. Biol. Cell 9, 1919–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byers H. R., Fujiwara K. 1982. Stress fibers in cells in situ: immunofluorescence visualization with antiactin, antimyosin, and anti-alpha-actinin. J. Cell Biol. 93, 804–811 10.1083/jcb.93.3.804 (doi:10.1083/jcb.93.3.804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White G. E., Gimbrone M. A. J., Fujiwara K. 1983. Factors influencing the expression of stress fibers in vascular endothelial cells in situ. J. Cell Biol. 97, 416–424 10.1083/jcb.97.2.416 (doi:10.1083/jcb.97.2.416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugimoto K., Fujii S., Yamashita K. 1991. Expression of stress fibers in bullfrog mesothelial cells in response to tension. Exp. Cell Res. 196, 353–361 10.1016/0014-4827(91)90271-U (doi:10.1016/0014-4827(91)90271-U) [DOI] [PubMed] [Google Scholar]
- 5.Murakami T., Ishikawa H. 1991. Stress fibers in situ in proximal tubules of the rat kidney. Cell Struct. Funct. 16, 231–240 10.1247/csf.16.231 (doi:10.1247/csf.16.231) [DOI] [PubMed] [Google Scholar]
- 6.Kumar S., Maxwell I. Z., Heisterkamp A., Polte T. R., Lele T. P., Salanga M., Mazur E., Ingber D. E. 2006. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 90, 3762–3773 10.1529/biophysj.105.071506 (doi:10.1529/biophysj.105.071506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson L. J., Rajfur Z., Maddox A. S., Freel C. D., Chen Y., Edlund M., Otey C., Burridge K. 2004. Simultaneous stretching and contraction of stress fibers in vivo. Mol. Biol. Cell 15, 3497–3508 10.1091/mbc.E03-09-0696 (doi:10.1091/mbc.E03-09-0696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geiger B., Spatz J. P., Bershadsky A. D. 2009. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 10, 21–33 10.1038/nrm2593 (doi:10.1038/nrm2593) [DOI] [PubMed] [Google Scholar]
- 9.Katoh K., Kano Y., Fujiwara K. 2000. Isolation and in vitro contraction of stress fibers. Methods Enzymol. 325, 369–380 10.1016/S0076-6879(00)25458-X (doi:10.1016/S0076-6879(00)25458-X) [DOI] [PubMed] [Google Scholar]
- 10.Isenberg G., Rathke P. C., Hülsmann N., Franke W. W., Wohlfarth-Bottermann K. E. 1976. Cytoplasmic actomyosin fibrils in tissue culture cells. Direct proof of contractility by visualization of ATP-induced contraction in fibrils isolated by laser microbeam dissection. Cell Tissue Res. 166, 427–443 [DOI] [PubMed] [Google Scholar]
- 11.Ishizaki T., Naito M., Fujisawa K., Maekawa M., Watanabe N., Saito Y., Narumiya S. 1997. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works down stream of Rho and induces focal adhesions. FEBS Lett. 404, 118–124 10.1016/S0014-5793(97)00107-5 (doi:10.1016/S0014-5793(97)00107-5) [DOI] [PubMed] [Google Scholar]
- 12.Leung T., Manser E., Tan L., Lim L. 1995. A novel serine/threonine kinase binding the ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J. Biol. Chem. 270, 29 051–29 054 10.1074/jbc.270.49.29051 (doi:10.1074/jbc.270.49.29051) [DOI] [PubMed] [Google Scholar]
- 13.Matsui T., et al. 1996. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 15, 2208–2216 [PMC free article] [PubMed] [Google Scholar]
- 14.Amano M., Chihara K., Kimura K., Fukata Y., Nakamura N., Matsuura Y., Kaibuchi K. 1997. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275, 1308–1311 10.1126/science.275.5304.1308 (doi:10.1126/science.275.5304.1308) [DOI] [PubMed] [Google Scholar]
- 15.Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, 20 246–20 249 [DOI] [PubMed] [Google Scholar]
- 16.Amano M., Mukai H., Ono Y., Chihara K., Matsui T., Hamajima Y., Okawa K., Iwamatsu A., Kaibuchi K. 1996. Identification of a putative target for Rho as the serine-threonin kinase protein kinase N. Science 271, 648–650 10.1126/science.271.5249.648 (doi:10.1126/science.271.5249.648) [DOI] [PubMed] [Google Scholar]
- 17.Ridley A. J., Hall A. 1992. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389–399 10.1016/0092-8674(92)90163-7 (doi:10.1016/0092-8674(92)90163-7) [DOI] [PubMed] [Google Scholar]
- 18.Feng J., Ito M., Ichikawa K., Isaka N., Nishikawa M., Hartshorne D. J., Nakano T. 1999. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J. Biol. Chem. 274, 37 385–37 390 10.1074/jbc.274.52.37385 (doi:10.1074/jbc.274.52.37385) [DOI] [PubMed] [Google Scholar]
- 19.Kawano Y., Fukata Y., Oshiro N., Amano M., Nakamura T., Ito M., Matsumura F., Inagaki M., Kaibuchi K. 1999. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase during cell migration and cytokinasis. J. Cell Biol. 147, 1023–1037 10.1083/jcb.147.5.1023 (doi:10.1083/jcb.147.5.1023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura K., et al. 1996. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273, 245–248 10.1126/science.273.5272.245 (doi:10.1126/science.273.5272.245) [DOI] [PubMed] [Google Scholar]
- 21.Ito M., Nakano T., Erdodi F., Hartshorne D. J. 2004. Myosin phosphatase: structure, regulation and function. Mol. Cell. Biochem. 259, 197–209 10.1023/B:MCBI.0000021373.14288.00 (doi:10.1023/B:MCBI.0000021373.14288.00) [DOI] [PubMed] [Google Scholar]
- 22.Muranyi A., Derkach D., Erdodi F., Kiss A., Ito M., Hartshorne D. J. 2005. Phosphorylation of Thr695 and Thr850 on the myosin phosphatase target subunit: inhibitory effects and occurrence in A7r5 cells. FEBS Lett. 579, 6611–6615 10.1016/j.febslet.2005.10.055 (doi:10.1016/j.febslet.2005.10.055) [DOI] [PubMed] [Google Scholar]
- 23.Ren X. D., Wang R., Li Q., Kahek L. A., Kaibuchi K., Clark R. A. 2004. Disruption of Rho signal transduction upon cell detachment. J. Cell. Sci. 117, 3511–3518 10.1242/jcs.01205 (doi:10.1242/jcs.01205) [DOI] [PubMed] [Google Scholar]
- 24.Wilson D. P., Susnjar M., Kiss E., Sutherland C., Walsh M. P. 2005. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem. J. 389, 763–774 10.1042/BJ20050237 (doi:10.1042/BJ20050237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riddick N., Ohtani K., Surks H. K. 2008. Targeting by myosin phosphatase-RhoA interacting protein mediates RhoA/ROCK regulation of myosin phosphatase. J. Cell. Biochem. 103, 1158–1170 10.1002/jcb.21488 (doi:10.1002/jcb.21488) [DOI] [PubMed] [Google Scholar]
- 26.Amano M., Fukata Y., Kaibuchi K. 2000. Regulation and functions of Rho-associated kinase. Exp. Cell Res. 25, 44–51 10.1006/excr.2000.5046 (doi:10.1006/excr.2000.5046) [DOI] [PubMed] [Google Scholar]
- 27.Kaibuchi K., Kuroda S., Amano M. 1999. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68, 459–486 10.1146/annurev.biochem.68.1.459 (doi:10.1146/annurev.biochem.68.1.459) [DOI] [PubMed] [Google Scholar]
- 28.Riento K., Ridley A. J. 2003. Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 4, 446–456 10.1038/nrm1128 (doi:10.1038/nrm1128) [DOI] [PubMed] [Google Scholar]
- 29.Katoh K., Kano Y., Amano M., Onishi H., Kaibuchi K., Fujiwara K. 2001. Rho-kinase-mediated contraction of isolated stress fibers. J. Cell. Biol. 153, 569–583 10.1083/jcb.153.3.569 (doi:10.1083/jcb.153.3.569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Totsukawa G., Wu Y., Sasaki Y., Hartshorne D. J., Yamakita Y., Yamashiro S., Matsumura F. 2004. Distinct roles of MLCK and ROCK in the regulation of membrane protrusions and focal adhesion dynamics during cell migration of fibroblasts. J. Cell Biol. 164, 427–439 10.1083/jcb.200306172 (doi:10.1083/jcb.200306172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katoh K., Kano Y., Amano M., Kaibuchi K., Fujiwara K. 2001. Stress fiber organization regulated by MLCK and Rho-kinase in cultured human fibroblast. Am. J. Cell Physiol. 280, C1669–C1679 [DOI] [PubMed] [Google Scholar]
- 32.Totsukawa G., Yamakita Y., Yamashiro S., Hartshorne D. J., Sasaki Y., Matsumura F. 2000. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 150, 797–806 10.1083/jcb.150.4.797 (doi:10.1083/jcb.150.4.797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanase M., et al. 2003. Functional diversity between Rho-kinase- and MLCK-mediated cytoskeletal actions in a myofibroblast-like hepatic stellate cell line. Biochem. Biophys. Res. Commun. 305, 223–228 10.1016/S0006-291X(03)00726-5 (doi:10.1016/S0006-291X(03)00726-5) [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Rico C., Pincet F., Tj P., Dufour S. 2010. Integrins stimulate E-cadherin-mediated intercellular adhesion by regulating Src-kinase activation and actomyosin contractility. J. Cell Sci. 123, 712–722 10.1242/jcs.047878 (doi:10.1242/jcs.047878) [DOI] [PubMed] [Google Scholar]
- 35.McKenzie J. A., Ridley A. J. 2007. Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J. Cell Physiol. 213, 221–228 10.1002/jcp.21114 (doi:10.1002/jcp.21114) [DOI] [PubMed] [Google Scholar]
- 36.Gitik M., Reichert F., Rotshenker S. 2010. Cytoskeleton plays a dual role of activation and inhibition in myelin and zymosan phagocytosis by microglia. FASEB J. 24, 2211–2221 10.1096/fj.09-146118 (doi:10.1096/fj.09-146118) [DOI] [PubMed] [Google Scholar]
- 37.Krndija D., Schmid H., Eismann J. L., Lother U., Adler G., Oswald F., Seufferlein T., von Wichert G. 2010. Substrate stiffness and the receptor-type tyrosine-protein phosphatase alpha regulate spreading of colon cancer cells through cytoskeletal contractility. Oncogene 29, 2724–2738 10.1038/onc.2010.25 (doi:10.1038/onc.2010.25) [DOI] [PubMed] [Google Scholar]
- 38.Paszek M. J., et al. 2005. Tensional homeostasis and the malignant phenotype. Cancer Cell. 8, 241–254 10.1016/j.ccr.2005.08.010 (doi:10.1016/j.ccr.2005.08.010) [DOI] [PubMed] [Google Scholar]
- 39.Ulrich T. A., De Juan Pardo E. M., Kumar S. 2009. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 69, 4167–4174 10.1158/0008-5472.CAN-08-4859 (doi:10.1158/0008-5472.CAN-08-4859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. 2003. Cell migration: integrating signals from front to back. Science 302, 1704–1709 10.1126/science.1092053 (doi:10.1126/science.1092053) [DOI] [PubMed] [Google Scholar]
- 41.Small J. V., Resch G. P. 2005. The comings and goings of actin: coupling protrusion and retraction in cell motility. Curr. Opin. Cell Biol. 17, 517–523 10.1016/j.ceb.2005.08.004 (doi:10.1016/j.ceb.2005.08.004) [DOI] [PubMed] [Google Scholar]
- 42.Small J. V., Rottner K., Kaverina I., Anderson K. I. 1998. Assembling an actin cytoskeleton for cell attachment and movement. Biochim. Biophys. Acta 1404, 271–281 10.1016/S0167-4889(98)00080-9 (doi:10.1016/S0167-4889(98)00080-9) [DOI] [PubMed] [Google Scholar]
- 43.Burridge K., Chrzanowska-Wodnicka M. 1996. Focal adhesions, contractility and signaling. Annu. Rev. Cell Dev. Biol. 12, 463–519 10.1146/annurev.cellbio.12.1.463 (doi:10.1146/annurev.cellbio.12.1.463) [DOI] [PubMed] [Google Scholar]
- 44.Burridge K., Fath K., Kelly K., Nuckolls G., Turner C. 1988. Focal adhesions: transmembrane junction between the extracellular matrix and the cytoskeleton. Annu. Rev. Cell Biol. 4, 487–525 [DOI] [PubMed] [Google Scholar]
- 45.Pellegrin S., Mellor H. 2007. Actin stress fibres. J. Cell Sci. 120, 3491–3499 10.1242/jcs.018473 (doi:10.1242/jcs.018473) [DOI] [PubMed] [Google Scholar]
- 46.Owaribe K., Masuda H. 1982. Isolation and characterization of circumferential microfilament bundles from retinal pigmented epithelial cells. J. Cell Biol. 95, 310–315 10.1083/jcb.95.1.310 (doi:10.1083/jcb.95.1.310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cande W. Z. 1980. A permeabilized cell model for studying cytokinesis using mammalian tissue culture cells. J. Cell Biol. 87, 326–335 10.1083/jcb.87.2.326 (doi:10.1083/jcb.87.2.326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mabuchi I., Tsukita S., Tsukita S., Sawai T. 1988. Cleavage furrow isolated from newt eggs: contraction, organization of the actin filaments, and protein components of the furrow. Proc. Natl Acad. Sci. USA 85, 5966–5970 10.1073/pnas.85.16.5966 (doi:10.1073/pnas.85.16.5966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodewald R., Newman S. B., Karnovsky M. J. 1976. Contraction of isolated brush borders from the intestinal epithelium. J. Cell Biol. 70, 541–554 10.1083/jcb.70.3.541 (doi:10.1083/jcb.70.3.541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burridge K. 1981. Are stress fibers contractile? Nature 294, 691–692 10.1038/294691a0 (doi:10.1038/294691a0) [DOI] [PubMed] [Google Scholar]
- 51.Kreis T. I., Birchmeier W. 1980. Stress fiber sarcomeres of fibroblasts are contractile. Cell 22, 555–561 10.1016/0092-8674(80)90365-7 (doi:10.1016/0092-8674(80)90365-7) [DOI] [PubMed] [Google Scholar]
- 52.Chen W. 1981. Mechanism of retraction of the trailing edge during fibroblast movement. J. Cell Biol. 90, 187–200 10.1083/jcb.90.1.187 (doi:10.1083/jcb.90.1.187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ridley A. J., Hall A. 1994. Signal transduction pathways regulating Rho-mediated stress fiber formation: requirement for a tyrosine kinase. EMBO J. 13, 2600–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawabata S., Usukura J., Moroe N., Ito M., Iwamatsu A., Kaibuchi K., Amano M. 2004. Interaction of Rho-kinase with myosin II at stress fibers. Genes Cells 9, 653–660 10.1111/j.1356-9597.2004.00749.x (doi:10.1111/j.1356-9597.2004.00749.x) [DOI] [PubMed] [Google Scholar]
- 55.Kureishi Y., Kobayashi S., Amano M., Kimura K., Kanaide H., Nakano T., Kaibuchi K., Ito M. 1997. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J. Biol. Chem. 272, 12 257–12 260 10.1074/jbc.272.19.12257 (doi:10.1074/jbc.272.19.12257) [DOI] [PubMed] [Google Scholar]
- 56.Amano M., Chihara K., Nakamura N., Fukata Y., Yano T., Shibata M., Ikebe M., Kaibuchi K. 1998. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells 3, 177–188 10.1046/j.1365-2443.1998.00181.x (doi:10.1046/j.1365-2443.1998.00181.x) [DOI] [PubMed] [Google Scholar]
- 57.Chihara K., et al. 1997. Cytoskeletal rearrangements and transcriptional activation of c-fos serum response element by Rho-kinase. J. Biol. Chem. 272, 25 121–25 127 10.1074/jbc.272.40.25121 (doi:10.1074/jbc.272.40.25121) [DOI] [PubMed] [Google Scholar]
- 58.Katoh K., Kano Y., Ookawara S. 2007. Rho-kinase dependent organization of stress fibers and focal adhesions in cultured fibroblasts. Genes Cells 12, 623–638 10.1111/j.1365-2443.2007.01073.x (doi:10.1111/j.1365-2443.2007.01073.x) [DOI] [PubMed] [Google Scholar]
- 59.Senju Y., Miyata H. 2009. The role of actomyosin contractility in the formation and dynamics of actin bundles during fibroblast spreading. J. Biochem. 145, 137–150 10.1093/jb/mvn151 (doi:10.1093/jb/mvn151) [DOI] [PubMed] [Google Scholar]