Abstract
It has been shown that prolonged exposure to a human face leads to shape-selective visual aftereffects. It seems that these face-specific aftereffects (FAEs) have multiple components, related to the adaptation of earlier and higher level processing of visual stimuli. The largest magnitude of FAE, using long-term adaptation periods, is usually observed at the retinotopic position of the preceding adaptor stimulus. However, FAE is also detected, to a smaller degree, at other retinal positions in a spatially invariant way and this component depends less on the adaptation duration. Several lines of evidences suggest that while the position-specific FAE involves lower level areas of the ventral processing stream, the position-invariant FAE depends on the activation of higher level face-processing areas and the fusiform gyrus in particular. In the present paper, we summarize the available behavioural, electrophysiological and neuroimaging results regarding the spatial selectivity of FAE and discuss their implications for the visual stability of object representations across saccadic eye movements.
Keywords: adaptation, aftereffect, face, invariance, spatiotopy, retinotopy
1. Introduction
The repeated presentation of a given visual stimulus leads to various perceptual consequences. A number of studies have demonstrated that brief (<1 s) prior exposure to the same or a related stimulus generally facilitates (in the form of faster and more accurate response) its subsequent recognition, a well-studied phenomenon called priming. Other repetition-related effects, called aftereffects, are created by the technique of selective adaptation. During such paradigms, the prolonged exposure of a given stimulus biases the perception of the subsequent pattern in a predictable way. For example, after being adapted to a grid pattern tilted to the right, a vertical pattern will look more like being tilted towards the left (the tilt aftereffect [1]).
The existence of adaptation to basic low-level visual dimensions—i.e. motion, orientation, size, curvature, spatial frequency, texture or perceived hue (for review see [2–6])—has been known for a long time, and the investigation of visual aftereffects provided crucial information about the mechanisms involved in the processing of specific visual attributes. On the other hand, we know surprisingly little about the mechanisms of neural adaptation underlying shape-specific aftereffects related to the analysis of complex object form. Recently, however, it has been shown that, similar to the aftereffects caused by adaptation to lower level visual features, prolonged exposure to a visual object—even if it is such a complex one as a human face—will also lead to shape-selective visual aftereffects. The first to report visual aftereffects in face perception were Webster & MacLin [7]. They used distorted faces (for example, with decreased distances between internal features in comparison with a normal, veridical face) and found that prolonged adaptation to such faces biased the perception of veridical faces in a direction opposite to the adapting distortion (i.e. perceived as having expanded features). Figure 1 summarizes the most important aftereffects regarding human faces.
Figure 1.
Illustration of some of the FAEs: distortion, identity, gender, facial expression and gaze-direction. Upper row: the adaptor stimuli; middle row: the ambiguous/neutral target stimulus; lower row: illustration of the percept after being adapted. For example, prolonged exposure to the second face of the upper row (Grace Kelly) would lead to the perception of the 50% morph stimulus (middle row) as biased towards another person (Halle Berry). For a demonstration of the effect, please visit http://cogsci.bme.hu/~gkovacs/FAE.html.
Since their discovery, face aftereffects (FAEs) have been used widely in the cognitive neuroscience community to tap into the mechanisms of face perception [7–17]. Probably the most important question related to the FAE is to what extent it reflects specific processes of face perception. The answer to this question determines the specificity of FAE, as theoretically it is possible that FAE is owing to a combination of localized aftereffects for low-level stimulus features, such as orientation, texture and spatial frequency. Such effects, inherited from earlier areas, are not without precedent in the visual system. For instance, contrast adaptation in the magnocellular layers of the primate lateral geniculate nucleus is known to be inherited from the retina, while adaptation-related changes in contrast sensitivity in the motion-sensitive area MT presumably also occur in early visual areas (for a review see [18]). Thus, it is of utmost importance to determine if FAE reflects the neural processes of face processing or of earlier feature processing. In their original study, Webster & MacLin [7] acknowledged that the observed FAE ‘… need not reflect specific processes of face processing’. They nevertheless argued that the asymmetry of FAE between normal and distorted faces (adaptation to a normal face did not lead to FAE) is a property that is different from the characteristics of low-level feature adaptation, hence FAE reflects processes that may not be specific, but at least are central to face perception.
One way to test the specificity of FAE is to measure its invariance for various low-level properties, such as size, orientation and position. The logic behind such experiments is that if FAE reflects the adaptation of neural processes specific to face perception then it should transfer across stimulus transformations to which it is known to be invariant. The involvement of hierarchically higher processing steps in FAE are suggested by the fact that face aftereffects are, to a large extent, invariant to changes in size [12,19–21], orientation [14,22], colour and contrast [23]. These findings suggest that face-selective neural processes at the higher stages of visual processing, tolerating the above changes of the stimuli, can adapt and might represent the neural basis of FAEs. Probably the most well-studied local feature, which has also led to the most controversial results, is the positional specificity/invariance of the FAE. In our present paper, we summarize the current results regarding the spatial selectivity of FAE and try to resolve the possible contradictions of the literature.
2. Data supporting that face aftereffects are position-invariant
(a). Behaviour
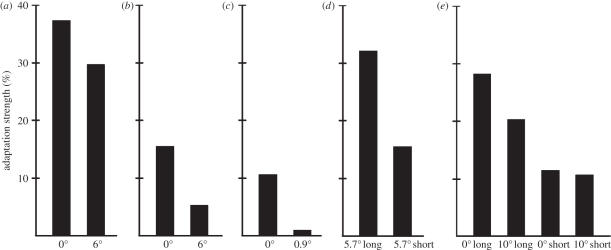
One of the most cited papers regarding FAE is the demonstration of norm-based coding of facial identity. Leopold et al. [13] were the first to demonstrate that adapting to a face for several seconds biases perception of a subsequent face so that an average face resembles the computationally opposite identity (figure 1b). They also tested if this FAE can be explained based on the combination of adaptation to low-level features. In their experiment, subjects were required to fixate a small red dot at a given location along the midline of the face during the adaptation phase and then they had to make a saccade to another fixation dot halfway between the eyes during the test face presentation (figure 2a). They found that adaptation to face identity generalized across retinal positions up to 6°. Later, Fang & He [8] and Fang et al. [24] used adapters floating in an approximately 6 × 6° area and also found viewpoint aftereffects for face stimuli (figure 2b).
Figure 2.
Behavioural results of several of the most important articles regarding the positional specificity of FAE. The numbers below the x-axis refer to the positional difference of adaptor and target stimuli (0° is a condition where adaptor and target stimuli were presented in the same retinal position). Long-adaptation time was at least 2 s, short-adaptation time was a few hundred milliseconds. For a detailed description please see text. (a) Leopold et al. [13], (b) Afraz & Cavanagh [28], (c) Xu et al. [57], (d) Fung & He [8], (e) Kovács et al. [27].
Thus, these studies suggest the role of higher level neurons with large receptive field sizes as possible correlates of FAE. However, in these studies, the adapting and test faces always overlapped spatially since the stimulus size was larger than (11° [13]) or comparable to (3° [8]) the visual field size within which translational tolerance was tested.
Cortical neurons of the macaque brain involved in the analysis of faces, such as the inferior temporal cortex (IT), usually have receptive fields that cover both sides of the fixation point, extending into the ipsilateral visual field [25,26]. Thus, an ultimate test for the role of these neurons in the creation of FAE would be to show its translation tolerance over the vertical midline. In a previous study, Kovács et al. [27] tested if adaptation effects on face gender perception were invariant to the hemifield of the test stimulus relative to the preceding adaptation stimulus. Using an adaptation stimulus of 500 ms, we found that the observed gender-specific FAE, although smaller than after 5 s adaptation time, transfers completely to the opposite hemifield (figure 2e). Afraz & Cavanagh [28] used a 5 s adaptation time and tested systematically the retinotopy of the face identity aftereffect. They also found a smaller but significant FAE even if the adaptor and test faces were presented 6° peripherally in opposite hemifields (figure 2b). In fact, the magnitude of FAE depended on the distance from the adapting stimulus to the same degree whether or not the test and adaptor faces were in the same hemifield. Altogether, these results suggest at least partial spatial tolerance of the FAE, extending across the vertical midline. This would support the theory that the adaptation of high-level neurons, having large receptive fields, leads to the FAEs.
(b). Neurophysiology
Despite the large body of experiments regarding the nature of behavioural FAE, there are relatively few studies testing the neural correlates of human FAE, using electrophysiological or neuroimaging methods. It has been shown that an early occipito-temporal, negative component of the event-related potential (ERP), appearing 170 ms after stimulus onset, shows a reduction in amplitude following prolonged adaptation to the gender of a given face in a category-specific manner [12,29]. Harris & Nakayama [30] have shown similar adaptation effects of the magnetoencephalographic equivalent of the N170 component, the M170, after the presentation of another face stimulus for as short as 200 ms. This N170 adaptation effect reflects the configural/holistic processing of faces, as suggested by its sensitivity to the orientation of the stimulus [31] and to the alignments of the upper and lower face halves in a composite face illusion paradigm [32], and it can also be observed when the two subsequent stimuli differ in their viewpoint ([33], but see [34] for a different conclusion regarding M170). Similar reduction of the N170 was also observed in gaze direction adaptation [16] and recognition tasks [35].
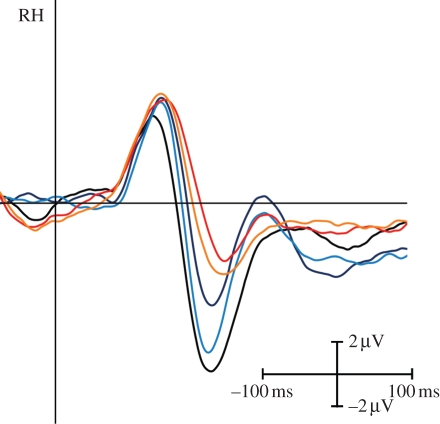
Since these previous experiments point to the relationship of FAE and of the N170 ERP component in a series of ERP-recording experiments, we tested the position specificity of electrophysiological adaptation effects (figure 3). Our ERP results paralleled the behavioural FAE in the sense that we observed similar adaptation effects measured on the amplitude of the N170 ERP component when the adaptor (presented for 500 ms) and the test stimuli were in the same or in the opposite visual hemifields [27]. Furthermore, we observed slightly larger adaptation effects on the N170 ERP component after short-term adaptation than after long-term adaptation. These results suggest that short-term adaptation duration leads to a position-invariant FAE, which is related to the reduction of the N170 ERP component. Consequently, the observed FAE is probably owing to the adaptation of the neurons responsible for the configural/holistic stages of face processing (for summary see [36,37]), rather than earlier processing steps.
Figure 3.
N170 as the major electrophysiological correlate of FAE for contralaterally presented target stimuli. Grand-average ERPs are presented over the occipito-temporal sites of the right hemisphere. Black is non-adapted control; red (500 ms adaptation time) and dark blue (5 s): adaptor and target are both in the same (left) hemifield; yellow (500 ms) and light blue (5 s): adaptor is presented in the ipsilateral hemifield. Modified from Kovács et al. [27].
But where are the neurons responsible for the behavioural and electrophysiological effects of face adaptation situated? Although the sensitivity of high-density electrophysiological studies has increased drastically in the past few years, we have no clear information about the cortical sources of the M/N170 component. Most current papers indicate that there are multiple generators of this component. In addition to the fusiform gyrus [38–42], the superior temporal sulcus [38,43,44] and occipital extrastriate areas [40] are identified as the major sources of N170. Thus, ERP/MEG recordings are not the ideal methods for determining the cortical location of the position-invariant FAE. In comparison with the ERP recordings, functional magnetic imaging (fMRI) provides data with better spatial resolution. Another advantage of fMRI over ERP recordings is that it has been widely used previously in the adaptation paradigms as well. The so-called fMRI adaptation (fMRIa) method has proved to be an efficient and popular way in studying visual functions and specifically face perception (for reviews see [45–48]). fMRIa paradigms are based on the finding that if two subsequent stimuli are processed by the same neural population, then adaptation reduces the neural activity evoked by the second stimulus and this, in turn, leads to a lower blood oxygen level-dependent (BOLD) signal.
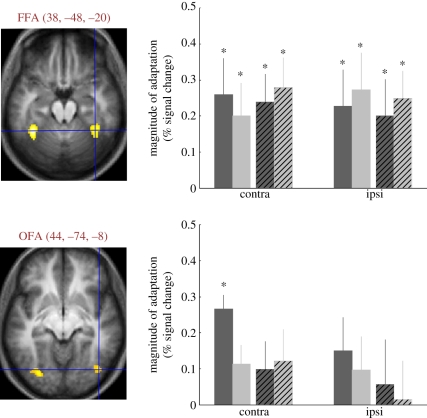
Thus, to determine the location of the neural correlates of position-invariant FAE, we replicated our previous FAE experiment in the MRI scanner (figure 4). Our results show that the right fusiform face area (FFA, for a summary see [49]) shows adaptation effects that are position-invariant and can be evoked by short (500 ms) as well as by long (4500 ms) adaptation durations [50]. The magnitude of the fMRIa effect was similar in all the studied conditions. Interestingly, this was true even if the stimuli were presented 5° peripherally in the right, ipsilateral visual hemifield. This suggests that the right FFA shows complete position invariance, including ipsilateral peripheral sites. This is in agreement with the results of previous studies, using stimulus repetition-evoked fMRIa, which suggest an invariance of FFA to the face's position, size [45,46], spatial scale [51] and viewing angle [24].
Figure 4.
The approximate location of the FFA in the middle fusiform gyrus and OFA in the inferior occipital gyrus. Mean MNI coordinates are indicated next to the labels. Diagrams indicate the mean decrease of BOLD signal when compared with the non-adapted condition. Asterisks mark significant differences (p < 0.05) between the non-adapted and adapted conditions. Dark-grey bars, long same; light-grey bars, long different; dark-grey crossed bars, short same; light-grey crossed bars, short different; contra and ipsi, contralateral and ipsilateral hemifields of the target stimulus. Long: 4500 ms adaptation time; short: 500 ms adaptation time. Same: adaptor and target in the same retinal position; different: adaptor and target in different hemifields. From the data of Kovács et al. [50].
3. Data supporting that face aftereffects are also specific for the retinal position
(a). Behaviour
Recent single-unit recording studies have provided evidence that some positional information is preserved even at the highest processing levels of the ventral processing stream of the macaque brain, in the inferior temporal cortex (for review see [52]). It was found that the receptive fields of inferior temporal neurons can differ in size, have ‘hot spots’, where they are most sensitive to stimulation and are typically biased towards the contralateral hemifield [53–55]. Therefore, if adaptation of these neurons is indeed involved in shape-selective aftereffects, they should show at least partial position-selectivity. Indeed, this is what we found with 5000 ms long adaptation times (figure 2e): the gender-specific aftereffects are larger when the adaptor and target faces are presented on the same retinal position when compared with when they are displayed in different hemifields [27,56].
Afraz & Cavanagh [28] tested further the retinotopy of the FAE. Using an identity-specific FAE, they presented the adaptor face peripherally and varied the position of the test anti-face around fixation. They found that the magnitude of the FAE depends on the relative distance of the adaptor and test faces (figure 2b). Their results indicated a position specificity of the FAE with 10.8° full width at half maximum (FWHM) of the position-tuning curve. The study suggesting the highest level of position specificity was performed on facial expression judgements. Xu et al. [57] used a simple concave or convex curve as adaptor and found that, as a consequence of 2000 ms adaptation, subjects perceived subsequent faces as significantly happier or sadder. They described that even a small (0.9°) displacement of the adaptor curve, relative to the mouth region of the test face, led to a complete elimination of the facial expression aftereffect (figure 2d). Thus, it seems that the abstract facial-expression aftereffect, induced by such a simple feature as a curved line, is much more local than the gender- and identity-specific FAE.
(b). Neurophysiology
In ERP recording experiments, it was found that after long-term (5000 ms) gender adaptation, the N170 amplitude reductions show strong position-specificity: they are larger when the adaptor and target faces are presented on the same retinal position when compared with when they are displayed in different hemifields (figure 3; [27,56]). Similarly, fMRI responses evoked by the contralateral test stimuli were significantly reduced in the right occipital face area (OFA) after long-term adaptation in a position-specific manner: we observed no fMRIa effect in OFA if the adaptor and test faces were presented in opposite hemifields (figure 4). The OFA has been shown to be involved in early processing of facial features in other fMRI and transcranial magnetic stimulation experiments and in lesion studies of acquired prosopagnosia [42,58–61]. Regarding the spatial properties of OFA, previous studies showed that its response has clear contralateral preference [62] and position-specific fMRIa [49]. Grill-Spector et al. [46] on the other hand suggested that the fMRIa in OFA translates across 5.6° of the visual field. Altogether, these results suggest the role of OFA in the formation of FAE and imply that the receptive field size of OFA neurons is larger than 5°, but it does not extend across the vertical meridian towards the ipsilateral hemifield.
4. Spatiotopic representation and face aftereffects
(a). Behaviour
What happens if the observer makes an eye movement between the adaptation and testing phases so that their gaze is directed to another location of the visual field? Suppose the subject is presented first an adaptor at a given peripheral fixation location and next, before the presentation of the test stimulus, (s)he moves her/his eye to the centre of the screen. If we observe aftereffects at the visual field position where the adaptor stimulus was previously presented, and which is now projected on a peripheral part of the retina, then the effect is independent of the actual retinal coordinates of the stimulus and is called spatiotopic (for reviews see [63–65]). However, if we only observe adaptation-related aftereffects on the central retinal location of the adaptor, which is now shifted to another position of the visual field as well, then it depends on the retinal location and is purely retinotopic.
Currently adaptation experiments suggest that the aftereffects observed for tilt [66,67], motion [68,69], duration [70], binocular rivalry [71] and shapes [67] have components that cannot be explained on the basis of retinotopic adaptations. These results together suggest the existence of spatiotopic representations, where position of objects is determined in world-based coordinates, for several types of stimulus categories.
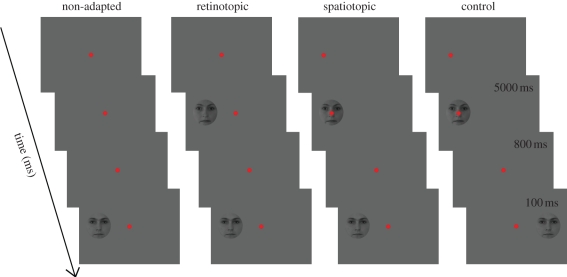
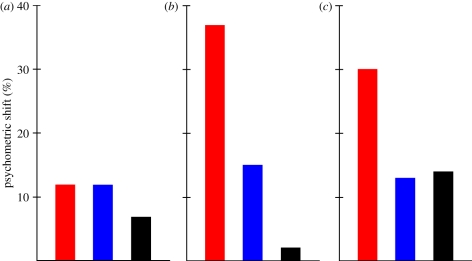
Regarding faces and the FAE, Melcher [67] used a variant of the gender adaptation experiment with the stimuli of Kovács et al. [12]. In his study, subjects had a three-alternative forced choice recognition task of male faces morphed together with female faces. He observed that after adaptation to a female face recognition performance increases, signalling the presence of specific aftereffects. To measure the spatiotopy of the effect his subjects had to make a 10° saccade between adaptation and test presentation and the position of the test face was either corresponding to the previous spatial location of the adaptor or it was in the opposite side of the fixation spot (figure 5). The results showed significant FAE both at the spatial location of the adaptor as well as in the opposite hemifield and these results support the existence of position-invariant mechanisms (figure 6). However, the effect was larger at the spatiotopic than at the control location and the magnitude of the effect was not significantly different from the FAE observed when subjects did not move their eyes and the adaptor and target faces were presented on the same peripheral retinal location. This suggests that there is a complete transfer of adaptation effect across saccades and supports the existence of spatiotopic representations for faces.
Figure 5.
The paradigms used for testing spatiotopic transfers of the FAE (based on [67,72]). In the spatiotopic and control conditions, the fixation spot moves from 10° periphery towards the centre. For details see text.
Figure 6.
The behavioural results regarding the spatiotopy of FAE. Red bars, retinotopic; blue bars, spatiotopic; black, control. (a) Melcher [67], (b) Van Boxtel et al. [71], (c) Afraz & Cavanagh [72].
Another paper, using a different approach with binocular rivalry, supports the existence of spatiotopic transfer for faces. van Boxtel et al. [71] investigated the influence of retinotopic and spatiotopic pre-adaptation on binocular rivalry. They presented a face and a house in a rivalry situation after being adapted to a face for as long as 30 s. As a result of adaptation, predominance during rivalry was biased away from the face stimulus towards the house. They found that previous face adaptation biases predominance when adaptor and rivalry locations are retinotopically as well as when spatiotopically matched, while no such effect occurs at other control locations. In contrast to the finding of Melcher [67], however, the retinotopic influence of rivalry was significantly larger than the spatiotopic. Another difference between spatiotopic and retinotopic effects regards the temporal development of the effect: while retinotopic effects are present since the beginning of the rivalry period it seems that spatiotopic effects require a minimum of 10 s of rivalry time to develop.
A further experiment, using a modified version of the Melcher [67] paradigm, questions the existence of spatiotopic transfer of FAE. Afraz & Cavanagh [72] used a two-alternative forced choice gender discrimination paradigm with the face morphs stimuli, generated by three-dimensional head models, of O'Toole et al. [73]. They found a significant FAE when subjects moved their eyes between the adaptation and test period from the periphery to the centre, but this effect was smaller than when they had to fixate continuously in the centre. These results were interpreted as clear evidence of the retinotopy of FAE. However, the fact that gender decision was also biased when the subjects made a 10° saccade during the inter-stimulus interval when compared with a non-adapted condition suggests that a significant part of FAE translates across retinal positions as well. The fact that they found no advantage of the spatiotopic location when compared with the control position suggests no spatiotopic transfer of FAE.
It is not yet clear why some studies find spatiotopic FAE while others do not. As Cavanagh et al. [63] argues, higher-level factors such as working memory or attention might be easily involved in the creation of binocular rivalry adaptation, explaining the spatiotopic effects. However, no such effects might explain the differences of the studies of Melcher [67] and Afraz & Cavanagh [72]. But in these two studies several confounds are to be found that make the final conclusions regarding the role of spatiotopic representation during the FAE rather difficult. First of all the task was different in the studies: identification versus gender discrimination. Both studies used the female prototype image of the female–male morphs as adaptors (i.e. adaptor and target faces were of the same identity). Melcher [67] asked the subjects to identify the target faces as one of the three possible male faces, hence asking identity decision and using identity-specific adaptation as well. Afraz & Cavanagh [72] on the other hand asked the subjects to perform two-alternative gender discrimination. As the images of the same persons were used as adaptors and test stimuli, they, in fact, tested identity contingent gender adaptation. Models of face perception suggest that different signals in faces (such as identity, age, gender and expression) are processed by independent systems (e.g. [74]). On the one hand, the FFA has been implicated in identity recognition [75–79]. The full positional invariance of this area for both contralateral and ipsilateral stimuli [50] suggests a high degree of spatial invariance. On the other hand, neuropsychological [80] and imaging [81,82] studies suggest that facial gender is processed in other cortical areas. Thus, it is possible that the two studies tapped into separate neural mechanisms of face perception, one responsible for recognition while the other responsible for gender decisions, and these two mechanisms have different retino/spatiotopic representations.
Another issue that makes the interpretation difficult regards the way they tested retinotopic adaptation and the non-adapted state (figure 5). Both studies used a peripheral adaptor and subsequent test stimulus in their retinotopic condition, when no eye movements of the subjects were required (the non-adapted condition was identical to this except for the presentation of adaptor). Thus, while in spatiotopic and control conditions, subjects made a saccade towards the centre; after the adaptation period (with foveally fixated adaptor stimulus) they had fixated at the central fixation spot throughout the whole of the retinotopic and non-adapted trials and the adaptor face was peripheral. In other words, three factors, the foveal/peripheral nature of the adaptor, the relative position of adaptor and test stimuli (unrelated, spatiotopic or retinotopic relation) and the presence/absence of eye movements co-varied: spatiotopic or control conditions were used with foveal adaptor and eye movements while retinotopic conditions were used with peripheral adaptor and without eye movements. This makes the comparison of retinotopic and spatiotopic conditions difficult. It is possible that eye movements interfere with the representation, reducing the effect in the spatiotopic and control conditions of Afraz & Cavanagh [72] when compared with the retinotopic conditions. But it is also possible that the neurons responsible for the FAE have information about the saccadic eye movements, via feedback connections, and this facilitates the transfer of FAE across spatial positions, as found by Melcher [67]. Thus, it will be important to reveal how task, eye movements and spatial position interact with the FAE. Whether the transfer of FAEs to spatially matching coordinates is a special case of positional invariance that is influenced by spatial attention [63] will be decided in future experiments.
(b). Neurophysiology
There are no electrophysiological or neuroimaging studies of the spatiotopic transfer of FAE as of today. The available results regarding motion processing suggest that the activity of the retinotopic portion of area MT depends on the gaze direction as well ([83–86], but see [87] for another conclusion). Similarly, Merriam et al. [88] found evidence of spatial remapping in higher-order retinotopic areas (specifically in area V3a and hV4) during the presentation of simple light flashes. McKyton & Zohary [89] tested similar questions in the object selective lateral occipital (LO) complex. They compared the fMRIa effect for man-made objects when their displacement was limited to either the retina or the screen, by manipulating eye position and object locations. Clear fMRIa was found in LO when the object's screen position was fixed, regardless of the object's retinal position, also suggesting spatiotopic representations. Sereno & Huang [90] mapped the organization of the multisensory ventral intraparietal area and found evidences of the location coding of visual stimuli with respect to the head, not with respect to the retina. Comparable paradigms and improved analysis techniques might help us to find such mechanisms in face perception in the future as well.
5. The importance of adaptation time
Several lines of data show that the presentation duration of the adaptor stimulus determines how it will interact with the subsequent target. We know that adaptation duration affects perceived FAE logarithmically, consistent with the classic time course of aftereffects for simple visual attributes [91–93]. Consistent with the idea that the duration of adaptation is a crucial factor, recent fMRI studies found that obtaining orientation-tuned adaptation signals in V1 requires long-term (several seconds) adaptation [94], whereas adaptation effects in extrastriate areas were already seen after short-term adaptation [95]. Moreover, Fang et al. [24] also showed that adaptation duration affects the properties of fMRIa in FFA and OFA. It seems that a reduction of adaptation duration allows the isolation of different steps of face processing [27,50]: while long-term adaptation affects neurons that are sensitive to the physical differences of the stimuli (for example in OFA or LO), as well as position-invariant neurons (in the FFA), short-term adaptation affects position- and viewpoint-invariant neurons (also cf. [27,50]).
Recent results using object morphing and adaptation confirmed the important role of temporal parameters in determining the behavioural effects. Daelli et al. [96] used long-term (3000 ms) adaptation and varied the delay of inter-stimulus interval between 50 and 3100 ms using an object-matching task. They found a crossover from adaptation aftereffects to priming effects as the delay lengthened: object-specific adaptation aftereffects vanished with time, unmasking a temporally sustained priming bias.
6. Summary and conclusions
It seems that FAEs have multiple components that are related to the adaptation of earlier and higher-level processing of visual stimuli. The largest magnitude of the FAE is invariably observed at the identical retinotopic position where the adaptor stimulus was presented. This component of the aftereffect depends strongly on the length of the adaptation period as well. These facts, together with the results of electrophysiological and neuroimaging experiments, suggest the involvement of lower processing levels, specifically of the contralateral OFA and earlier areas of the IT.
However, the FAE is also detected, to a smaller degree, at other retinal positions showing spatially invariant processing and this component, in contrast to the spatially specific one, occurs even with short adaptation periods. These facts and experiments testing fMRIa support the contribution of higher-level face-processing areas and the right FFA in particular.
These conclusions are in line with previous data suggesting a progressive construction of spatially invariant representations along the visual-form pathway [64,67], and are consistent with the suggestion that low-level areas are largely retinotopic but that high-level features can be matched for the same object even when the retinal position is different [97]. Furthermore, it is interesting to note that similar ideas have been put forward regarding spatial selectivity for action as well: the right posterior parietal cortex represents space in both hemispheres, while other action-related areas, such as the frontal eye field, show contralateral selectivity [98,99]. The question remains to what extent the spatial invariance of object representations might play a role in the stability of the world across eye movements.
Acknowledgements
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (KO 3918/1-1) and by the Deutscher Akademischer Austauschdienst (DAAD-MöB/821). We thank Daniel Kaiser for his help.
Footnotes
One contribution of 11 to a Theme Issue ‘Visual stability’.
References
- 1.Gibson J., Radner M. 1937. Adaptation, aftereffect and contrast in the perception of tilted lines. I. Quantitative studies. J. Exp. Psychol. 20, 453–467 10.1037/h0059826 (doi:10.1037/h0059826) [DOI] [Google Scholar]
- 2.Anstis S. M., Verstraten F. A. J., Mather G. 1998. The motion aftereffect. Trends Cogn. Sci. 2, 111–117 10.1016/S1364-6613(98)01142-5 (doi:10.1016/S1364-6613(98)01142-5) [DOI] [PubMed] [Google Scholar]
- 3.Antal A., Varga E. T., Nitsche M. A., Chadaide Z., Paulus W., Kovács G., Vidnyánszky Z. 2004. Modulation of motion aftereffect by transcranial direct current stimulation over MT+/V5 in humans. Neuroreport 15, 2491–2494 [DOI] [PubMed] [Google Scholar]
- 4.Clifford C. W. 2002. Perceptual adaptation: motion parallels orientation. Trends Cogn. Sci. 6, 136–143 10.1016/S1364-6613(00)01856-8 (doi:10.1016/S1364-6613(00)01856-8) [DOI] [PubMed] [Google Scholar]
- 5.Durgin F. H., Proffitt D. R. 1996. Visual learning in the perception of texture: simple and contongent after effects of texture density. Spatial Vis. 9, 423–474 10.1163/156856896X00204 (doi:10.1163/156856896X00204) [DOI] [PubMed] [Google Scholar]
- 6.Frisby J. P. 1979. Seeing: illusion, brain and mind. Oxford, UK: Oxford University Press [Google Scholar]
- 7.Webster M. A., MacLin O. 1999. Figural aftereffects in the perception of faces. Psychonom. Bull. Rev. 6, 647–653 [DOI] [PubMed] [Google Scholar]
- 8.Fang F., He S. 2005. Viewer-centered object representation in the human visual system revealed by viewpoint aftereffects. Neuron 45, 793–800 10.1016/j.neuron.2005.01.037 (doi:10.1016/j.neuron.2005.01.037) [DOI] [PubMed] [Google Scholar]
- 9.Fox C. J., Barton J. J. S. 2007. What is adapted in face adaptation? The neural representations of expression in the human visual system. Brain Res. 1127, 80–89 10.1016/j.brainres.2006.09.104 (doi:10.1016/j.brainres.2006.09.104) [DOI] [PubMed] [Google Scholar]
- 10.Jaquet E., Rhodes G., Hayward W. G. 2007. Opposite aftereffects for Chinese and Caucasian faces are selective for social category information and not just physical face differences. Q. J. Exp. Psychol. 60, 1457–1467 10.1080/17470210701467870 (doi:10.1080/17470210701467870) [DOI] [PubMed] [Google Scholar]
- 11.Jenkins R., Beaver J. D., Calder A. J. 2006. I thought you were looking at me: direction-specific aftereffects in gaze perception. Psychol. Sci. 17, 506–513 10.1111/j.1467-9280.2006.01736.x (doi:10.1111/j.1467-9280.2006.01736.x) [DOI] [PubMed] [Google Scholar]
- 12.Kovács G., Zimmer M., Bankó E., Harza I., Antal A., Vidnyánszky Z. 2006. Electrophysiological correlates of visual adaptation to faces and body parts in humans. Cerebral Cortex 16, 742–753 10.1093/cercor/bhj020 (doi:10.1093/cercor/bhj020) [DOI] [PubMed] [Google Scholar]
- 13.Leopold D. A., O'Toole A. J., Vetter T., Blanz V. 2001. Prototype-referenced shape encoding revealed by high-level aftereffects. Nat. Neurosci. 4, 89–94 10.1038/82947 (doi:10.1038/82947) [DOI] [PubMed] [Google Scholar]
- 14.Rhodes G., Jeffery L., Watson T. L., Clifford C. W. G., Nakayama K. 2003. Fitting the mind to the world: face adaptation and attractiveness aftereffects. Psychol. Sci. 14, 558–566 10.1046/j.0956-7976.2003.psci_1465.x (doi:10.1046/j.0956-7976.2003.psci_1465.x) [DOI] [PubMed] [Google Scholar]
- 15.Ryu J. J., Borrmann K., Chaudhuri A. 2008. Imagine Jane and identify John: face identity aftereffects induced by imagined faces. PLoS ONE 3, e2195. 10.1371/journal.pone.0002195 (doi:10.1371/journal.pone.0002195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schweinberger S. R., Kloth N., Jenkins R. 2007. Are you looking at me? Neural correlates of gaze adaptation. NeuroReport 18, 693–696 10.1097/WNR.0b013e3280c1e2d2 (doi:10.1097/WNR.0b013e3280c1e2d2) [DOI] [PubMed] [Google Scholar]
- 17.Webster M. A., Kaping D., Mizokami Y., Duhamel P. 2004. Adaptation to natural facial categories. Nature 428, 557–561 10.1038/nature02420 (doi:10.1038/nature02420) [DOI] [PubMed] [Google Scholar]
- 18.Kohn A. 2007. Visual adaptation: physiology, mechanisms, and functional benefits. J. Neurophysiol. 97, 3155–3164 10.1152/jn.00086.2007 (doi:10.1152/jn.00086.2007) [DOI] [PubMed] [Google Scholar]
- 19.Anderson N. D., Wilson H. R. 2005. The nature of synthetic face adaptation. Vis. Res. 25, 1815–1828 10.1016/j.visres.2005.01.012 (doi:10.1016/j.visres.2005.01.012) [DOI] [PubMed] [Google Scholar]
- 20.Rhodes G., Jeffery L., Watson T. L., Jaquet E., Winkler C., Clifford C. W. G. 2004. Orientation-contingent face aftereffects and implications for face-coding mechanisms. Curr. Biol. 14, 2119–2123 10.1016/j.cub.2004.11.053 (doi:10.1016/j.cub.2004.11.053) [DOI] [PubMed] [Google Scholar]
- 21.Zhao L., Chubb C. 2001. The size-tuning of the face-distortion after-effect. Vis. Res. 41, 2979–2994 10.1016/S0042-6989(01)00202-4 (doi:10.1016/S0042-6989(01)00202-4) [DOI] [PubMed] [Google Scholar]
- 22.Watson T. L., Clifford C. W. 2003. Pulling faces: an investigation of face-distortion aftereffect. Perception 32, 1109–1116 10.1068/p5082 (doi:10.1068/p5082) [DOI] [PubMed] [Google Scholar]
- 23.Yamashita J. A., Hardy J. L., De Valois K. K., Webster M. A. 2005. Stimulus selectivity of figural aftereffects for faces. J. Exp. Psychol. Hum. Percept. Perform. 31, 420–437 10.1037/0096-1523.31.3.420 (doi:10.1037/0096-1523.31.3.420) [DOI] [PubMed] [Google Scholar]
- 24.Fang F., Murray S. O., He S. 2007. Duration-dependent fMRI adaptation and distributed viewer-centered face representation in human visual cortex. Cerebral Cortex 17, 1402–1411 10.1093/cercor/bhl053 (doi:10.1093/cercor/bhl053) [DOI] [PubMed] [Google Scholar]
- 25.Desimone R., Albright T. D., Gross C. G., Bruce C. 1984. Stimulus-selective properties of inferior temporal neurons in the macaque. J. Neurosci. 4, 2051–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross C. G., Rocha-Miranda C. E., Bender D. B. 1972. Visual properties of neurons in inferotemporal cortex of the macaque. J. Neurophysiol. 35, 96–111 [DOI] [PubMed] [Google Scholar]
- 27.Kovács G., Zimmer M., Harza I., Vidnyánszky Z. 2007. Adaptation duration affects the spatial selectivity of facial aftereffects. Vis. Res. 47, 3141–3149 10.1016/j.visres.2007.08.019 (doi:10.1016/j.visres.2007.08.019) [DOI] [PubMed] [Google Scholar]
- 28.Afraz S. R., Cavanagh P. 2008. Retinotopy of the face aftereffect. Vis. Res. 48, 42–54 10.1016/j.visres.2007.10.028 (doi:10.1016/j.visres.2007.10.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kloth N., Schweinberger S. R., Kovács G. 2010. Neural correlates of generic versus gender-specific face adaptation. J. Cogn. Neurosci. 22, 2345–2356 10.1162/jocn.2009.21329 (doi:10.1162/jocn.2009.21329) [DOI] [PubMed] [Google Scholar]
- 30.Harris A., Nakayama K. 2007. Rapid face-selective adaptation of an early extrastriate component in MEG. Cerebral Cortex 17, 63–70 10.1093/cercor/bhj124 (doi:10.1093/cercor/bhj124) [DOI] [PubMed] [Google Scholar]
- 31.Jacques C., d'Arripe O., Rossion B. 2007. The time course of the face inversion effect during individual face discrimination. J. Vis. 7, 1–9 10.1167/7.8.3 (doi:10.1167/7.8.3) [DOI] [PubMed] [Google Scholar]
- 32.Jacques C., Rossion B. 2009. The initial representation of individual faces in the right occipito-temporal cortex is holistic: electrophysiological evidence from the composite face illusion. J. Vis. 9, 8, 1–16 10.1167/9.6.8 (doi:10.1167/9.6.8) [DOI] [PubMed] [Google Scholar]
- 33.Caharel S., d'Arripe O., Ramon M., Jacques C., Rossion B. 2009. Early adaptation to unfamiliar faces across viewpoint changes in the right hemisphere: evidence from the N170 ERP component. Neuropsychologia 47, 639–643 10.1016/j.neuropsychologia.2008.11.016 (doi:10.1016/j.neuropsychologia.2008.11.016) [DOI] [PubMed] [Google Scholar]
- 34.Ewbank M. P., Smith W. A. P., Hancock E. R., Andrews T. J. 2008. The M170 reflects a viewpoint-dependent representation for both familiar and unfamiliar faces. Cerebral Cortex 18, 364–370 10.1093/cercor/bhm060 (doi:10.1093/cercor/bhm060) [DOI] [PubMed] [Google Scholar]
- 35.Ganis G., Schendan H. E. 2008. Visual mental imagery and perception produce opposite adaptation effects on early brain potentials. Neuroimage 42, 1714–1727 10.1016/j.neuroimage.2008.07.004 (doi:10.1016/j.neuroimage.2008.07.004) [DOI] [PubMed] [Google Scholar]
- 36.Minnebusch D. A., Daum I. 2009. Neuropsychological mechanisms of face and body perception. Neurosci. Biobehav. Rev. 33, 1133–1144 10.1016/j.neubiorev.2009.05.008 (doi:10.1016/j.neubiorev.2009.05.008) [DOI] [PubMed] [Google Scholar]
- 37.Rossion B., Gauthier I. 2002. How does the brain process upright and inverted faces? Behav. Cogn. Neurosci. Rev. 1, 62–74 10.1177/1534582302001001004 (doi:10.1177/1534582302001001004) [DOI] [PubMed] [Google Scholar]
- 38.Corrigan N. M., et al. 2009. An investigation of the relationship between fMRI and ERP source localized measurements of brain activity during face processing. Brain Topogr. 22, 83–96 10.1007/s10548-009-0086-5 (doi:10.1007/s10548-009-0086-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halgren E., Raij T., Marinkovic K., Jousmäki V., Hari R. 2000. Cognitive response profile of the human fusiform face area as determined by MEG. Cerebral Cortex 10, 69–81 [DOI] [PubMed] [Google Scholar]
- 40.Itier R. J., Herdman A. T., George N., Cheyne D., Taylor M. J. 2006. Inversion and contrast-reversal effects on face processing assessed by MEG. Brain Res. 1115, 108–120 10.1016/j.brainres.2006.07.072 (doi:10.1016/j.brainres.2006.07.072) [DOI] [PubMed] [Google Scholar]
- 41.Rossion B., Delvenne J. F., Debatisse D., Goffaux V., Bruyer R., Crommelinck M., Guerit J. M. 1999. Spatio-temporal localization of the face inversion effect: an event-related potentials study. Biol. Psychol. 50, 173–189 10.1016/S0301-0511(99)00013-7 (doi:10.1016/S0301-0511(99)00013-7) [DOI] [PubMed] [Google Scholar]
- 42.Rossion B., Caldara R., Seghier M., Schuller A. M., Lazeyras F., Mayer E. 2003. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain 126, 2381–2395 10.1093/brain/awg241 (doi:10.1093/brain/awg241) [DOI] [PubMed] [Google Scholar]
- 43.Itier R. J., Taylor M. J. 2004. Source analysis of the N170 to faces and objects. Neuroreport 15, 1261–1265 [DOI] [PubMed] [Google Scholar]
- 44.Itier R. J., Alain C., Sedore K., McIntosh A. R. 2007. Early face processing specificity: it's in the eyes! J. Cogn. Neurosci. 19, 1815–1826 10.1162/jocn.2007.19.11.1815 (doi:10.1162/jocn.2007.19.11.1815) [DOI] [PubMed] [Google Scholar]
- 45.Andrews T. J., Ewbank M. P. 2004. Distinct representations for facial identity and changeable aspects of faces in the human temporal lobe. NeuroImage 23, 905–913 10.1016/j.neuroimage.2004.07.060 (doi:10.1016/j.neuroimage.2004.07.060) [DOI] [PubMed] [Google Scholar]
- 46.Grill-Spector K., Kushnir T., Edelman S., Avidan G., Itzchak Y., Malach R. 1999. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron 24, 187–203 10.1016/S0896-6273(00)80832-6 (doi:10.1016/S0896-6273(00)80832-6) [DOI] [PubMed] [Google Scholar]
- 47.Grill-Spector K., Henson R., Martin A. 2006. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci. 10, 14–23 10.1016/j.tics.2005.11.006 (doi:10.1016/j.tics.2005.11.006) [DOI] [PubMed] [Google Scholar]
- 48.Krekelberg B., Boynton G. M., van Wezel R. J. A. 2006. Adaptation: from single cells to BOLD signals. Trends Neurosci. 29, 250–256 10.1016/j.tins.2006.02.008 (doi:10.1016/j.tins.2006.02.008) [DOI] [PubMed] [Google Scholar]
- 49.Kanwisher N., Yovel G. 2006. The fusiform face area: a cortical region specialized for the perception of faces. Phil. Trans. R. Soc. B 361, 2109–2128 10.1098/rstb.2006.1934 (doi:10.1098/rstb.2006.1934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kovács G., Cziráki C., Vidnyánszky Z., Schweinberger S. R., Greenlee M. W. 2008. Position-specific and position-invariant face aftereffects reflect the adaptation of different cortical areas. Neuroimage 43, 156–164 10.1016/j.neuroimage.2008.06.042 (doi:10.1016/j.neuroimage.2008.06.042) [DOI] [PubMed] [Google Scholar]
- 51.Eger E., Henson R. N., Driver J., Dolan R. J. 2004. BOLD repetition decreases in object-responsive ventral visual areas depend on spatial attention. J. Neurophysiol. 92, 1241–1247 10.1152/jn.00206.2004 (doi:10.1152/jn.00206.2004) [DOI] [PubMed] [Google Scholar]
- 52.Rousselet G. A., Thorpe S. J., Fabre-Thorpe M. 2004. How parallel is visual processing in the ventral pathway? Trends Cogn. Sci. 8, 363–370 10.1016/j.tics.2004.06.003 (doi:10.1016/j.tics.2004.06.003) [DOI] [PubMed] [Google Scholar]
- 53.DiCarlo J. J., Maunsell J. H. R. 2003. Anterior inferior temporal neurons of monkeys engaged in object recognition can be highly sensitive to object retinal position. J. Neurophysiol. 89, 3264–3278 10.1152/jn.00358.2002 (doi:10.1152/jn.00358.2002) [DOI] [PubMed] [Google Scholar]
- 54.Op De Beeck H., Vogels R. 2000. Spatial sensitivity of macaque inferior temporal neurons. J. Comput. Neurol. 426, 505–518 10.1002/1096-9861(20001030)426 (doi:10.1002/1096-9861(20001030)426) [DOI] [PubMed] [Google Scholar]
- 55.Rolls E. T., Aggelopoulos N. C., Zheng F. 2003. The receptive fields of inferior temporal cortex neurons in natural scenes. J. Neurosci. 23, 339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kovács G., Zimmer M., Harza I., Antal A., Vidnyánszky Z. 2005. Position-specificity of facial adaptation. Neuroreport 16, 1945–1949 [DOI] [PubMed] [Google Scholar]
- 57.Xu H., Dayan P., Lipkin R. M., Qian N. 2008. Adaptation across the cortical hierarchy: low-level curve adaptation affects high-level facial-expression judgments. J. Neurosci. 28, 3374–3383 10.1523/JNEUROSCI.0182-08.2008 (doi:10.1523/JNEUROSCI.0182-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gauthier I., Skudlarski P., Gore J. C., Anderson A. W. 2000. Expertise for cars and birds recruits brain areas involved in face recognition. Nat. Neurosci. 3, 191–197 10.1038/72140 (doi:10.1038/72140) [DOI] [PubMed] [Google Scholar]
- 59.Pitcher D., Walsh V., Yovel G., Duchaine B. 2007. TMS evidence for the involvement of the right occipital face area in early face processing. Curr. Biol. 17, 1568–1573 10.1016/j.cub.2007.07.063 (doi:10.1016/j.cub.2007.07.063) [DOI] [PubMed] [Google Scholar]
- 60.Schiltz C., Rossion B. 2006. Faces are represented holistically in the human occipito-temporal cortex. Neuroimage 32, 1385–1394 10.1016/j.neuroimage.2006.05.037 (doi:10.1016/j.neuroimage.2006.05.037) [DOI] [PubMed] [Google Scholar]
- 61.Sorger B., Goebel R., Schiltz C., Rossion B. 2007. Understanding the functional neuroanatomy of acquired prosopagnosia. Neuroimage 35, 836–852 10.1016/j.neuroimage.2006.09.051 (doi:10.1016/j.neuroimage.2006.09.051) [DOI] [PubMed] [Google Scholar]
- 62.Hemond C. C., Kanwisher N. G., Op de Beeck H. P. 2007. A preference for contralateral stimuli in human object- and face-selective cortex. PLoS ONE 2, e574. 10.1371/journal.pone.0000574 (doi:10.1371/journal.pone.0000574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cavanagh P., Hunt A. R., Afraz A., Rolfs M. 2010. Visual stability based on remapping of attention pointers. Trends Cogn. Sci. 14, 147–153 10.1016/j.tics.2010.01.007 (doi:10.1016/j.tics.2010.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melcher D., Colby C. L. 2008. Trans-saccadic perception. Trends Cogn. Sci. 12, 466–473 10.1016/j.tics.2008.09.003 (doi:10.1016/j.tics.2008.09.003) [DOI] [PubMed] [Google Scholar]
- 65.Wurtz R. H. 2008. Neuronal mechanisms of visual stability. Vis. Res. 48, 2070–2089 10.1016/j.visres.2008.03.021 (doi:10.1016/j.visres.2008.03.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cha O., Chong S. C. In press. Background is remapped across saccades. J. Vis. 10 10.1167/10.7.516 (doi:10.1167/10.7.516) (VSS abstracts) [DOI] [PubMed] [Google Scholar]
- 67.Melcher D. 2005. Spatiotopic transfer of visual-form adaptation across saccadic eye movements. Curr. Biol. 15, 1745–1748 10.1016/j.cub.2005.08.044 (doi:10.1016/j.cub.2005.08.044) [DOI] [PubMed] [Google Scholar]
- 68.Afraz S. R., Kiani R., Vaziri-Pashkam M., Esteky H. 2004. Motion-induced overestimation of the number of items in a display. Perception 33, 915–925 10.1068/p5296 (doi:10.1068/p5296) [DOI] [PubMed] [Google Scholar]
- 69.Ezatti A., Golzar A., Afraz A. S. R. 2008. Topography of the motion aftereffect with and without eye movements. J. Vis. 8, 1–16 10.1167/8.14.23 (doi:10.1167/8.14.23) [DOI] [PubMed] [Google Scholar]
- 70.Burr D., Tozzi A., Morrone M. C. 2007. Neural mechanisms for timing visual events are spatially selective in real-world coordinates. Nat. Neurosci. 10, 423–425 10.1038/nn1874 (doi:10.1038/nn1874) [DOI] [PubMed] [Google Scholar]
- 71.van Boxtel J. J. A., Alais D., van Ee R. 2008. Retinotopic and non-retinotopic stimulus encoding in binocular rivalry and the involvement of feedback. J. Vis. 8, 1–10 10.1167/8.5.17 (doi:10.1167/8.5.17) [DOI] [PubMed] [Google Scholar]
- 72.Afraz A., Cavanagh P. 2009. The gender-specific face aftereffect is based in retinotopic not spatiotopic coordianets across several natural image transformations. J. Vis. 9, 1–17 10.1167/9.10.10 (doi:10.1167/9.10.10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O'Toole A. J., Vetter T., Blanz V. 1999. Three-dimensional shape and two-dimensional surface reflectance contributions to face recognition: an application of three-dimensional morphing. Vis. Res. 39, 3145–3155 10.1016/S0042-6989(99)00034-6 (doi:10.1016/S0042-6989(99)00034-6) [DOI] [PubMed] [Google Scholar]
- 74.Bruce V., Young A. 1986. Understanding face recognition. Br. J. Psychol. 77, 305–327 [DOI] [PubMed] [Google Scholar]
- 75.Druzgal T. J., D'Esposito M. 2001. Activity in fusiform face area modulated as a function of working memory load. Brain Res. Cogn. Brain Res. 10, 355–364 10.1016/S0926-6410(00)00056-2 (doi:10.1016/S0926-6410(00)00056-2) [DOI] [PubMed] [Google Scholar]
- 76.Golarai G., Hong S., Haas B. W., Galaburda A. M., Mills D. L., Bellugi U., Grill-Spector K., Reiss A. L. 2010. The fusiform face area is enlarged in Williams syndrome. J. Neurosci. 30, 6700–6712 10.1523/JNEUROSCI.4268-09.2010 (doi:10.1523/JNEUROSCI.4268-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golby A. J., Gabrieli J. D., Chiao J. Y., Eberhardt J. L. 2001. Differential responses in the fusiform region to same-race and other-race faces. Nat. Neurosci. 4, 845–850 10.1038/nn90565 (doi:10.1038/nn90565) [DOI] [PubMed] [Google Scholar]
- 78.Nichols E. A., Kao Y. C., Verfaellie M., Gabrieli J. D. 2006. Working memory and long-term memory for faces: evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus 16, 604–616 10.1002/hipo.20190 (doi:10.1002/hipo.20190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ranganath C., DeGutis J., D'Esposito M. 2004. Category-specific modulation of inferior temporal activity during working memory encoding and maintenance. Brain Res. Cogn. Brain Res. 20, 37–45 10.1016/j.cogbrainres.2003.11.017 (doi:10.1016/j.cogbrainres.2003.11.017) [DOI] [PubMed] [Google Scholar]
- 80.Tranel D., Damasio A. R., Damasio H. 1988. Intact recognition of facial expression, gender, and age in patients with impaired recognition of face identity. Neurology 38, 690–696 [DOI] [PubMed] [Google Scholar]
- 81.Ishai A. 2008. Let's face it: it's a cortical network! Neuroimage 40, 415–419 10.1016/j.neuroimage.2007.10.040 (doi:10.1016/j.neuroimage.2007.10.040) [DOI] [PubMed] [Google Scholar]
- 82.Ng M., Ciaramitaro V. M., Anstis S., Boynton G. M., Fine I. 2006. Selectivity for the configural cues that identify the gender, ethnicity, and identity of faces in human cortex. Proc. Natl Acad. Sci. USA 103, 19 552–19 557 10.1073/pnas.0605358104 (doi:10.1073/pnas.0605358104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.d'Avossa G., Tosetti M., Crespi S., Biagi L., Burr D. C., Morrone M. C. 2007. Spatiotopic selectivity of BOLD responses to visual motion in human area MT. Nat. Neurosci. 10, 249–255 10.1038/nn1824 (doi:10.1038/nn1824) [DOI] [PubMed] [Google Scholar]
- 84.Goossens J., Dukelow S. P., Menon R. S., Vilis T., van den Berg A. 2006. Representation of head-centric flow in the human motion complex. J. Neurosci. 26, 5616–5627 10.1523/JNEUROSCI.0730-06.2006 (doi:10.1523/JNEUROSCI.0730-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kimmig H., Ohlendorf S., Speck O., Sprenger A., Rutschmann R. M., Haller S., Greenlee M. W. 2008. fMRI evidence for sensorimotor transformations in human cortex during smooth pursuit eye-movements. Neuropsychologia 46, 2203–2213 10.1016/j.neuropsychologia.2008.02.021 (doi:10.1016/j.neuropsychologia.2008.02.021) [DOI] [PubMed] [Google Scholar]
- 86.Ong W. S., Hooshvar N., Zhang M., Bisley J. V. 2009. Psychological evidence for spatiotopic processing in area MT in a short-term memory for motion task. J. Neurophysiol. 102, 2435–2440 10.1152/jn.00684.2009 (doi:10.1152/jn.00684.2009) [DOI] [PubMed] [Google Scholar]
- 87.Gardner J. L., Merriam E. P., Movshon J. A., Heeger D. J. 2008. Maps of visual space in human occipital cortex are retinotopic, not spatiotopic. J. Neurosci. 28, 3988–3999 10.1523/JNEUROSCI.5476-07.2008 (doi:10.1523/JNEUROSCI.5476-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Merriam E. P., Genovese C. R., Colby C. L. 2007. Remapping in human visual cortex. J. Neurophysiol. 97, 1738–1755 10.1152/jn.00189.2006 (doi:10.1152/jn.00189.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McKyton A., Zohary E. 2007. Beyond retinotopic mapping: the spatial representation of objects in the human lateral occipital cortex. Cerebral Cortex 17, 1164–1172 10.1093/cercor/bhl027 (doi:10.1093/cercor/bhl027) [DOI] [PubMed] [Google Scholar]
- 90.Sereno M. I., Huang R. S. 2006. A human parietal face area contains aligned head-centered visual and tactile maps. Nat. Neurosci. 9, 1337–1343 10.1038/nn1777 (doi:10.1038/nn1777) [DOI] [PubMed] [Google Scholar]
- 91.Greenlee M. W., Georgeson M. A., Magnussen S., Harris J. P. 1999. The time course of adaptation to spatial contrast. Vis. Res. 31, 223–236 10.1016/0042-6989(91)90113-J (doi:10.1016/0042-6989(91)90113-J) [DOI] [PubMed] [Google Scholar]
- 92.Leopold D. A., Rhodes G., Müller K. M., Jeffrey L. 2005. The dynamics of visual adaptation to faces. Proc. R. Soc. B 272, 897–904 10.1098/rspb.2004.3022 (doi:10.1098/rspb.2004.3022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rhodes G., Jeffery L., Clifford C. W. G., Leopold D. A. 2007. The timecourse of higher-level face aftereffects. Vis. Res. 47, 2291–2296 10.1016/j.visres.2007.05.012 (doi:10.1016/j.visres.2007.05.012) [DOI] [PubMed] [Google Scholar]
- 94.Fang F., Murray S. O., Kersten D., He S. 2005. Orientation-tuned fMRI adaptation in human visual cortex. J. Neurophysiol. 94, 4188–4195 10.1152/jn.00378.2005 (doi:10.1152/jn.00378.2005) [DOI] [PubMed] [Google Scholar]
- 95.Henson R. N. A. 2003. Neuroimaging studies of priming. Progr. Neurobiol. 70, 53–81 10.1016/S0301-0082(03)00086-8 (doi:10.1016/S0301-0082(03)00086-8) [DOI] [PubMed] [Google Scholar]
- 96.Daelli V., van Rijsbergen N. J., Treves A. 2010. How recent experience affects the perception of ambiguous objects. Brain Res. 1322, 81–91 10.1016/j.brainres.2010.01.060 (doi:10.1016/j.brainres.2010.01.060) [DOI] [PubMed] [Google Scholar]
- 97.Burr D. C., Morrone M. C. 2011. Spatiotopic coding and remapping in humans. Phil. Trans. R. Soc. B 366, 504–515 10.1098/rstb.2010.0244 (doi:10.1098/rstb.2010.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prime S. L., Vesia M., Crawford J. D. 2008. Transcranial magnetic stimulation over posterior parietal cortex disrupts transsaccadic memory of multiple objects. J. Neurosci. 28, 6938–6949 10.1523/JNEUROSCI.0542-08.2008 (doi:10.1523/JNEUROSCI.0542-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pisella L., Alahyane N., Blangero A., Thery F., Blanc S., Pelisson D. 2011. Right-hemispheric dominance for visual remapping in humans. Phil. Trans. R. Soc. B 366, 572–585 10.1098/rstb.2010.0258 (doi:10.1098/rstb.2010.0258) [DOI] [PMC free article] [PubMed] [Google Scholar]