Abstract
We review evidence showing a right-hemispheric dominance for visuo-spatial processing and representation in humans. Accordingly, visual disorganization symptoms (intuitively related to remapping impairments) are observed in both neglect and constructional apraxia. More specifically, we review findings from the intervening saccade paradigm in humans—and present additional original data—which suggest a specific role of the asymmetrical network at the temporo-parietal junction (TPJ) in the right hemisphere in visual remapping: following damage to the right dorsal posterior parietal cortex (PPC) as well as part of the corpus callosum connecting the PPC to the frontal lobes, patient OK in a double-step saccadic task exhibited an impairment when the second saccade had to be directed rightward. This singular and lateralized deficit cannot result solely from the patient's cortical lesion and, therefore, we propose that it is due to his callosal lesion that may specifically interrupt the interhemispheric transfer of information necessary to execute accurate rightward saccades towards a remapped target location. This suggests a specialized right-hemispheric network for visuo-spatial remapping that subsequently transfers target location information to downstream planning regions, which are symmetrically organized.
Keywords: visual remapping, optic ataxia, hemineglect, constructional apraxia, posterior parietal cortex, right-hemispheric dominance
1. Introduction
The representation of the visual fields in the early areas of the occipital cortex is retinotopic, i.e. centred on the instantaneous eye position. After each eye movement, this instantaneous representation is overwritten by a new one centred on the new eye position. In addition, retinal information is sampled at high resolution by the fovea, which represents only a few degrees of visual angle. A complete and coherent representation of the visual scene thus requires numerous exploratory saccades as well as the integration of these different points of view over time and space. Such integration requires dynamic spatial maps in which the neuronal visual activity is maintained and updated in spatial coherence with each new eye position (remapping processes). Accordingly, monkey electrophysiology has described dynamic oculocentric representations in which the neuronal response can outlast the duration of a visual stimulus of interest within the retinotopic receptive field, and this ‘memory’ activity can be transferred to another neuron in order to recode the location of the (extinguished) stimulus with respect to the new ocular position. Such neuronal activity has been described in oculomotor centres such as the superior colliculus [1], the frontal eye fields [2,3] and the lateral intraparietal (LIP) area. The area LIP contains neurons whose activity even begins in anticipation of a saccade that will bring the location of the extinguished visual stimulus into their receptive fields (review in [4,5]; figure 1). Dynamic oculocentric representations in other cortical regions (occipital or frontal cortex) might, therefore, depend on the remapped information sent from LIP [6].
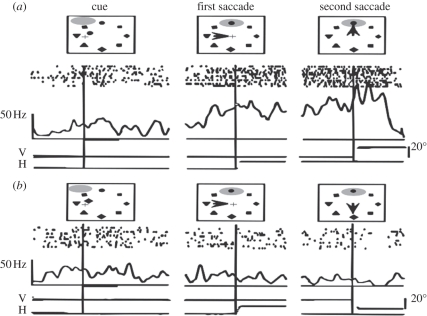
Figure 1.
Response of a monkey parietal neuron during a double-step task instructed by a cue in a condition where the stimulus entering the neuron's receptive field is relevant to the task (condition a) and in a condition where it is irrelevant to the task (condition b). The cue (left panel) indicates the target to fixate, after having fixated at the array centre. The triangle (left panel) indicates the initial position of the eyes, and the receptive field of the neuron is represented by a shaded ellipse and moves with the eyes. In both conditions (a) and (b), the receptive field of the neuron matches, after the first saccade, the location of the permanent stimulus presented at the top of a circular array of eight stimuli (centre panel). When the second saccade has to be guided to this top position (condition a), the firing of the neuron precedes and remains elevated after the second saccade. However, when the second saccade has to be guided to another position (condition b), the neuron remains silent despite the presence of the same stimulus in its receptive field. (Adapted from [5]).
Experimentally, remapping processes have been studied using the ‘double-step saccade paradigm’ in monkeys [1,2,4,7–13]. The double-step paradigm has also been tested in humans through lesion studies to test the role of specific brain areas in remapping [14–16]. In the general task, two targets are successively presented in the visual periphery. The subject is asked to foveate the targets through two successive saccades. ‘ON trials’, in which the two visual targets remain on the screen until the end of the saccadic sequence, are run as a control condition in order to assess the general ability of the subject to plan and execute the two requested saccades based on peripheral visual information. In ‘OFF trials’, both targets are extinguished before the first saccade is completed (figure 2a); the second saccade does not correspond to the initially sampled retinal vector but has to rely on the memorized location of target 2, updated with respect to the new eye position after the first saccade (to target 1). A deficit in trials in which target 2 is ‘OFF’, therefore, reveals a specific impairment for the memory-based remapping processes owing to the lesion of the patient being studied. For example, the first neuropsychological observation reported in the literature was a single-case study of a neglect patient [14]. This patient, with right fronto-parietal lesions, was able to generate sequences of leftward followed by rightward saccades (L-R) as well as opposite sequences (R-L), when the targets remained visible in ON trials. However, in OFF trials, his rightward second saccades, preceded by a leftward saccade, were aborted (figure 2b). In summary, there was a deficit for a second saccade in the direction opposite to the one predicted simply from an attentional deficit for contralesional stimuli. This inability to produce rightward second saccades in remapping conditions was observed irrespective of whether target 2 was initially presented in the right or the left visual field: between-hemifeld and within-hemifield conditions of L-R trials were both impaired in the patient. Duhamel et al. [14] concluded that this neglect patient was impaired in registering extraretinal information about the motor vector of a leftward (contralesional) saccade and using it to update the spatial representation of the next target. Later, Heide et al. [16] tested a group of patients with lesions to the prefrontal cortex (PFC) as well as a group with lesions to the posterior parietal cortex (PPC). Patients with lesions to the PFC were impaired in both OFF and ON conditions (labelled with and without retino-spatial dissonance, respectively) and were therefore not considered as having a specific remapping impairment. Only the patients with PPC lesions exhibited a specific deficit only in OFF trials, demonstrating an impairment of remapping. In this group of patients, the authors observed not only aborted but also dysmetric second saccades, erroneously performed according to the retinal vector of the second target [16].
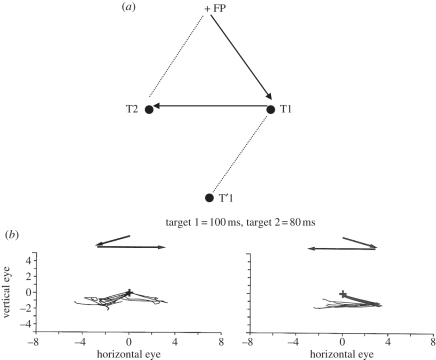
Figure 2.
(a) The two targets (T1 and T2) are flashed successively while the gaze is at a central fixation point (FP). Two successive saccades toward T1 and T2 are instructed and performed after T2 has disappeared. The motor vector of the second saccade (T1 → T2) is different from the retinal vector of the second target (FP → T2 or T1 → T′1). However, in this condition, the saccade towards position T2 is achieved correctly both in humans and in animals. This observation thus suggests the existence of remapping mechanisms allowing the oculomotor system to anticipate the new retinal position of T2 by integrating the displacement on the retina produced by the first saccade towards position T1. (Redrawn from [18].) (b) Schematic of the aborted rightward second saccades after first leftward saccades in a patient with right fronto-parietal lesions, whereas trials of double-step saccadic sequences with rightward first saccade and leftward second saccade were performed correctly (adapted from [14]).
Note that Heide et al. [16] tested the same between-hemifield and within-hemifield L-R and R-L saccadic conditions as Duhamel et al. [14]. However, they labelled ‘L-R’ and ‘R-L’ the conditions in which the two targets were initially presented in opposite visual fields (between-hemifield conditions), ‘L-L’ the within-hemifield L-R saccadic conditions and ‘R-R’ the within-hemifield R-L saccadic conditions. This labelling thus corresponds to the targets' initial spatial locations (right and/or left visual field) before the execution of the first saccade instead of saccadic directions (but is also different to figure 4b because Heide et al. [14] tested only centripetal within-hemifield saccadic conditions). In the study by Heide et al. [16], patients with right PPC lesions were impaired in ‘L-R’ and ‘L-L', conditions, confirming with a group study the single-case deficit reported by Duhamel et al. [14] in updating the entire visual space after leftward saccades. However, their patients with right PPC lesions also demonstrated a significant increase in errors in the ‘R-L’ condition in the OFF trials compared with the ON trials. Patients with left PPC lesions were impaired only in the between-hemifield R-L condition but not in the within-hemifield R-L condition. In summary, patients with left PPC lesions exhibit an impairment in remapping contralesional saccades only in the between-hemifield condition, while patients with right PPC lesions are only able to remap in the ‘R-R’ within-hemifield condition [17]. Heide & Kömpf [18] mentioned that in the between-hemifield conditions, in which the first target appeared in the ipsilesional and the second target was briefly flashed in the contralesional visual field, the percentage of aborted second saccades correlated with the score of neglect (or extinction) in both the parietal and frontal patients, reflecting more an attentional than a remapping deficit. If one does not consider the between-hemifield conditions, one could simply sum up these results by proposing that patients with left PPC lesions exhibit no remapping impairments, whereas patients with right PPC lesions are impaired in updating contralesional saccades. In any case, the results of Heide et al. [16] reflect an obvious asymmetry between left and right PPC lesions in remapping impairments, which matches the clinical consequences of lesions of the temporo-parietal junction (TPJ) and is probably due to an asymmetry in visual space representation in humans [17]. Using a paradigm of inhibition of return across saccades, van Koningsbruggen et al. [19] have recently demonstrated this asymmetry: whereas transcranial magnetic stimulation (TMS) on the right PPC impaired visual remapping (as already shown by Morris et al. [20] using a double-step saccadic task), TMS on the left PPC did not. Furthermore, Prime et al. [21] have also shown that only TMS on the right PPC, and not on the left PPC, disrupts spatial working memory not only in static conditions but even more so across saccades. Nevertheless, the asymmetry in remapping impairments between left and right PPC lesions was not highlighted in the original paper of Heide et al. [16]. Instead, they drew a general conclusion in line with Duhamel et al. [14], that patients with PPC lesions are impaired in remapping contralesional saccades.
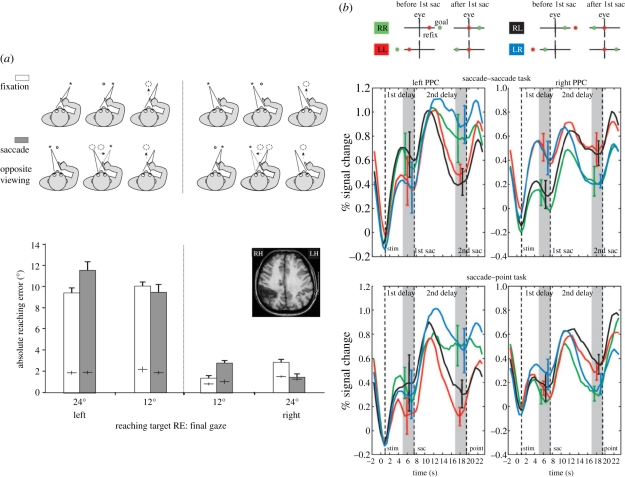
Figure 4.
(a) Patient OK with optic ataxia typically misreaches targets in his contralesional visual field (fixation condition). The pointing errors depend on target location relative to final gaze position, and are much larger for leftward locations. The same pattern of pointing errors is observed when the target is initially presented in one visual field, turned off and then crossed by a saccade bringing its location within the opposite visual field (saccade condition). In particular, when the target is presented in the patient's contralesional visual field, turned off and then crossed by a saccade bringing its location within the ipsilesional visual field, his pointing become accurate. This demonstrates that visual remapping has correctly transferred the target location into the healthy hemisphere, before the damaged locus for visuo-manual transformation (Adapted from [46]). (b) Left and right parietal BOLD activation (mean and s.e. across six subjects) are plotted with time for RR, LL, RL and LR conditions (here, the first letter signifies initial location of the first target (R, right hemifield; L, left hemifield), and the second letter refers to the remapped location of the remembered second target location. Note that this labelling is different from the one used by Heide et al. [16] (see text for details). The LL and RR conditions, which do not involve interhemispheric transfer of brain activity at the parietal level, are novel conditions that had not been tested in patients with cortical lesions before the present report of patient OK). Dashed lines indicate the presentation of stimuli, the time of the first saccade and the time of the second saccade, respectively. Grey areas indicate the periods over which the differences between the LR and RL condition were taken. Top panel: the intervening saccade is followed by a second saccade towards the remapped target location. Bottom panel: the intervening saccade is followed by a pointing movement towards the remapped target location. In both conditions, the remembered location of the goal target is transferred across cerebral hemispheres within the human PPC after an intervening saccade. (Adapted from [49]).
In a following chapter, however, Heide & Kömpf wrote ‘our data confirm the key role of the PPC in the analysis of visual space with a dominance of the right hemisphere’ ([18], p. 166). In this same chapter, they also provided new information on the lesions and symptoms of their patient groups: the focus of the PPC lesions was located ‘in the inferior parietal lobule along the border between the angular and supramarginal gyrus, extending cranially towards the intraparietal sulcus (IPS), caudally to the TPJ, and posteriorly into the angular gyrus’ ([18], p. 158). Compatible with this lesion site, patients in the right PPC lesion group initially presented with unilateral spatial neglect syndrome. Furthermore, Heide & Kömpf [18] mentioned that their deficit in the double-step saccade task correlated with the patients' impairments in copying Rey's complex figure, but not with other tests that measure severity of the left hemineglect. Accordingly, Pisella & Mattingley [17] have suggested that an impairment of remapping processes may contribute to a series of specific symptoms pertaining to unilateral visual neglect syndrome which cannot be explained by the attentional bias hypothesis alone (e.g. revisiting, spatial transpositions, loss of consciousness and disorganization across the entire visual field, figure 3a). Pisella et al. [23] have shown that patients with parietal neglect demonstrate spatial working memory impairments within the entire visual space that is additional to the left–right attentional gradient (figure 3b). Note that these two components of parietal neglect can be attributed to damage of the ventral and dorsal PPC networks, respectively. These two networks have been distinguished by cerebral activity [24] elicited during a Posner task [25]: dorsal PPC activity increased during attentional orienting towards contralateral visual space whereas activity in the right ventral PPC was specific to the detection of unexpected stimulus appearance within the entire visual field (all invalid trials). The ventral network (including the TPJ in the right hemisphere) has been shown to be involved in ‘non-spatially lateralized processes’ such as sustaining and reorienting attention to spatial locations across the entire visual field, midline judgements and spatial working memory [24,26–30]. In contrast, the functionally symmetrical dorsal network (including the superior parietal lobule (SPL) and the IPS) is involved in attentional shifts and visuo-motor transformations. Damage to this network potentially induces contralesional optic ataxia (OA) and contralesional visual extinction [31,32]. Corbetta et al. [33] have provided evidence that a lesion restricted to the right TPJ causes clinical neglect because of (i) the direct damage to the ventral network and (ii) the resulting indirect imbalance of neuronal activity in the dorsal network, with increased activity in the left hemisphere resulting in lateralized bias of attention towards the right visual space. Accordingly, unilateral spatial neglect syndrome is known to occur following lesions of the right TPJ [34–36], and isolated symptoms of visual disorganization, known as constructional apraxia, are observed after recovery of the ipsilesional bias of attention characterizing neglect [37].
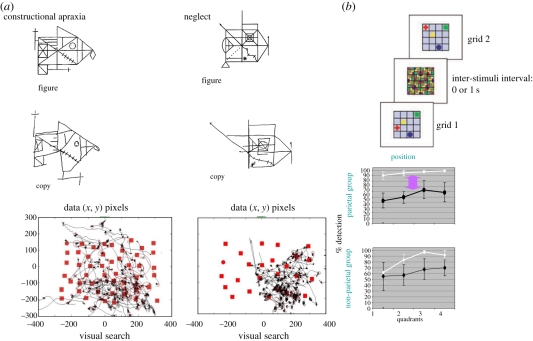
Figure 3.
(a) Spatial transpositions on the copying of a Rey figure following right posterior parietal lesions. In his copy, the patient with neglect (right panel) not only omits most elements in the left side of the figure but also inappropriately adds to the right side some elements on the left side (from [22]). The patient with constructional apraxia without neglect (left panel) copies almost all the figure components but exhibits errors in their relative locations (from [18]). Other patients with constructional apraxia and neglect (unpublished) had to search for the target (circle), which normally easily ‘pops up’ among the distracters (squares) The lines in the graphs represent the continuous eye position recorded until the patient provided his response (target present or absent). As shown by the ocular tracking, both patients with constructional apraxia and neglect showed much revisiting behaviour during their visual search, with lack of exploration of the left part of the visual scene exhibited in addition in the neglect patient. (b) In addition to the attentional left–right gradient, deficient spatial working memory for the whole visual space is demonstrated by the difference between the change detection conditions with (1 s of inter-stimuli interval: black lines) and without (white lines) delay, in parietal neglect only. Note that the location change always occurred in one object only, within a vertical quadrant (=column of the grid), as illustrated. (Adapted from [23]).
A further problem is the functional consequence of visual remapping impairments. Spatial remapping has been classically considered to be the mechanism underlying perceptual stability of the visual world during and across eye movements [38]. However, Bays & Husain [39] have proposed that spatial remapping is not involved in visual stability but rather in visuo-motor processes such as adaptation, motor control and spatial working memory. In the literature, an impairment of visual remapping is often used to explain perceptual disturbances such as blurred vision and dizziness, and more specifically spatial mislocalizations across saccades [18,40]. Remapping impairments following PPC lesions have been postulated to contribute to impaired visual perception, consciousness and to several symptoms of visual disorganization pertaining to Balint's syndrome [41], e.g. visual transposition errors, impaired copies of drawing, revisiting behaviour in counting dots and difficulty in executing a coherent and efficient exploration of visual scenes (figure 3a). In particular, an impairment of visual remapping has been postulated to increase the handicap of neglect patients consecutive to right inferior parietal damage [17], being responsible, for example, for the disorganized copying of Rey's figure [16], revisiting behaviour in visual search [42] and deficient spatial working memory ([23]; figure 3b). More recently, deficient visual remapping has been demonstrated in constructional apraxia [37], an isolated deficit of visual disorganization observed in the entire visual field without a lateralized bias of attention (figure 3a). Symptoms of disorganized copying and revisiting without a lateralized bias of attention or a lack of visual synthesis during ocular exploration of visual scenes are also observed in addition to simultanagnosia in patients with posterior cortical atrophy causing extensive bilateral damage of the PPC [43]. In contrast, they are not observed in patients whose focal lesions, restricted to the dorsal PPC and IPS, cause chronic OA and attentional deficits [44], which were initially exhibited clinically as visual extinction (in the case of unilateral damage) or simultanagnosia (in the case of bilateral damage). Moreover, these patients presenting with OA without neglect, resulting from dorsal PPC lesions (i.e. lesions of the SPL and the IPS), have shown preserved visual remapping in the context of a trans-saccadic pointing task ([45,46]; figure 4a). As a consequence, the dorsal PPC region, damaged in OA patients and involved in visuo-manual transformations, cannot be considered to be a region crucially involved in visual remapping. Interestingly, visual remapping was less evident in a patient (AT) with more extensive bilateral parietal damage extending to the dorsal occipital areas and to the TPJ [45]. Preserved visual remapping in patients with OA may therefore result from the remapping activity found in occipital areas [6,47] or in the TPJ, which may involve a specialized representation of the entire visual field within the right hemisphere [17].
The critical neural substrates and pathways for oculocentric remapping processes are still debated and largely unknown, especially in humans. The existence of dynamic spatial representations in humans, such as those seen in monkey LIP area, has been demonstrated, for example, by showing interhemispheric transfer of memorized visual information across the saccade in the context of visual perception [47,48] or goal-directed action [46,49] (figure 4a,b). In these studies, a visual stimulus of interest presented in periphery is initially represented in the contralateral hemisphere. Then, the visual stimulus extinguishes and the subject is requested to produce a saccade that crosses the stimulus location and brings its location into the opposite visual space (across-hemifield visual remapping conditions). Behaviour [46] and brain activity [47–49] have demonstrated that, consequent to the saccade, the representation of the (extinguished) visual stimulus was transferred to the ipsilateral hemisphere. Neuroimaging studies have shown this interhemispheric transfer between homologous cortical regions, at the level of the posterior parietal cortices (PPC; [48,49]; figure 2a) or the occipital striate and extrastriate cortices [47]. This has suggested a necessary role of the corpus callosum for across-hemifield visual remapping (e.g. in RL and LR saccade–saccade and saccade–pointing conditions but not in LL and RR in figure 4b). This recoding of target locations with respect to the new eye position can occur between visuo-spatial maps (to update spatial working memory) and/or between visuo-motor maps (to update saccadic or pointing plans) and/or from a representational map to visuo-motor centres in the opposite hemisphere (this may be necessary if the next target is located in the opposite visual space after the saccade or has to be reached by the hand represented in the opposite hemisphere). Interhemispheric transfer demonstrated by neuroimaging studies at the level of occipital areas may reflect exchanges necessary for visuo-spatial coding [30,38]. At the level of the PPC, interhemispheric transfer may rather reflect the visuo-motor updating of the saccadic or pointing goals, i.e. the visuo-motor preparation of a movement in the opposite direction when the saccade brings the next visual goal location into the opposite oculocentric space [45,46,49] (figure 4a,b). However, this hypothesis is more uncertain since the conflict between ‘attentional’ (visuo-spatial) and ‘intentional’ (visuo-motor) views of the PPC remains unresolved ([50] and [51], respectively). As reviewed above, the consequences of PPC lesions in humans suggest that, within the PPC, symmetrical visuo-motor maps and asymmetrical (right-hemispheric dominant) visuo-spatial maps coexist.
In humans, the PPC as the main cortical region for visual remapping has been investigated without a distinction between the ventral and dorsal parts of the PPC and, to our knowledge, no study has yet investigated the importance of the preservation of the corpus callosum. Split-brain monkeys exhibit only moderate (increased variability) and temporary deficits in the across-hemifield visual remapping conditions [9,10,13]. This moderate effect of disconnecting the cerebral hemispheres can be explained by a redundancy of neural circuits [52], allowing any given monkey LIP neuron to access information from throughout the visual field such that it can respond to visual stimuli presented anywhere [12]. Specifically, any given LIP neuron may be interconnected with other neurons of the same and the opposite hemisphere via the subcortical pathway demonstrated by Wurtz et al. [42] the superior colliculus sends information about the saccade just being executed (efferent copy) to the frontal eye fields and—from there—to the PPC in order to update the cortical oculocentric representations of space. This subcortical pathway goes through the central thalamus whose lesion has been shown to affect saccade-associated efference copy signals [53]. It is therefore probable that an interhemispheric transfer occurs between cortical symmetrical representations of contralateral space via a subcortical (bilateral) pathway. In humans, it is also possible that an interhemispheric transfer occurs from a cortical or subcortical representation of the right visual space to a right-hemispheric cortical representation of the entire space for visual remapping (unilateral subcortical or callosal transfer). If remapping occurs in such a right-hemispheric representational map that is not directly related to motor programming, then a secondary interhemispheric transfer would be necessary in order to plan rightward saccades thereafter.
Patient OK presents with dorsal PPC lesions in the right hemisphere leading to OA without clinical neglect (no line bisection bias nor omissions in line cancellation) nor any spatial disorganization symptoms. Postulating a dependency of remapping processes on the right TPJ and a functional link between remapping impairments and spatial disorganization symptoms, we predicted that this patient would not be severely impaired in the double-step saccadic task. In addition, OK has focal damage of the corpus callosum (whose effects have not been observed in previous studies [46]). Investigating visual remapping in this particularly rare patient could provide information with respect to the crucial region and neural pathway for remapping, and to the neural pathways involved in preparing different oculomotor responses towards remapped locations.
2. Patient and methods
This study was conducted with the informed consent of the patient, in agreement with the French Law (4 March 2002) and the Helsinki declaration with respect to patients' rights. At the time of testing, patient OK was a 38-year-old male who had suffered from ischaemic damage to the right dorsal PPC network [24]. The lesion included the SPL, the IPS and a slight extension to the dorsal and anterior part of the inferior parietal lobule (corresponding to Brodmann areas 5, 7 and to the upper and anterior part of area 40). MRI scans also revealed focal damage to the part of the corpus callosum connecting the PPC to the frontal lobes (figure 5). Clinically, he mainly presented with the visuo-manual symptoms of unilateral (left) OA [45] and subclinical deficit of covert attentional shift towards the contralesional hemifield, as classically exhibited by OA patients [41,44]. Consequently, an impairment of covert attentional orienting to the left was revealed in an experimental condition of letter discrimination in peripheral vision among flankers [55], despite no visual extinction or neglect during clinical testing (line bisection was slightly biased towards the left like normal subjects and patient OK starts line cancellation on the left and exhibits no omission in this visual search task). Five healthy subjects (two males and three females, age range: 21–42, mean age: 31) were also tested as a control group.
Figure 5.
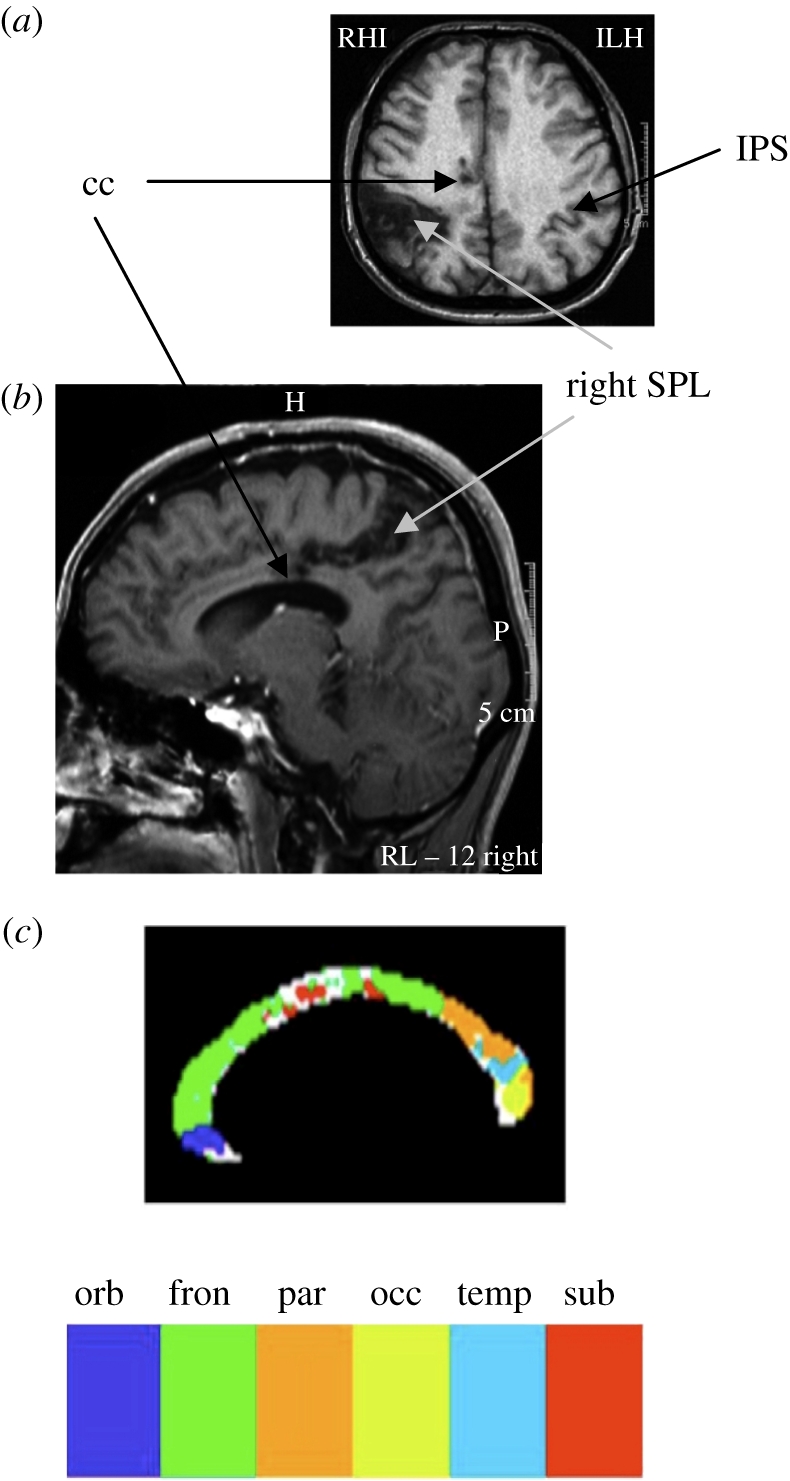
(a) Horizontal and (b) sagittal slices of the MRI scan of patient OK revealed focal damage to the corpus callosum (black arrow, cc) and to the right dorsal posterior parietal cortex (grey arrow, right SPL). (c) Sub-regions of the corpus callosum based on fibre tracking [54].
A detailed description of the experimental set-up and eye movement monitoring and analyses can be found in Alahyane et al. [56]. Subjects were seated in a dimly lit room in front of a concave spherical board containing red light-emitting diodes (LEDs; diameter 3 mm) used as visual targets. The centre of the board was aligned with the subjects' naso-occipital axis at 1.10 m (sphere radius) from the cyclopean eye. The head was stabilized by means of a chin rest.
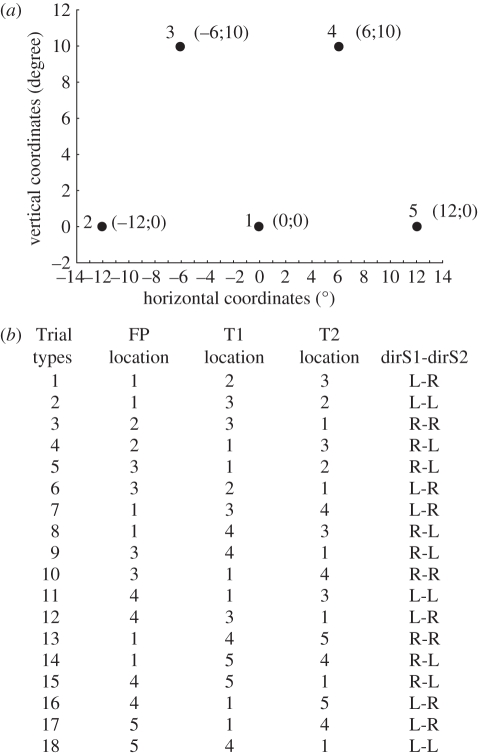
At trial onset, a fixation LED turned on (fixation point: FP); after a 1200 ms delay, FP turned off and simultaneously another LED turned on (target 1, presented for 140 ms), followed by a third LED (target 2). The subjects were instructed to fixate the FP and then to successively make a saccade towards the locations of the two peripheral LEDs (target 1 and target 2) following their presentation order. The matrix of five LEDs used in this study is presented in figure 6a. Trials were made up of the 18 possible combinations of three LEDs forming the edges of three triangles (figure 6a), with all oblique and horizontal edges subtending 12° of visual angle. Among the 18 possible combinations, six instructed a leftward saccade followed by a rightward saccade (L-R), six instructed a rightward saccade followed by a leftward saccade (R-L), three instructed two leftward sequential saccades (L-L) and three instructed two rightward sequential saccades (R-R). In addition, the paradigm included two types of trials. In ‘ON’ trials, target 2 remained lit until the end of the trial, allowing both the first and second saccades to be planned based on visual information (retinal vector). In OFF trials, target 2 was presented only for 80 ms and thus was extinguished before the first saccade reached the location of target 1. In these OFF trials, the planning of the second saccade thus necessarily relied on a remapping of the memorized visual information about the location of target 2. The entire experiment consisted of 216 trials among which the 18 possible combinations (figure 6b) were randomly presented and repeated four times as ON trials and eight times as OFF trials. This experiment was run after a training session that included ON trials only.
Figure 6.
(a) The five target locations used for the experiment. (b) The table represents the 18 different trial conditions corresponding to combinations of a fixation point (FP), a first target (T1) and a second target (T2) at adjacent locations so that each trial required a sequence of two saccades of 12° amplitude. As an example, the schematic of figure 2a corresponds to trial 5 or 15.
Horizontal and vertical eye movements were recorded by videooculography using an EyeLink I system (SMI, Germany), at a frequency of 250 Hz with an accuracy of 0.1°. A Data Wave computer program (Berthoud, USA) controlled the randomized presentation of the LEDs, sampled eye position data (sampling frequency = 500 Hz), displayed eye movements after each trial and stored the data on disk for offline analysis. Saccade onsets and offsets were detected offline automatically based on a velocity threshold of 40° s−1 and were verified visually by the experimenter. Trials with artefacts or erroneous first saccades (directed towards target 2 instead of target 1) were removed from the analysis.
The absolute error between the location of target 2 and the final position of the eye was computed. The effects of the trial type (ON/OFF), the direction of the first saccade (left/right) and the direction of the second saccade (left/right) on this dependent variable were first tested in the patient using a factorial analysis of variance (ANOVA). A repeated-measures ANOVA was then used, as proposed by Mycroft et al. [57], to additionally compare this single case to the control group.
3. Results
Among the 216 trials presented to the patient, 59 trials were excluded from the analysis because the first saccade was not correctly directed towards target 1: of these, 51 were excluded because the first saccade was aimed directly towards target 2 (either followed or not by a second saccade towards target 1) and eight because the saccade towards target 1 was very inaccurate (absolute error greater than 7°). These erroneous responses occurred more often in trials without remapping (32% of the total number of ON trials and 20% of the total number of OFF trials) and more often when the first saccade was rightward (36% for R-R and 33% for R-L) than leftward (14% for L-R and 11% for L-L). This pattern of errors, therefore, cannot account for the remapping impairment observed specifically for the second saccade direction, as described below.
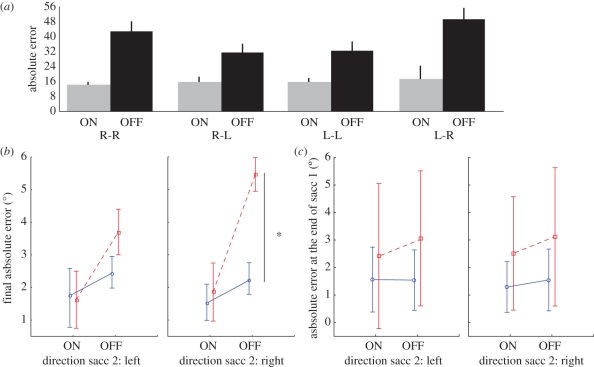
The factorial ANOVA could nevertheless be performed on the remaining 157 trials of the patient and revealed main effects of trial type (ON–OFF) (F1,157 = 51.4; p < 0.05) and of the direction of the second saccade (F1,157 = 4.5; p < 0.05). A trend for the interaction between trial type and the two second saccade direction (F1,157 = 3.6; p = 0.059) was also observed and allowed us to perform planned comparisons to compare the four conditions that combine the two trial types (ON/OFF) and the two second saccade directions (L/R). These tests revealed a larger increase of final errors in the OFF condition—rightward second saccade (figure 7a); the OFF condition involving a rightward second saccade was significantly less accurate than both the OFF condition involving a leftward second saccade (F1,157 = 13.8; p < 0.05) and the ON condition involving a rightward second saccade (F1,157 = 36.8; p < 0.05).
Figure 7.
(a) Histogram of the absolute errors (degree) observed for the second saccade in each combination of type of trials (ON/OFF), and directions of saccades 1 and 2 for patient OK. (b) Mean absolute error (degree) and 95% confidence intervals observed for the second saccade in each combination of type of trials (ON/OFF) and direction of saccade 2 (left/right) plotted separately for patient OK and the control group. Blue lines, control group; red dashed lines, patient OK. (c) Mean absolute error (degree) and 95% confidence intervals observed for the first saccade in each combination of type of trials (ON/OFF) and direction of saccade 2 (left/right) plotted separately for patient OK and the control group. Blue lines, control group; red lines, patient OK.
A repeated-measures ANOVA with group (patient versus controls) as a factor was then performed with the method proposed by Mycroft et al. [57] in order to determine whether the decrease in accuracy was ‘pathological’ (different from controls) in this specific condition of a rightward second saccade. This analysis revealed a significant interaction: trial type × direction of second saccade × group (F1,4 = 32.0) superior to the F′ corrected for the difference of variances between groups (F′ = 27; σ2 = 4). Planned comparisons were used to compare, between groups, the four conditions combining the two trial types (ON–OFF) and the two second saccade directions (L-R). These tests revealed that the main effect of trial type was due to a pathological inaccuracy in the OFF condition—rightward second saccade in the patient with respect to the control group (F1,4 = 35.2 > F′; figure 7b). There was no significant difference between the patient and the control group in ON trials or in the OFF—leftward second saccade trials (all Fs < 10, p > 0.05). Note that the overall larger increase in error in the patient (with respect to controls, figure 7b) between ON and OFF conditions corresponds to an unspecific increase in variability when the task increases in difficulty (in the OFF conditions, duration of targets presentation is shortened and thus memory is added). Indeed, errors of patient OK were already larger than those of controls at the end of the first saccade in the OFF conditions (figure 7c). Patient OK shows a mean error of 3.1° with respect to target 1 location in conditions OFF (same value when the second saccade was directed leftward or rightward), which is outside the confidence interval of the controls (same errors of 1.6° on average, and the same 95% confidence interval ranging from 0.3° to 2.7°, when the second saccade was directed leftward or rightward).The fact that it is already present for the first saccade demonstrates that it is not specific for the remapping process but rather linked to the disappearance of the targets in conditions OFF (unspecific increase in task difficulty). In contrast, the larger (and significant) effect specific to rightward second saccades is due to an additional deficit that seems to be relevant to the issue of visual remapping.
In sum, the larger inaccuracy observed in the OFF condition involving a second rightward saccade cannot be explained solely by the direction of second saccade nor by an overall increase in inaccuracy between ON and OFF trials (owing to the general increase in difficulty in OFF trials, i.e. when target 2 is only briefly presented and the second saccade is memory-guided). Instead, this pattern of results reveals a specific and lateralized impairment of the memory-based remapping processes.
4. Discussion
In this study, the ‘saccadic double-step’ paradigm (figure 2a) [8] was tested in a patient (OK) who had damage to the dorsal part of the right PPC and to the corpus callosum (figure 5). Patient OK exhibited an asymmetrical increase in final errors in trials requiring remapping processes. More specifically, in the OFF condition, the rightward second saccades had the largest errors (figure 7). We will address successively different classical models and show that none fits with this specific and lateralized impairment of the remapping of memorized visual information in patient OK. At the end, we will propose a new functional scheme that is compatible both with this finding and with previous studies.
First, the present results cannot be explained by a basic impairment of saccadic preparation and/or attentional selection owing to the PPC lesion. Indeed, following right parietal lesions, the impairment should concern the leftward saccades [58,59], as observed in another study in this patient in a task involving the selection of a target among flankers [55].
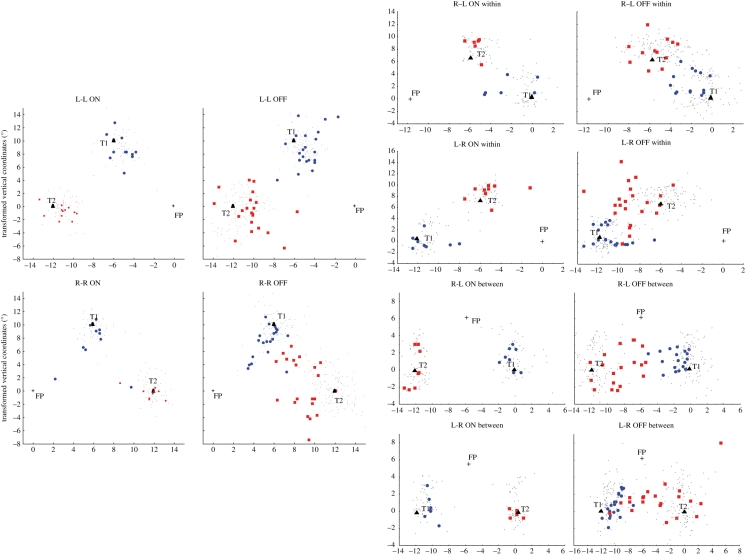
Second, a deficit depending on the direction of the second saccade cannot be explained by classical views of how cortical lesions impair remapping processes. Indeed, a remapping impairment owing to the lesion of the right PPC has been related to spatial updating associated with the execution of the first saccade: either the damage of the right hemisphere is viewed to prevent from the updating of contralesional (leftward) first saccades [11,14,16] or the representation of the visual stimuli is viewed to be transferred into the left oculocentric field encoded in the damaged right hemisphere after a first (rightward) saccade [37,60]. In contrast, the present results did not reveal a significant interaction between trial type and the direction of the first saccade. Moreover, the erroneous rightward second saccades observed in patient OK (figure 8) were not directed towards the retinal location of target 2 ([16]; figure 2a) nor aborted ([14]; figure 2b).
Figure 8.
Plot of final eye positions for saccades 1 and 2 in patient OK and controls, separately for R-R, L-L, within-hemifield and between-hemifield L-R and R-L conditions, in ON and OFF trials. Horizontal and vertical coordinates have been transformed to present the data on the same triangle of target locations. Grey dots, control; blue circles, saccade 1 OK; red squares, saccade 2 OK.
In the classical models, visual remapping involves contralateral (visual and/or motor) representations. Therefore, these models would not predict any deficit for rightward second saccades, which imply representation of the second target location in the left (non-damaged) hemisphere, in the case of right unilateral cortical lesions. Moreover, conditions involving two successive leftward saccades, supposed to be represented within the damaged right hemisphere, are correctly performed, while conditions involving two successive rightward saccades, supposed to be represented within the non-damaged left hemisphere, are impaired. In sum, the cortical lesion of patient OK cannot by itself explain his double-step saccadic deficit, hence the location of his cortical lesion (SPL–IPS and slight extension into Brodmann area 40) is irrelevant for this task. Alternatively, this impairment of patient OK could be related to his callosal lesion. Such a disconnection hypothesis fits more with the type of errors observed (figure 8) [9,10]. There are still two possibilities: either the disconnection prevents visual remapping per se from occurring optimally or it affects a callosal transfer that occurs after visual remapping.
The callosal lesion could prevent an interhemispheric transfer of the target 2 location for visual remapping. However, such an interhemispheric transfer is proposed to be necessary when the target 2 is initially presented in one visual field and its location is remapped into the opposite visual field after the first saccade (between-hemifield LR and RL conditions [9,49]; figure 4a). A specialization of the right hemisphere in humans for visual remapping would not help, since it predicts a unilateral interhemispheric transfer that would be necessary in all conditions in which target 1 or target 2 is initially presented in the right visual field, in order to be transferred to the right hemisphere. Inconsistent with both predictions, in addition to L-R conditions, patient OK is impaired in the R-R condition (figure 8) and not in R-L.
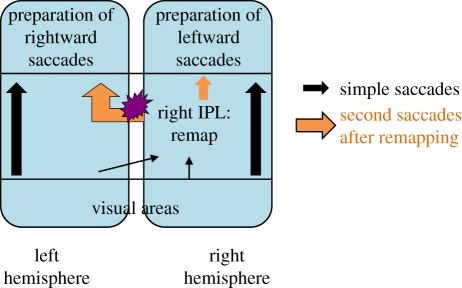
Finally, if the results of patient OK in the double-step saccadic paradigm cannot be explained by an impairment of visual remapping per se, they may then correspond to a deficit that occurs after the remapping. The model presented in the following provides an explanation of patient OK's results in the present (saccade) and previous (pointing) experiments and fits with most other data of the literature. This model is based both on a specific focal damage of the corpus callosum in OK and on a right-hemispheric predominance for space representation and integration in humans (figure 9). Patient OK is impaired when he has to accurately guide rightward second saccades, and this impairment is specific to remapping conditions. The callosal lesion could prevent an interhemispheric transfer necessary for sending the remapped location of target 2 towards the regions involved in saccadic motor preparation in the left hemisphere. The results of visual remapping achieved after a first saccade would no longer transfer into a map directly assigned to saccadic planning. Along an attentional view of the PPC [51], a memory-based remapping of visual information may rather first involve a specific ‘saliency’ map [5] in which the retinal location of target 2 throughout the visual field can be memorized and updated. Such a saliency map may have to subsequently transfer the remapped visual information to ‘motor’ areas, in order to plan and execute subsequent behaviour, such as a second saccade. According to the hypothesis that remapping impairments contribute to hemineglect following lesions of the right inferior parietal lobule (IPL) [17,18,61], a specialized region of the right IPL (which could be the right angular gyrus (Brodmann area 39) according to Heide & Kömpf [18]) may contain this saliency map necessary for visual exploration and for the integration of space. Given the impairment of patients with lesions of the PFC when the saccadic sequence is visually guided (ON trials) [16], one can further speculate that the visual information will have to be transmitted from the right IPL to the left PFC via the corpus callosum in OFF trials—rightward second saccades. These trials, which are the ones specifically impaired in patient OK, would thus involve an interhemispheric transfer (probably via the part of the corpus callosum damaged in the patient), while those involving a leftward second saccade, preserved in patient OK, would be processed within the right hemisphere (figure 9). Consistent with this scheme, the callosal lesion of patient OK specifically matches a region identified by Huang et al. [54] as projecting towards the frontal lobes (figure 5). This scheme also implies that the representation within the right IPL of a target 2 presented in the right visual field (and thus initially represented in the left occipital visual areas) does not involve an interhemispheric transfer via the same part of the corpus callosum that is damaged in the patient. This is consistent with the lesion of patient OK preserving the most posterior part of the corpus callosum (figure 5). The present results thus leave three possibilities for the interhemispheric transfer, providing access of the ipsilateral retinal locations to the right human IPL (or to LIP neurons in the monkey; [12]): between homologous occipital areas, between left occipital areas and the right IPL or through a subcortical pathway.
Figure 9.
Schematic of our explanatory hypothesis.
A previous study has tested the post-saccadic memory-based remapping in the context of a pointing task in patient OK [46] (figure 4a). When visual targets were initially presented in the ipsilesional visual field and remapped into the contralesional oculocentric space, the patient exhibited pointing errors (i.e. OA, as if pointing target had been presented initially in the contralesional visual field). Conversely, when visual targets were initially presented in the contralesional visual field and remapped into the ipsilesional oculocentric space, the patient exhibited no pointing errors. It was thus concluded that OA is linked to the represented oculocentric target location rather than retinal target location [46]. These results also implied that interhemispheric transfer of pointing targets' location was preserved in both directions, and hence occurred at a site in between the retinotopic representation of the target location and the dorsal part of the PPC involved in visuo-manual transformation (damaged in patient OK [46]). This reinforces our interpretation of the present results excluding a remapping deficit per se: the callosal lesion disrupts the specific transfer of the remapped target location towards oculomotor frontal structures necessary to guide saccadic behaviour (figure 9) and not pointing behaviour. The same neural network would thus be used for visual remapping in the context of a saccade–saccade task and a saccade–pointing task (figure 4b), the corpus callosum lesion of patient OK affecting the double-step saccadic task only at the execution stage.
The present results of patient OK reinforce the literature reviewed in the introduction (e.g. [17–19,21,27]) in favour of the existence of an asymmetrical neural network for visual remapping. However, we should mention that we cannot definitively state that this asymmetrical network is the one regularly used in the context of a double-step saccade task in healthy subjects. It remains possible that the regular network used for saccade–saccade and saccade–pointing remapping is symmetrical and involves the dorsal PPC (as suggested by Medendorp et al. [49], figure 4b). In patients with lesions of the dorsal PPC, lacking immediate visuo-motor guidance [55,62,63], this visuo-motor remapping could be achieved via the asymmetrical ventral PPC network as a compensatory network. Note that the use of alternative spatial representations, operating on longer time scales, has been demonstrated for simple actions in patients with OA. Indeed, OA is a deficit of immediate and automatic visuo-motor guidance, and motor performance of these patients paradoxically improves with a memorization delay ([64,65]; reviews in [62,66]).
The contexts in which visual remapping processes proposed in our model (figure 9) are used will, therefore, require future investigation. The functional link made between visual remapping in the context of a sequence of two saccades and ocular exploration of complex visual scenes is also questionable. So, is the functional link between visual remapping in motor contexts (saccadic exploration, pointing, drawing, etc.) and in contexts of visual perception and awareness (as suggested by required saccades that were not executed which could reflect omissions). Indeed, the implicit functional link between visual remapping across saccades and spatial working memory (which operate at different time scales) has been recently challenged by the results of Vuilleumier et al. [60], who have been the first to test a perceptual spatial working memory task (verbal report of change detection in a visual display) across saccades in neglect patients (not selected with respect to their lesion locations). The same results have been published more recently in patients with constructional apraxia following the right IPL lesion [37]. These results contrast with the literature of the double-step saccade task reviewed in §1: they show impairments after a rightward (ipsilesional) first saccade [37,60]. Their interpretation was that after a rightward saccade, the representation of the initial visual scene that has to be compared with the following scene is transferred into the left oculocentric field encoded in the damaged right hemisphere. In contrast, Duhamel et al. [14] proposed that the damage of the right hemisphere prevents the updating of leftward (contralesional) first saccades. The network involved in remapping might therefore be different between goal-directed action (single saccade or pointing) in which only the goal need to be represented (egocentric coding), and perceptual and behavioural contexts (visual search, drawing copy) in which a pattern of salient stimuli has to be represented (allocentric coding). Alternatively, these two apparently contradictory deficits of neglect patients might correspond to two successive steps of visual remapping: (i) the integration of the efferent copy in order to determine the next location targeted that would then become the new centre of the map and (ii) the remapping of salient stimuli locations when this new centre has been reached. Note that the first (pre-saccadic) shift occurs in the same direction as the saccade, while the post-saccadic shift logically occurs in the opposite direction. One could speculate that perceptual stability and spatial working memory across saccades rely more on the predictive remapping mechanisms (both involving the comparison of the predicted visual scene with the actual one after the saccade), whereas motor behaviour relies more on the post-saccadic memory-based remapping of selected goals as tested by the double-step saccadic task.
In conclusion, even if many issues remain to be further investigated, the literature and the behaviour of patient OK confirm the specific contribution of the human right cerebral hemisphere in some processes of visual remapping. Lateralization for spatial processing in the human right cerebral hemisphere (and for language in the other hemisphere) would include the remapping of memorized visual information.
Footnotes
One contribution of 11 to a Theme Issue ‘Visual stability’.
References
- 1.Mays L. E., Sparks D. L. 1980. Dissociation of visual and saccade-related responses in superior colliculus neurons. J. Neurophysiol. 43, 207–232 [DOI] [PubMed] [Google Scholar]
- 2.Goldberg M. E., Bruce C. J. 1990. Primate frontal eye fields III. Maintenance of a spatially accurate saccade signal. J. Neurophysiol. 64, 489–508 [DOI] [PubMed] [Google Scholar]
- 3.Tian J., Schlag J., Schlag-Rey M. 2000. Testing quasi-visual neurons in the monkey's frontal eye field with the triple-step paradigm. Exp. Brain Res. 130, 433–440 10.1007/s002210050047 (doi:10.1007/s002210050047) [DOI] [PubMed] [Google Scholar]
- 4.Colby C. L., Duhamel J.-R., Goldberg M. E. 1995. Oculocentric spatial representation in parietal cortex. Cereb. cortex 5, 470–481 10.1093/cercor/5.5.470 (doi:10.1093/cercor/5.5.470) [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb J. P., Kusunoki M., Goldberg M. E. 1998. The representation of visual salience in monkey parietal cortex. Nature 391, 481–484 10.1038/35135 (doi:10.1038/35135) [DOI] [PubMed] [Google Scholar]
- 6.Nakamura K., Colby C. L. 2002. Updating of the visual representation in monkey striate and extrastriate cortex during saccades. Proc. Natl Acad. Sci. USA 99, 4026–4031 10.1073/pnas.052379899 (doi:10.1073/pnas.052379899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnadt J. W., Andersen R. A. 1988. Memory related motor planning activity in posterior parietal cortex of macaque. Exp. Brain Res. 70, 216–220 10.1007/BF00271862 (doi:10.1007/BF00271862) [DOI] [PubMed] [Google Scholar]
- 8.Hallett P. E., Lightstone A. D. 1976. Saccadic eye movements to flashed targets. Vision Res. 16, 107–114 10.1016/0042-6989(76)90084-5 (doi:10.1016/0042-6989(76)90084-5) [DOI] [PubMed] [Google Scholar]
- 9.Berman R. A., Heiser L. M., Saunders R. C., Colby C. L. 2005. Dynamic circuitry for updating spatial representations. I. Behavioral evidence for interhemispheric transfer in the split-brain macaque. J. Neurophysiol. 94, 3228–3248 10.1152/jn.00028.2005 (doi:10.1152/jn.00028.2005) [DOI] [PubMed] [Google Scholar]
- 10.Colby C. L., Berman R. A., Heiser L. M., Saunders R. C. 2005. Corollary discharge and spatial updating: when the brain is split, is space still unified? Prog. Brain Res. 149, 187–205 10.1016/S0079-6123(05)49014-7 (doi:10.1016/S0079-6123(05)49014-7) [DOI] [PubMed] [Google Scholar]
- 11.Li C. S., Andersen R. A. 2001. Inactivation of macaque lateral intraparietal area delays initiation of the second saccade predominantly from contralesional eye positions in a double-step task. Exp. Brain Res. 137, 45–57 10.1007/s002210000546 (doi:10.1007/s002210000546) [DOI] [PubMed] [Google Scholar]
- 12.Heiser L. M., Colby C. L. 2006. Spatial updating in area LIP is independent of saccade direction. J. Neurophysiol. 95, 2751–2767 10.1152/jn.00054.2005 (doi:10.1152/jn.00054.2005) [DOI] [PubMed] [Google Scholar]
- 13.Berman R. A., Heiser L. M., Dunn C. A., Saunders R. C., Colby C. L. 2007. Dynamic circuitry for updating spatial representations. III. From neurons to behavior. J. Neurophysiol. 98, 105–121 10.1152/jn.00330.2007 (doi:10.1152/jn.00330.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duhamel J.-R., Goldberg M. E., Fitzgibbon E. J., Sirigu A., Grafman J. 1992. Saccadic dysmetria in a patient with a right frontoparietal lesion. Brain 115, 1387–1402 10.1093/brain/115.5.1387 (doi:10.1093/brain/115.5.1387) [DOI] [PubMed] [Google Scholar]
- 15.Heide W., Binkofski F., Seitz R. J., Posse S., Nitschke M. F., Freund H. J., Kömpf D. 2001. Activation of frontoparietal cortices during memorized triple-step sequences of saccadic eye movements: an fMRI study. Eur. J. Neurosci. 13, 1177–1189 10.1046/j.0953-816x.2001.01472.x (doi:10.1046/j.0953-816x.2001.01472.x) [DOI] [PubMed] [Google Scholar]
- 16.Heide W., Blankenburg M., Zimmermann E., Kömpf D. 1995. Cortical control of double-step saccades: implications for spatial orientation. Ann. Neurol. 38, 739–748 10.1002/ana.410380508 (doi:10.1002/ana.410380508) [DOI] [PubMed] [Google Scholar]
- 17.Pisella L., Mattingley J. B. 2004. The contribution of spatial remapping impairments to unilateral visual neglect. Neurosci. Biobehav. Rev. 28, 181–200 10.1016/j.neubiorev.2004.03.003 (doi:10.1016/j.neubiorev.2004.03.003) [DOI] [PubMed] [Google Scholar]
- 18.Heide W., Kömpf D. 1997. Specific parietal lobe contribution to spatial constancy across saccades. In Parietal lobe contributions to orientation in 3D space (eds Thier P., Karnath H.-O.), pp. 149–172 Heidelberg, Germany: Springer-Verlag [Google Scholar]
- 19.van Koningsbruggen M. G., Gabay S., Sapir A., Henik A., Rafal R. D. 2010. Hemispheric asymmetry in the remapping and maintenance of visual saliency maps: a TMS study. J. Cogn. Neurosci. 22, 1730–1738 10.1162/jocn.2009.21356 (doi:10.1162/jocn.2009.21356) [DOI] [PubMed] [Google Scholar]
- 20.Morris A. P., Chambers C. D., Mattingley J. B. 2007. Parietal stimulation destabilizes spatial updating across saccadic eye movements. Proc. Natl Acad. Sci. USA 104, 9069–9074 10.1073/pnas.0610508104 (doi:10.1073/pnas.0610508104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prime S. L., Vesia M., Crawford J. D. 2008. Transcranial magnetic stimulation over posterior parietal cortex disrupts transsaccadic memory of multiple objects. J. Neurosci. 28, 6938–6949 10.1523/JNEUROSCI.0542-08.2008 (doi:10.1523/JNEUROSCI.0542-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rode G., Luauté J., Klos T., Courtois-Jacquin S., Revol P., Pisella L., Holmes N. P., Boisson D., Rossetti Y. 2007. Bottom-up visuo-manual adaptation: consequences for spatial cognition. In Attention and performance (eds Haggard P., Rossetti Y., Kawato M.), ch. 10, pp. 207–229 Oxford, UK: Oxford University Press; (XXI: sensorimotor foundations of higher cognition.) [Google Scholar]
- 23.Pisella L., Berberovic N., Mattingley J. B. 2004. Impaired working memory for location but not for colour or shape in visual neglect: a comparison of parietal and non-parietal lesions. Cortex 40, 379–390 10.1016/S0010-9452(08)70132-1 (doi:10.1016/S0010-9452(08)70132-1) [DOI] [PubMed] [Google Scholar]
- 24.Corbetta M., Kincade J. M., Ollinger J. M., McAvoy M. P., Shulman G. L. 2000. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat. Neurosci. 3, 292–297 10.1038/73009 (doi:10.1038/73009) [DOI] [PubMed] [Google Scholar]
- 25.Posner M. I. 1980. Orienting of attention. Q. J. Exp. Psychol. 32, 3–25 10.1080/00335558008248231 (doi:10.1080/00335558008248231) [DOI] [PubMed] [Google Scholar]
- 26.Fink G. R., Marshall J. C., Shah N. J., Weiss P. H., Halligan P. W., Grosse-Ruyken M., Ziemons K., Zilles K., Freund H. J. 2000. Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology 54, 1324–1331 [DOI] [PubMed] [Google Scholar]
- 27.Malhotra P., Coulthard E. J., Husain M. 2009. Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain 132, 645–660 10.1093/brain/awn350 (doi:10.1093/brain/awn350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mannan S. K., Mort D. J., Hodgson T. L., Driver J., Kennard C., Husain M. 2005. Revisiting previously searched locations in visual neglect: role of right parietal and frontal lesions in misjudging old locations as new. J. Cogn. Neurosci. 17, 340–354 10.1162/0898929053124983 (doi:10.1162/0898929053124983) [DOI] [PubMed] [Google Scholar]
- 29.Prime S. L., Niemeier M., Crawford J. D. 2006. Transsaccadic integration of visual features in a line intersection task. Exp. Brain Res. 169, 532–548 10.1007/s00221-005-0164-1 (doi:10.1007/s00221-005-0164-1) [DOI] [PubMed] [Google Scholar]
- 30.Ruff C. C., Blankenburg F., Bjoertomt O., Bestmann S., Weiskopf N., Driver J. 2009. Hemispheric differences in frontal and parietal influences on human occipital cortex: direct confirmation with concurrent TMS-fMRI. J. Cogn. Neurosci. 21, 1146–1161 10.1162/jocn.2009.21097 (doi:10.1162/jocn.2009.21097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blangero A., et al. 2009. Systematic retinotopic error vectors in unilateral optic ataxia. Cortex 46, 77–93 10.1016/j.cortex.2009.02.015 (doi:10.1016/j.cortex.2009.02.015) [DOI] [PubMed] [Google Scholar]
- 32.Pascual-Leone A., Gomez-Tortosa E., Grafman J., Always D., Nichelli P., Hallett M. 1994. Induction of visual extinction by rapid-rate transcranial magnetic stimulation of parietal lobe. Neurology 44, 494–498 [DOI] [PubMed] [Google Scholar]
- 33.Corbetta M., Kincade M. J., Lewis C., Snyder A. Z., Sapir A. 2005. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat. Neurosci. 8, 1603–1610 10.1038/nn1574 (doi:10.1038/nn1574) [DOI] [PubMed] [Google Scholar]
- 34.Karnath H. O., Ferber S., Himmelbach M. 2001. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature 411, 950–953 10.1038/35082075 (doi:10.1038/35082075) [DOI] [PubMed] [Google Scholar]
- 35.Mort D. J., Malhotra P., Mannan S. K., Rorden C., Pambakian A., Kennard C., Husain M. 2003. The anatomy of visual neglect. Brain 126, 1986–1997 10.1093/brain/awg200 (doi:10.1093/brain/awg200) [DOI] [PubMed] [Google Scholar]
- 36.Vallar G., Perani D. 1986. The anatomy of unilateral neglect after right-hemisphere stroke lesions. A clinical/CT-scan correlation study in man. Neuropsychologia 24, 609–622 10.1016/0028-3932(86)90001-1 (doi:10.1016/0028-3932(86)90001-1) [DOI] [PubMed] [Google Scholar]
- 37.Russell C., Deidda C., Malhotra P., Crinion J. T., Merola S., Husain M. 2010. A deficit of spatial remapping in constructional apraxia after right-hemisphere stroke. Brain 133, 1239–1251 10.1093/brain/awq052 (doi:10.1093/brain/awq052) [DOI] [PubMed] [Google Scholar]
- 38.Melcher D., Colby C. 2008. Trans-saccadic perception. Trends Cogn. Sci. 12, 466–473 10.1016/j.tics.2008.09.003 (doi:10.1016/j.tics.2008.09.003) [DOI] [PubMed] [Google Scholar]
- 39.Bays P. M., Husain M. 2007. Spatial remapping of the visual world across saccades. Neuroreport 18, 1207–1213 10.1097/WNR.0b013e328244e6c3 (doi:10.1097/WNR.0b013e328244e6c3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wurtz R. H. 2008. Neuronal mechanisms of visual stability. Vision Res. 48, 2070–2089 10.1016/j.visres.2008.03.021 (doi:10.1016/j.visres.2008.03.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pisella L., Sergio L., Blangero A., Torchin H., Vighetto A., Rossetti Y. 2009. Optic ataxia and the function of the dorsal stream: contribution to perception and action. Neuropsychologia 47, 3033–3044 10.1016/j.neuropsychologia.2009.06.020 (doi:10.1016/j.neuropsychologia.2009.06.020) [DOI] [PubMed] [Google Scholar]
- 42.Husain M., Mannan S., Hodgson T., Wojciulik E., Driver J., Kennard C. 2001. Impaired spatial working memory across saccades contributes to abnormal search in parietal neglect. Brain 124, 941–952 10.1093/brain/124.5.941 (doi:10.1093/brain/124.5.941) [DOI] [PubMed] [Google Scholar]
- 43.Pisella L., Blangero A., Tilikete C., Biotti D., Rode G., Vighetto A., Mattingley J. B., Rossetti Y. In press. Attentional disorders. In Handbook of cognitive neuroscience (eds Ochsner K., Kosslyn S.). New York, NY: Oxford University Press [Google Scholar]
- 44.Striemer C., Blangero A., Rossetti Y., Boisson D., Rode G., Vighetto A., Pisella L., Danckert J. 2007. Deficits in peripheral visual attention in patients with optic ataxia. Neuroreport 18, 1171–1175 10.1097/WNR.0b013e32820049bd (doi:10.1097/WNR.0b013e32820049bd) [DOI] [PubMed] [Google Scholar]
- 45.Khan A. Z., Pisella L., Rossetti Y., Vighetto A., Crawford J. D. 2005. Impairment of gaze-centered updating of reach targets in bilateral parietal-occipital damaged patients. Cereb. Cortex 15, 1547–1560 10.1093/cercor/bhi033 (doi:10.1093/cercor/bhi033) [DOI] [PubMed] [Google Scholar]
- 46.Khan A. Z., Pisella L., Vighetto A., Cotton F., Luauté J., Boisson D., Salemme R., Crawford J. D., Rossetti Y. 2005. Optic ataxia errors depend on remapped, not viewed, target location. Nat. Neurosci. 8, 418–420 10.1038/nn1425 (doi:10.1038/nn1425) [DOI] [PubMed] [Google Scholar]
- 47.Merriam E. P., Genovese C. R., Colby C. L. 2007. Remapping in human visual cortex. J. Neurophysiol. 97, 1738–1755 10.1152/jn.00189.2006 (doi:10.1152/jn.00189.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merriam E. P., Genovese C. R., Colby C. L. 2003. Spatial updating in human parietal cortex. Neuron 39, 361–373 10.1016/S0896-6273(03)00393-3 (doi:10.1016/S0896-6273(03)00393-3) [DOI] [PubMed] [Google Scholar]
- 49.Medendorp W. P., Goltz H. C., Vilis T., Crawford J. D. 2003. Gaze-centered updating of visual space in human parietal cortex. J. Neurosci. 23, 6209–6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andersen R. A., Buneo C. A. 2002. Intentional maps in posterior parietal cortex. Annu. Rev. Neurosci. 25, 189–220 10.1146/annurev.neuro.25.112701.142922 (doi:10.1146/annurev.neuro.25.112701.142922) [DOI] [PubMed] [Google Scholar]
- 51.Colby C. L., Goldberg M. E. 1999. Space and attention in parietal cortex. Annu. Rev. Neurosci. 22, 319–349 10.1146/annurev.neuro.22.1.319 (doi:10.1146/annurev.neuro.22.1.319) [DOI] [PubMed] [Google Scholar]
- 52.Berman R. A., Colby C. 2009. Attention and active vision. Vision Res. 49, 1233–1248 10.1016/j.visres.2008.06.017 (doi:10.1016/j.visres.2008.06.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaymard B., Rivaud S., Pierrot-Deseilligny C. 1994. Impairment of extraretinal eye position signals after central thalamic lesions in humans. Exp. Brain Res. 102, 1–9 (Erratum in Exp. Brain Res. 1995;104:3621). [DOI] [PubMed] [Google Scholar]
- 54.Huang H., et al. 2005. DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. Neuroimage 26, 195–205 10.1016/j.neuroimage.2005.01.019 (doi:10.1016/j.neuroimage.2005.01.019) [DOI] [PubMed] [Google Scholar]
- 55.Blangero A., Khan A. Z., Salemme R., Deubel H., Schneider W. X., Rode G., Vighetto A., Rossetti Y., Pisella L. 2010. Pre-saccadic perceptual facilitation can occur without covert orienting of attention. Cortex 46, 1132–1137 10.1016/j.cortex.2009.06.014 (doi:10.1016/j.cortex.2009.06.014) [DOI] [PubMed] [Google Scholar]
- 56.Alahyane N., Koene A., Pélisson D. 2004. Transfer of adaptation from visually guided saccades to averaging saccades elicited by double visual targets. Eur. J. Neurosci. 20, 827–836 10.1111/j.1460-9568.2004.03536.x (doi:10.1111/j.1460-9568.2004.03536.x) [DOI] [PubMed] [Google Scholar]
- 57.Mycroft R. H., Mitchell D. C., Kay J. 2002. An evaluation of statistical procedures for comparing an individual's performance with that of a group of controls. Cogn. Neuropsychol. 19, 291–299 10.1080/02643290143000150 (doi:10.1080/02643290143000150) [DOI] [PubMed] [Google Scholar]
- 58.Pierrot-Deseilligny C., Ploner C. J., Muri R. M., Gaymard B., Rivaud-Pechoux S. 2002. Effects of cortical lesions on saccadic: eye movements in humans. Ann. N. Y. Acad. Sci. 956, 216–229 10.1111/j.1749-6632.2002.tb02821.x (doi:10.1111/j.1749-6632.2002.tb02821.x) [DOI] [PubMed] [Google Scholar]
- 59.Rafal R. D. 2006. Oculomotor functions of the parietal lobe: effects of chronic lesions in humans. Cortex 42, 730–739 10.1016/S0010-9452(08)70411-8 (doi:10.1016/S0010-9452(08)70411-8) [DOI] [PubMed] [Google Scholar]
- 60.Vuilleumier P., Sergent C., Schwartz S., Valenza N., Girardi M., Husain M., Driver J. 2007. Impaired perceptual memory of locations across gaze-shifts in patients with unilateral spatial neglect. J. Cogn. Neurosci. 19, 1388–1406 10.1162/jocn.2007.19.8.1388 (doi:10.1162/jocn.2007.19.8.1388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kennard C., Mannan S. K., Nachev P., Parton A., Mort D. J., Rees G., Hodgson T. L., Husain M. 2005. Cognitive processes in saccade generation. Ann. N. Y. Acad. Sci. 1039, 176–183 10.1196/annals.1325.017 (doi:10.1196/annals.1325.017) [DOI] [PubMed] [Google Scholar]
- 62.Rossetti Y., Pisella L., Vighetto A. 2003. Optic ataxia revisited: visually guided action versus immediate visuo-motor control. Exp. Brain Res. 153, 171–179 10.1007/s00221-003-1590-6 (doi:10.1007/s00221-003-1590-6) [DOI] [PubMed] [Google Scholar]
- 63.Gaveau V., Pélisson D., Blangero A., Urquizar C., Prablanc C., Vighetto A., Pisella L. 2008. Saccadic control and eye–hand coordination in optic ataxia. Neuropsychologia 46, 475–486 10.1016/j.neuropsychologia.2007.08.028 (doi:10.1016/j.neuropsychologia.2007.08.028) [DOI] [PubMed] [Google Scholar]
- 64.Milner A. D., Dijkermann C., Pisella L., McIntosh R., Tilikete C., Vighetto A., Rossetti Y. 2001. Grasping the past: delaying the action improves visuo-motor performance. Curr. Biol. 11, 1896–1901 10.1016/S0960-9822(01)00591-7 (doi:10.1016/S0960-9822(01)00591-7) [DOI] [PubMed] [Google Scholar]
- 65.Revol P., Rossetti Y., Vighetto A., Rode G., Boisson D., Pisella L. 2003. Pointing errors in immediate and delayed conditions in unilateral optic ataxia. Spat. Vis. 16, 347–364 10.1163/156856803322467572 (doi:10.1163/156856803322467572) [DOI] [PubMed] [Google Scholar]
- 66.Pisella L., Binkofski F., Lasek K., Toni I., Rossetti Y. 2006. No double-dissociation between optic ataxia and visual agnosia: multiple sub-streams for multiple visuo-manual integrations. Neuropsychologia 44, 2734–2748 10.1016/j.neuropsychologia.2006.03.027 (doi:10.1016/j.neuropsychologia.2006.03.027) [DOI] [PubMed] [Google Scholar]