Abstract
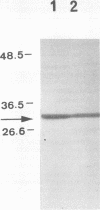
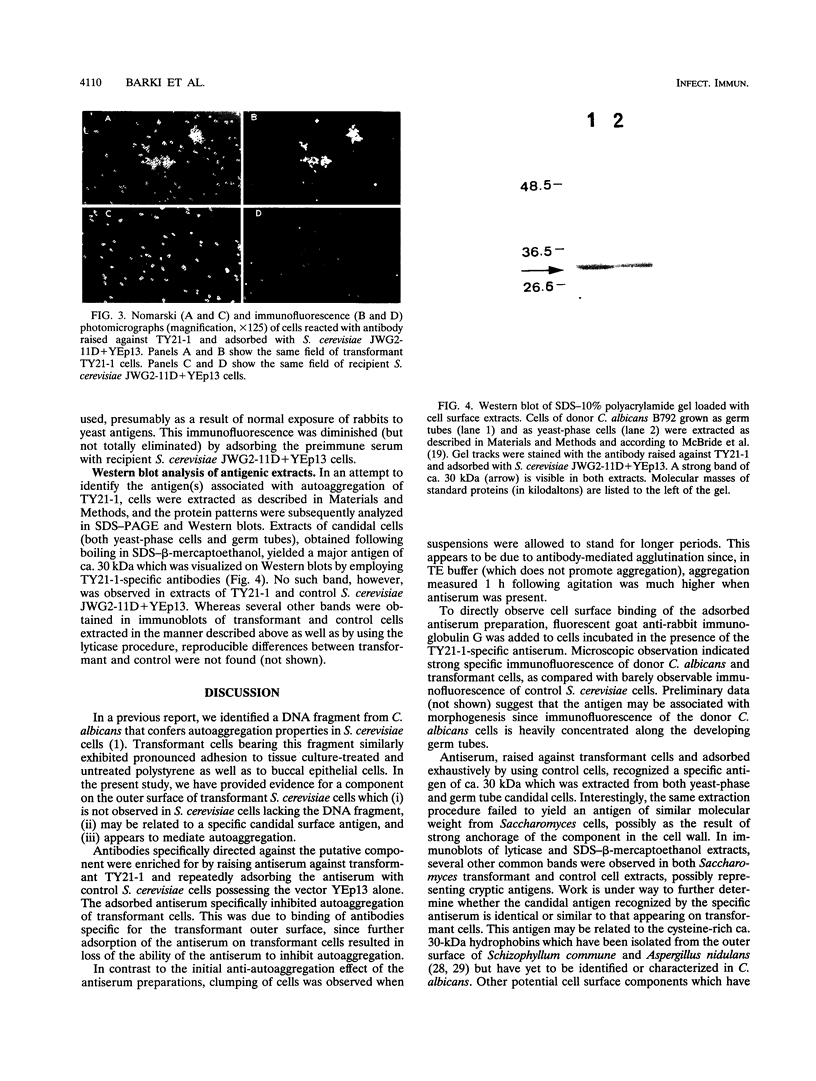
In a previous study (M. Barki, Y. Koltin, M. Yanko, A. Tamarkin, and M. Rosenberg, J. Bacteriol. 175:5683-5689, 1993), a 3.3-kb DNA fragment from Candida albicans which confers adhesion and autoaggregation in Saccharomyces cerevisiae was isolated and partially characterized. In this report, evidence is presented that the adhesion-autoaggregation phenotype observed in S. cerevisiae cells transformed with the candidal DNA fragment is due to expression of a C. albicans surface antigen. Rabbit antiserum, prepared against transformant S. cerevisiae cells, was adsorbed with S. cerevisiae bearing the vector alone. Immunofluorescence micrography showed that the adsorbed antiserum bound to the surface of transformant S. cerevisiae cells as well as to C.albicans cells, but only marginally to the S. cerevisiae control. The absorbed antiserum specifically inhibited autoaggregation of transformant cells. Further adsorption of the antiserum with transformant cells eliminated both inhibition and immunofluorescence. Autoaggregative activity and immunofluorescence of transformant cells were abolished following proteolytic treatment. Western blot (immunoblot) analysis of candidal extracts revealed that the absorbed antiserum recognized a major candidal antigen of ca. 30 kDa which was present on both yeast-phase and germ tube cells. The data suggest that the observed adhesion-autoaggregation phenotype is due to the presence of a specific candidal antigen on the outer surface of the transformant cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barki M., Koltin Y., Yanko M., Tamarkin A., Rosenberg M. Isolation of a Candida albicans DNA sequence conferring adhesion and aggregation on Saccharomyces cerevisiae. J Bacteriol. 1993 Sep;175(17):5683–5689. doi: 10.1128/jb.175.17.5683-5689.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchara J. P., Tronchin G., Annaix V., Robert R., Senet J. M. Laminin receptors on Candida albicans germ tubes. Infect Immun. 1990 Jan;58(1):48–54. doi: 10.1128/iai.58.1.48-54.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Calderone R. A., Braun P. C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991 Mar;55(1):1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R. A., Wadsworth E. Characterization of mannoproteins from a virulent Candida albicans strain and its derived, avirulent strain. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S423–S427. doi: 10.1093/cid/10.supplement_2.s423. [DOI] [PubMed] [Google Scholar]
- Chaffin W. L., Collins B., Marx J. N., Cole G. T., Morrow K. J., Jr Characterization of mutant strains of Candida albicans deficient in expression of a surface determinant. Infect Immun. 1993 Aug;61(8):3449–3458. doi: 10.1128/iai.61.8.3449-3458.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moreno M. R., Smith J. F., Smith R. V. Silver staining of proteins in polyacrylamide gels: increased sensitivity through a combined Coomassie blue-silver stain procedure. Anal Biochem. 1985 Dec;151(2):466–470. doi: 10.1016/0003-2697(85)90206-4. [DOI] [PubMed] [Google Scholar]
- Hazen K. C., Brawner D. L., Riesselman M. H., Jutila M. A., Cutler J. E. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect Immun. 1991 Mar;59(3):907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C., Hazen B. W. Hydrophobic surface protein masking by the opportunistic fungal pathogen Candida albicans. Infect Immun. 1992 Apr;60(4):1499–1508. doi: 10.1128/iai.60.4.1499-1508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C., Lay J. G., Hazen B. W., Fu R. C., Murthy S. Partial biochemical characterization of cell surface hydrophobicity and hydrophilicity of Candida albicans. Infect Immun. 1990 Nov;58(11):3469–3476. doi: 10.1128/iai.58.11.3469-3476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C. Participation of yeast cell surface hydrophobicity in adherence of Candida albicans to human epithelial cells. Infect Immun. 1989 Jul;57(7):1894–1900. doi: 10.1128/iai.57.7.1894-1900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. J., Calderone R. A., Cutler J. E., Kanabe T., Riesselman M. H., Robert R., Senet J. M., Annaix V., Bouali A., Mahaza C. Molecular basis of Candida albicans adhesion. J Med Vet Mycol. 1992;30 (Suppl 1):95–122. [PubMed] [Google Scholar]
- Klotz S. A., Smith R. L. A fibronectin receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J Infect Dis. 1991 Mar;163(3):604–610. doi: 10.1093/infdis/163.3.604. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopez-Ribot J. L., Casanova M., Martinez J. P., Sentandreu R. Characterization of cell wall proteins of yeast and hydrophobic mycelial cells of Candida albicans. Infect Immun. 1991 Jul;59(7):2324–2332. doi: 10.1128/iai.59.7.2324-2332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Shibata N., Podzorski R. P., Herron M. J. Candida mannan: chemistry, suppression of cell-mediated immunity, and possible mechanisms of action. Clin Microbiol Rev. 1991 Jan;4(1):1–19. doi: 10.1128/cmr.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollert M. W., Wadsworth E., Calderone R. A. Reduced expression of the functionally active complement receptor for iC3b but not for C3d on an avirulent mutant of Candida albicans. Infect Immun. 1990 Apr;58(4):909–913. doi: 10.1128/iai.58.4.909-913.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotrosen D., Calderone R. A., Edwards J. E., Jr Adherence of Candida species to host tissues and plastic surfaces. Rev Infect Dis. 1986 Jan-Feb;8(1):73–85. doi: 10.1093/clinids/8.1.73. [DOI] [PubMed] [Google Scholar]
- Smail E. H., Jones J. M. Demonstration and solubilization of antigens expressed primarily on the surfaces of Candida albicans germ tubes. Infect Immun. 1984 Jul;45(1):74–81. doi: 10.1128/iai.45.1.74-81.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Robert R. Dynamic changes of the cell wall surface of Candida albicans associated with germination and adherence. Eur J Cell Biol. 1989 Dec;50(2):285–290. [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Robert R., Senet J. M. Adherence of Candida albicans germ tubes to plastic: ultrastructural and molecular studies of fibrillar adhesins. Infect Immun. 1988 Aug;56(8):1987–1993. doi: 10.1128/iai.56.8.1987-1993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels J. G., de Vries O. M., Asgeirsdóttir S. A., Springer J. The thn mutation of Schizophyllum commune, which suppresses formation of aerial hyphae, affects expression of the Sc3 hydrophobin gene. J Gen Microbiol. 1991 Oct;137(10):2439–2445. doi: 10.1099/00221287-137-10-2439. [DOI] [PubMed] [Google Scholar]
- Wessels JGH., De Vries OMH., Asgeirsdottir S. A., Schuren FHJ. Hydrophobin Genes Involved in Formation of Aerial Hyphae and Fruit Bodies in Schizophyllum. Plant Cell. 1991 Aug;3(8):793–799. doi: 10.1105/tpc.3.8.793. [DOI] [PMC free article] [PubMed] [Google Scholar]