Abstract
Drug-delivery systems with a unique capability to respond to a given stimulus can improve therapeutic efficacy. However, development of such systems is currently heavily reliant on responsive polymeric materials and pursuing this singular strategy limits the potential for clinical translation. In this report, with a model system used for drug-release studies, we demonstrate a new strategy: how a temperature-responsive non-toxic, volatile liquid can be encapsulated and stored under ambient conditions and subsequently programmed for controlled drug release without relying on a smart polymer. When the stimulus temperature is reached, controlled encapsulation of different amounts of dye in the capsules is achieved and facilitates subsequent sustained release. With different ratios of the liquid (perfluorohexane): dye in the capsules, enhanced controlled release with real-time response is provided. Hence, our findings offer great potential for drug-delivery applications and provide new generic insights into the development of stimuli drug-release systems.
Keywords: stimuli-responsive drug delivery, controlled release, hollow capsules
1. Introduction
Drug-delivery systems with a unique capability to respond to a given stimulus can improve therapeutic efficacy and hold great promise as smart materials for drug delivery and controlled-release applications [1–3]. However, development of such systems is currently heavily reliant on responsive polymeric materials [4,5] that are responsive, for example, to enzymes [6], light [7], temperature [8], calcium [9] and ultrasound [10]. Such systems usually incorporate responsive elements, e.g. micelles, vesicles and hydrogels, and have been designed to undergo conformational or phase changes in response to a given stimulus, providing controlled release of a therapeutic substance from a polymer matrix [8,11]. The ability of these polymeric carriers to encapsulate, protect and facilitate controlled release provides a means of regulating pharmacokinetics and improving therapeutic efficacy.
Some crucial disadvantages are linked with the use of responsive polymers and these can limit their utility in biomedical applications. For example, there is very little practical potential to use salt-responsive capsule systems in the human body owing to the absence of variation in ionic strength and their low-release efficiency [12]. The radiation of light may cause damage to organisms and normal cells, and its penetration depth is weak in tissues [13,14]. Furthermore, toxic by-products and the biocompatibility of responsive polymers may be a significant impediment to the development of these delivery systems and for patient use [15,16]. Thus, fabrication of new stimuli capsule systems is very important to speedily enable their application in drug delivery.
In this report, perfluorohexane (PFH) is used as the responsive element in a new stimulus drug-delivery system independent of the action of smart polymers. Such non-toxic, biocompatible fluorinated compounds have been used in clinical applications such as liquid ventilation and ultrasound contrast enhancement [17–19], and for exchange encapsulation [20]. PFH is a low surface tension (12 m Nm−1) biologically inert liquid at normal ambient temperature and can be easily transformed to its gaseous phase on account of its high volatility and high oxygen solubility [21]. Our aim was to integrate PFH into a core-shell structure and then investigate how the stimulus effects can be controlled by temperature variation.
2. Materials and methods
2.1. Materials
Polymethylsilsesquioxane (PMSQ) powder was provided by Wacker Chemie AG, GmbH; PFH was obtained from F2 Chemicals Ltd, and they were used without further treatment. Both materials are chemically stable and biocompatible. Ethanol was provided from BDH Laboratory Supplies and was used to prepare PMSQ solutions. PMSQ was dissolved in ethanol with magnetic stirring in a conical flask for 1800 s at 22°C to ensure full miscibility of the polymer.
2.2. Characterization of polymer solution
Liquid properties such as viscosity, surface tension, density and electrical conductivity were characterized as follows. Viscosity was determined using a U-tube viscometer and a VISCOEASY rotational viscometer. Surface tension was measured using a Kruss Tensiometer. Density was measured using a standard 25 ml density bottle. Electrical conductivity was determined using a HI-8733 conductivity probe. Ethanol was used to calibrate the various instruments mentioned above and all experiments were performed at 22°C. The characterized properties are shown in table 1.
Table 1.
Relevant physical properties of the liquids used in experimental work. The properties of PFH were provided by F2 Chemicals Ltd.
| material | density (kg m−3) | viscosity (mPa s) | surface tension (m Nm−1) | electrical conductivity (Sm−1) |
|---|---|---|---|---|
| perfluorohexane | 1710 | 1.1 | 12 | <1 × 10−11 |
| PMSQ 18 wt % | 805 | 1.8 | 23 | 9 × 10−5 |
2.3. Formation of capsules
PFH and PMSQ polymer solution, which are immiscible, were electrosprayed simultaneously to obtain the core-shell structure. The experimental set-up consists of a pair of concentric stainless steel needles, with the inner needle raised by 2 mm above the exit of the outer needle. The inner needle has an inner diameter of 150 µm and an outer diameter of 300 µm. The outer needle has inner and outer diameters of 685 and 1100 µm, respectively. PMSQ solution was introduced through the outer needle, while the inner needle was supplied with PFH. The flow rates of the two liquids were controlled by high-precision programmable syringe pumps (Harvard PHD 4400, Apparatus, Edenbridge, UK). Volume capacity syringes of 10 and 5 ml were separately connected with the inner and outer needles using silicone tubing. The flow rate of syringe 2 (PMSQ solution) was fixed at 650 µl min−1, while the flow rate of syringe 1 (PFH solution) was fixed at 150 µl min−1. An electric field (4.5 kV) between the needles and a ring-shaped ground electrode (external and internal diameters of 20 and 15 mm, respectively) was controlled by a high-voltage generator (Glassman Europe Limited, Bramley, UK). The distance from the exit of the outer needle to the ground electrode (the working distance) was fixed at 12 mm in all the experiments. The flow of the liquids under the influence of the electric field was visualized using a video camera (LEICA S6D JVC-colour).
2.4. Characterization of capsules
The size of the fabricated particles was studied as-formed by optical microscopy (Nikon Eclipse ME-600, Nikon Co, Tokyo, Japan) and subsequently, after drying, by scanning electron microscopy (SEM; JEOL JSM-6301F field-emission scanning electron microscope). Two millilitre samples of the capsules were collected in liquid and put on glass slides. After drying for 48 h in a desiccator, the dried samples were vacuum-coated with a thin layer of gold for 90 s to obtain SEM images. To calculate the size of the capsules, 200 capsules were analysed from the SEM images. All measurements on the micrographs were carried out using the standard IMAGE-PROPLUS software (Media Cybernetics, L.P. Del Mar, CA, USA).
In order to evaluate the PFH loading content, three samples were kept at 37, 45 and 57°C to evaporate the encapsulated PFH, respectively, and were re-weighed every 300 s. The PFH content is defined as the ratio of the actual amount of PFH encapsulated in PMSQ and the actual weight of the capsules (equation 2.1).
 |
2.1 |
Evans blue dye was used to determine the loading capacity. This PMSQ-dye combination is used as a model in drug-release experiments and generates experimentally verified drug-release profiles, which agree with established drug-release models [22]. The capsules were collected in a solution of evans blue dye that cannot diffuse into the capsules initially because the reservoir is occupied by PFH. By heating the PFH capsules to different temperatures and times, the dye was incorporated in the capsules and the estimation of dye content was carried out by determining the difference in dye concentration before and after heating using an UV spectrometer (UV-2401PC spectrophotometer, Shimadzu). The loading efficiency was defined as the ratio of actual and theoretical amounts of drug encapsulated in the polymer shell:
 |
2.2 |
In vitro release of dye from polymer capsules was carried out in 20 ml of phosphate buffer (pH 7.4) under stirring at 100 rpm in a flask. Typically, the temperature of the suspension was kept at 37 ± 0.5°C. At designated time intervals, the vial was taken out and centrifuged. The supernatants of dye were directly analysed by UV spectroscopy at a wavelength of 611 nm. After each measurement, the release medium was replaced. The concentration of dye in the supernatant was determined by UV absorption, reference to a standard curve. The in vitro release was obtained in release medium of different temperatures (37, 45 and 57°C) and analysed following the same procedure for 37 ± 0.5°C.
3. Results and discussion
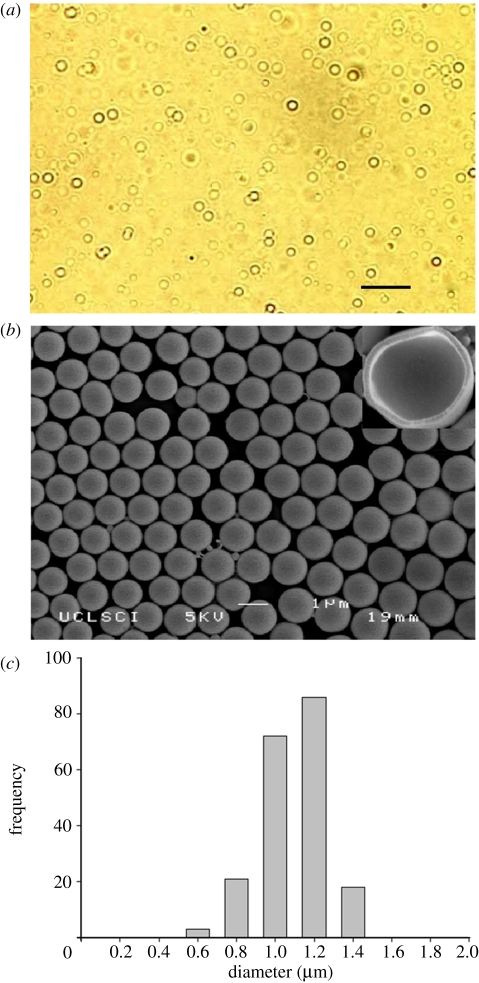
The polymer spheres with PFH in the core are stable and sediment in water due to their relatively high density. Figure 1a shows an optical micrograph of these particles in suspension, while figure 1b shows the dried microspheres. A hollow cavity is obtained after PFH evaporation. As shown in figure 1c, the PFH capsules are 1.2 µm in diameter with a narrow size distribution. In this work, the shell thickness of the capsules was kept at 120 ± 16 nm. Both capsule size and shell thickness can be easily varied with the processing method used [23].
Figure 1.
PFH-loaded PMSQ capsules: (a) optical micrograph taken immediately after preparation (scale bar, 5 µm), (b) SEM with typical cross-section in top right-hand side corner, and (c) size distribution.
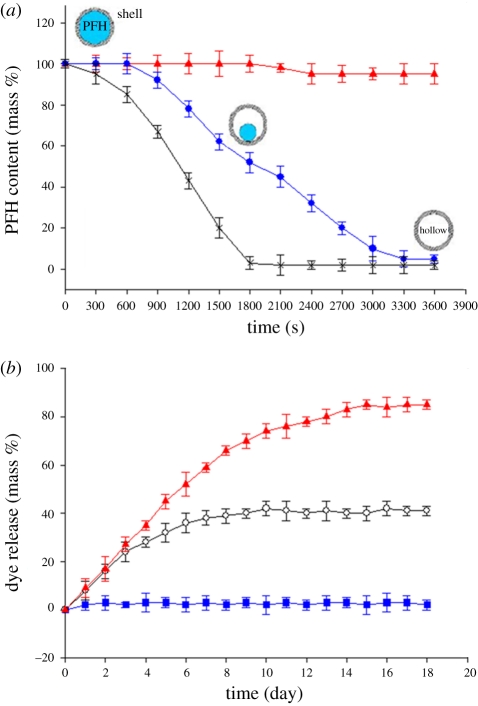
In order to first establish the influence of temperature on the PFH capsules, samples were sprayed onto glass slides and incubated at 37, 45 and 57°C to evaporate the encapsulated PFH. The capsules were re-weighed at regular intervals (figure 2a). At 37°C, the amount of PFH was constant for 1800 s and then slightly decreased. In contrast, at 45°C, the percentage of PFH remained constant for 600 s and was fully released after 3000 s. At 57°C, all the PFH was lost in 1500 s.
Figure 2.
(a) Thermogravimetric traces showing the PFH content retained as a function of time at different temperatures (red triangles, 37°C; blue circles, 45°C; black crosses, 57°C). (b) Dye release at 37°C from capsules originally incubated at 45°C for different times (blue squares, 300 s; black circles, 1800 s; red triangles, 3600 s). Error bars represent the standard deviation from three experiments.
The effect of the storage and release of dye on the structure of PMSQ can be compared by considering the stability of this polymer at the temperatures used in this work. PMSQ capsules were collected in a cross-linker (distilled water) and possessed good mechanical properties and chemical durability [24]. In addition, PFH is an unreactive, immiscible and inert liquid, and does not change the composition of the shell material [23]. After the release of dye molecules, no apparent difference in morphology can be observed within the experimental time span. Furthermore, it has been reported that glass-transition temperature of PMSQ polymers is in the range of 80–120°C [25,26], and its coefficient of thermal expansion is approximately 19 × 10−6°C [27] and on pyrolysis of PMSQ to ceramic, the first mass loss of PMSQ can be observed only at higher than 199°C [28].
Suspension of hollow capsules in a solution of the material to be encapsulated results in the yield spontaneous filling of the capsules [29,30] and different amounts of drug encapsulation can be obtained by varying the size of the hollow spheres [31,32]. In addition, with our capsules, the capacity for filling depends on the PFH content retained in the capsule. In vitro dye release at 37°C from capsules after undergoing different incubation times (ti) at 45°C is shown in figure 2b. For ti = 300 s, no dye release was observed because encapsulated PFH is stable in the polymer shell and the liquid medium. On increase of ti to 1800 s, a sustained release behaviour can be observed accompanied by a steady decrease in the release rate and only 40 per cent of the dye (equation (2.2)) was released. At ti = 3600 s, after sustained release at a higher rate over 15 days, the amount of total dye release reached 82 per cent. These results are in overall agreement with figure 2b, but also show that the dye content incorporated into capsules is lower than the predicted values which, for example, suggests that dye in the capsules can reach approximately 48 per cent when heated at 45°C for 1800 s. The 8 per cent discrepancy is because of the evaporation rate of PFH being faster under ambient conditions than that in solution. In addition, oxygen concentration in the ambient environment can have a significant influence on the evaporation rate of PFH [19]. However, this system exhibits temperature-responsive controllable encapsulation, and therefore offers the ability to incorporate high or low doses of a drug in the capsule. In addition, as discussed above PMSQ is stable at 37, 45 and 57°C and does not react with the PFH during the loading and the releasing process. The temperatures used here are much lower than the critical temperature needed to affect the PMSQ shell. Therefore, the dye loading and release processes are dominated by the concentration difference between the core and the sink.
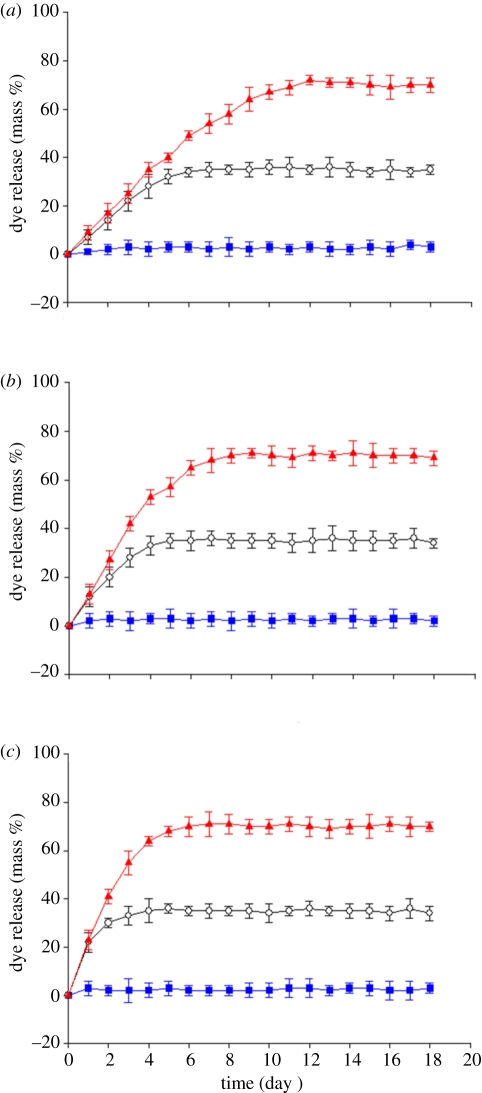
To further quantify the temperature-stimulated release characteristics of the PFH capsules, three sets of capsules, containing different amounts of dye were investigated. Figure 3a–c shows the cumulative dye release of the three systems, which were allowed to absorb dye at 37, 45 and 57°C, in each case for 1500 s. Therefore, the starting dye contents (mass %) of these capsules were 0, 35 and 72, respectively. In all cases, release experiments were performed at 37, 45 and 57°C. The results clearly show that the three systems exhibited sustained release properties but, crucially the dye-release rates from these systems were different. At ambient temperature, the capsules were stable in the medium and therefore possess good storage characteristics.
Figure 3.
Release behaviour of capsules filled with dye at different temperatures: (a) 37°C, (b) 45°C and (c) 57°C, while being subsequently held in the phosphate buffer release medium at 37–57°C. Error bars represent the standard deviation from three experiments. Blue squares, 37°C; black circles, 45°C; red triangles, 57°C.
If the initial incubation temperature was greater than 37°C, dye was released at a faster rate. Thus, at 37°C, the maximum dye content released reaches 72 per cent in 11 days for the capsules incubated at 57°C. This is because 72 per cent dye in the capsules is released by natural diffusion until equilibrium is reached with respect to the dye content in the capsule and medium [33]. When the temperature of the medium was increased, the dye-release rate from the system increased substantially. The released amount reached 72 per cent after 7 days at 45°C, while at 57°C, 72 per cent is achieved after 4 days. This difference in release rates is mainly attributed to the fact that PFH is sensitive to the temperature of the medium. At a higher temperature (45 and 57°C), the PFH in the capsule is active and this can result in enhanced dye release. However, the total amounts released from the three systems are very similar although the release experiments were carried out at different temperatures. Thus, 72 per cent of the dye was released on incubating at 57°C, while at 45°C only 35 per cent of the dye was released. A temperature of 37°C is not enough to evaporate PFH from the core of the capsules.
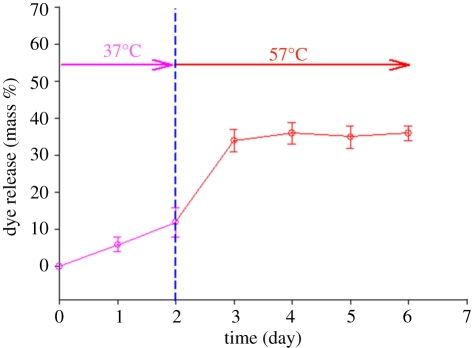
Experiments were also conducted to investigate the effect of changing the temperature of the release medium. The dye was infused into the capsule by heating it at 45°C for 1500 s. Subsequently, the temperature was increased from 37°C to 57°C after 2 days to stimulate the PFH for enhanced release and this is crucial in a number of clinical situations [10]. Herein, the release of the dye from the capsules at different temperatures is illustrated in figure 4. There is a clear and significant enhancement of dye release on increasing the temperature from 37 to 57°C, starting with sustained release at 37°C. At 57°C, the release of the dye is strongly temperature-dependent. The temperature increase triggers the liquid to vapour phase transformation of PFH by inducing thermal instability. Consequently, the PFH in the core of the capsules is activated as demonstrated by a fast release of dye at 57°C (figure 4). However, although the mechanism with respect to real-time stimuli-effect has been demonstrated, the operation temperatures are higher than the normal body temperature, but such a strategy can be useful in medical practice, e.g. tumour treatment and stimuli-responsive capsules [34,35].
Figure 4.
Cumulative release of dye from capsules at 37°C and 57°C. Initial incubation was at 45°C for 1500 s. Error bars represent the standard deviation from three experiments.
4. Conclusions
In this work, we have developed a new system based on temperature-responsive carriers which enables both stimulus-encapsulation and stimulus-release of a drug. The system does not require the use of smart polymers but instead relies on a non-toxic, biocompatible, volatile liquid. The capsule preparation method and encapsulation process is simple and amenable to mass production. The capsules remain stable under storage at normal temperatures in suspension. Hence, we propose that this work offers significant potential benefits for drug-delivery applications and the design of stimuli-responsive systems.
Acknowledgements
The authors would like to thank the EPSRC and the Royal Academy of Engineering for supporting this work through Prof. Edirisinghe's Platform Grant (EP/E045839) and Dr Stride's Research Fellowship, respectively, and also UCL for supporting Ming-Wei Chang through an Overseas Research Scholarship.
References
- 1.Cai K., Luo Z., Hu Y., Chen X., Liao Y., Yang L., Deng L. 2009. Magnetically triggered reversible controlled drug delivery from microfabricated polymeric multireservoir devices. Adv. Mater. 21, 4045–4049 10.1002/adma.200900593 (doi:10.1002/adma.200900593) [DOI] [Google Scholar]
- 2.Ehrbar M., Schoenmakers R., Christen E. H., Fussenegger M., Weber W. 2008. Drug-sensing hydrogels for the inducible release of biopharmaceuticals. Nat. Mater. 7, 800–804 10.1038/nmat2250 (doi:10.1038/nmat2250) [DOI] [PubMed] [Google Scholar]
- 3.Vallet-Regi M., Balas F., Arcos D. 2007. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 46, 7548–7558 10.1002/anie.200604488 (doi:10.1002/anie.200604488) [DOI] [PubMed] [Google Scholar]
- 4.Bajpai A. K., Shukla S. K., Bhanu S., Kankane S. 2008. Responsive polymers in controlled drug delivery. Progr. Polym. Sci. 33, 1088–1118 10.1016/j.progpolymsci.2008.07.005 (doi:10.1016/j.progpolymsci.2008.07.005) [DOI] [Google Scholar]
- 5.Hest J. C. M. V. 2009. Materials science: pulsating vesicles. Nature 461, 45–47 10.1038/461045a (doi:10.1038/461045a) [DOI] [PubMed] [Google Scholar]
- 6.Um S. H., Lee J. B., Park N., Kwon S. Y., Umbach C. C., Luo D. 2006. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater. 5, 797–801 10.1038/nmat1741 (doi:10.1038/nmat1741) [DOI] [PubMed] [Google Scholar]
- 7.Yang J., et al. 2009. Smart drug-loaded polymer gold nanoshells for systemic and localized therapy of human epithelial cancer. Adv. Mater. 21, 4339–4342 10.1002/adma.200900334 (doi:10.1002/adma.200900334) [DOI] [PubMed] [Google Scholar]
- 8.Stuart M. A. C., et al. 2010. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 9, 101–113 10.1038/nmat2614 (doi:10.1038/nmat2614) [DOI] [PubMed] [Google Scholar]
- 9.Ehrick J. D., Deo S. K., Browning T. W., Bachas L. G., Madou M. J., Daunert S. 2005. Genetically engineered protein in hydrogels tailors stimuli-responsive characteristics. Nat. Mater. 4, 298–302 10.1038/nmat1352 (doi:10.1038/nmat1352) [DOI] [PubMed] [Google Scholar]
- 10.Kim H.-J., Matsuda H., Zhou H., Honma I. 2006. Ultrasound-triggered smart drug release from a poly(dimethylsiloxane)–mesoporous silica composite. Adv. Mater. 18, 3083–3088 10.1002/adma.200600387 (doi:10.1002/adma.200600387) [DOI] [Google Scholar]
- 11.Ganta S., Devalapally H., Shahiwala A., Amiji M. 2008. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Controll. Rel. 126, 187–205 10.1016/j.jconrel.2007.12.017 (doi:10.1016/j.jconrel.2007.12.017) [DOI] [PubMed] [Google Scholar]
- 12.Wang Z. J., Qian L., Wang X. L., Yang F., Yang X. R. 2008. Construction of hollow DNA/PLL microcapsule as a dual carrier for controlled delivery of DNA and drug. Colloids Surf. A 326, 29–36 10.1016/j.colsurfa.2008.05.010 (doi:10.1016/j.colsurfa.2008.05.010) [DOI] [Google Scholar]
- 13.Geest B. G. D., Sanders N. N., Sukhorukov G. B., Demeester J., Smedt S. C. D. 2007. Release mechanisms for polyelectrolyte capsules. Chem. Soc. Rev. 36, 636–649 10.1039/b600460c (doi:10.1039/b600460c) [DOI] [PubMed] [Google Scholar]
- 14.O'Donovan P., et al. 2005. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science 309, 1871–1874 10.1126/science.1114233 (doi:10.1126/science.1114233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S., Kim J.-H., Jeon O., Kwon I. C., Park K. 2009. Engineered polymers for advanced drug delivery. Eur. J. Pharm. Biopharm. 71, 420–430 10.1016/j.ejpb.2008.09.021 (doi:10.1016/j.ejpb.2008.09.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putnam D. 2008. Drug delivery: the heart of the matter. Nat. Mater. 7, 836–837 10.1038/nmat2309 (doi:10.1038/nmat2309) [DOI] [PubMed] [Google Scholar]
- 17.Bleyl J. U., Ragaller M., Tscho T. U., Regner M., Kanzow M., Hubler M., Rasche S., Albrecht M. 1999. Vaporized perfluorocarbon improves oxygenation and pulmonary function in an ovine model of acute respiratory distress syndrome. Anesthesiology 91, 461–469 10.1097/00000542-199908000-00021 (doi:10.1097/00000542-199908000-00021) [DOI] [PubMed] [Google Scholar]
- 18.Couture O., Bevan P. D., Cherin E., Cheung K., Burns P. N., Foster F. S. 2006. Investigating perfluorohexane particles with high-frequency ultrasound. Ultrasound Med. Biol. 32, 73–82 10.1016/j.ultrasmedbio.2005.09.010 (doi:10.1016/j.ultrasmedbio.2005.09.010) [DOI] [PubMed] [Google Scholar]
- 19.Spieth P., Knels L., Kasper M., Quelhas A. D., Bärbelwiedemann Lupp A., Hübler M., Neto A. G., Koch T., Abreu M. G. D. 2007. Effects of vaporized perfluorohexane and partial liquid ventilation on regional distribution of alveolar damage in experimental lung injury. Intens. Care Med. 33, 308–314 10.1007/s00134-006-0428-7 (doi:10.1007/s00134-006-0428-7) [DOI] [PubMed] [Google Scholar]
- 20.Chang M.-W., Stride E., Edirisinghe M. 2009. A novel process for drug encapsulation using a liquid to vapour phase change material. Soft Matter 5, 5029–5036 10.1039/b913178g (doi:10.1039/b913178g) [DOI] [Google Scholar]
- 21.Manaa Z., Pellequera Y., Lamprecht A. 2007. Oil-in-oil microencapsulation technique with an external perfluorohexane phase. Int. J. Pharm. 338, 231–237 10.1016/j.ijpharm.2007.02.010 (doi:10.1016/j.ijpharm.2007.02.010) [DOI] [PubMed] [Google Scholar]
- 22.Pancholi K., Stride E., Edirisinghe M. 2009. In vitro method to characterize diffusion of dye from polymeric particles: a model for drug release. Langmuir 25, 10 007–10 013 10.1021/la900694k (doi:10.1021/la900694k) [DOI] [PubMed] [Google Scholar]
- 23.Chang M.-W., Stride E., Edirisinghe M. 2010. Controlling the thickness of hollow polymeric microspheres prepared by electrohydrodynamic atomization. J. R. Soc. Interface 7, S451–S460 10.1098/rsif.2010.0092.focus (doi:10.1098/rsif.2010.0092.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye C., Chen A., Colombo P., Martinez C. 2010. Ceramic microparticles and capsules via microfluidic processing of a preceramic polymer. J. R. Soc. Interface 7, S461–S473 10.1098/rsif.2010.0133.focus (doi:10.1098/rsif.2010.0133.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Q. R., Kim H.-C., Huang E., Mecerreyes D., Hedrick J. L., Volksen W., Frank C. W., Miller R. D. 2003. Miscibility in organic/inorganic hybrid nanocomposites suitable for microelectronic applications: comparison of modulated differential scanning calorimetry and fluorescence spectroscopy macromolecules. Macromolecules 36, 7661–7671 10.1021/ma034034d (doi:10.1021/ma034034d) [DOI] [Google Scholar]
- 26.Seki H., Kajiwara T., Abe Y., Gunji T. 2010. Synthesis and structure of ladder polymethylsilsesquioxanes from sila-functionalized cyclotetrasiloxanes. J. Organometall. Chem. 695, 1363–1369 10.1016/j.jorganchem.2010.02.008 (doi:10.1016/j.jorganchem.2010.02.008) [DOI] [Google Scholar]
- 27.Ro H. W., et al. 2007. The direct patterning of nanoporous interlayer dielectric insulator films by nanoimprint lithography. Adv. Mater. 19, 2919–2924 10.1002/adma.200602872 (doi:10.1002/adma.200602872) [DOI] [Google Scholar]
- 28.Narisawa M., Kado H., Mabuchi H., Kim W. 2010. Accelerated ceramization of polymethylsilsesquioxane by aluminum-based filler reductant. Appl. Organometall. Chem. 24, 612–617 10.1002/aoc.1554 (doi:10.1002/aoc.1554) [DOI] [Google Scholar]
- 29.Im S. H., Jeong U., Xia Y. 2005. Polymer hollow particles with controllable holes in their surfaces. Nat. Mater. 4, 671–675 10.1038/nmat1448 (doi:10.1038/nmat1448) [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y., Shi J., Shen W., Dong X., Feng J., Ruan M., Li Y. 2005. Stimuli-responsive controlled drug release from a hollow mesoporous silica sphere/polyelectrolyte multilayer core-shell structure. Angew. Chem. Int. Ed. 44, 5083–5087 10.1002/anie.200501500 (doi:10.1002/anie.200501500) [DOI] [PubMed] [Google Scholar]
- 31.Shchukin D. G., Sukhorukov G. B., Möhwald H. 2003. Smart inorganic/organic nanocomposite hollow microcapsules. Angew. Chem. Int. Ed. 115, 4610–4613 10.1002/ange.200352068 (doi:10.1002/ange.200352068) [DOI] [PubMed] [Google Scholar]
- 32.Still T., Sainidou R., Retsch M., Jonas U., Spahn P., Hellmann G. P., Fytas G. 2008. The ‘music’ of core–shell spheres and hollow capsules: influence of the architecture on the mechanical properties at the nanoscale. Nano Lett. 8, 3194–3199 10.1021/nl801500n (doi:10.1021/nl801500n) [DOI] [PubMed] [Google Scholar]
- 33.Zhao W., Chen H., Li Y., Li L., Lang M., Shi J. 2008. Uniform rattle-type hollow magnetic mesoporous spheres as drug delivery carriers and their sustained-release property. Adv. Funct. Mater. 18, 2780–2788 10.1002/adfm.200701317 (doi:10.1002/adfm.200701317) [DOI] [Google Scholar]
- 34.Berger S., Zhang H., Pich A. 2009. Microgel-based stimuli-responsive capsules. Adv. Funct. Mater. 19, 554–559 10.1002/adfm.200801203 (doi:10.1002/adfm.200801203) [DOI] [Google Scholar]
- 35.Kennedy J. E., Harr G. R., Cranston D. 2003. High intensity focused ultrasound: surgery of the future? Br. J. Radiol. 76, 590–599 10.1259/bjr/17150274 (doi:10.1259/bjr/17150274) [DOI] [PubMed] [Google Scholar]