Abstract
Oceanic rafting is thought to play a fundamental role in assembling the biological communities of isolated coastal ecosystems. Direct observations of this key ecological and evolutionary process are, however, critically lacking. The importance of macroalgal rafting as a dispersal mechanism has remained uncertain, largely owing to lack of knowledge about the capacity of fauna to survive long voyages at sea and successfully make landfall and establish. Here, we directly document the rafting of a diverse assemblage of intertidal organisms across several hundred kilometres of open ocean, from the subantarctic to mainland New Zealand. Multispecies analyses using phylogeographic and ecological data indicate that 10 epifaunal invertebrate species rafted on six large bull kelp specimens for several weeks from the subantarctic Auckland and/or Snares Islands to the Otago coast of New Zealand, a minimum distance of some 400–600 km. These genetic data are the first to demonstrate that passive rafting can enable simultaneous trans-oceanic transport and landfall of numerous coastal taxa.
Keywords: dispersal, biogeography, macroalgae, drifting, invertebrate, marine
1. Introduction
Biologists have long speculated that passive rafting plays a fundamental role in assembling isolated (e.g. island) biotas [1–4], with the wide distributions of many ‘non-dispersive’ coastal species frequently accredited to this phenomenon [5]. Direct observations of rafting fauna, however, have been extremely rare [6–9], and the origins [7,8] and destinations [6,9] of rafts have remained largely speculative. Indeed, the importance of rafting is more typically invoked on the basis of observations of detached macroalgae and other flotsam at sea [10–20], or macroalgal fragments washed ashore [21,22]. Numerous studies have, additionally, inferred rafting dispersal on the basis of circumstantial evidence from biogeographic range data [10,23,24] or from evidence of genetic similarity among disjunct coastal populations [25–29]. Our genetic study is the first to explicitly demonstrate a trans-oceanic origin for rafted, beach-cast organisms.
The biotic communities associated with southern bull kelp provide a highly informative system for studies of trans-oceanic rafting. These isolated coastal benthic communities are linked primarily by the Antarctic Circumpolar Current (ACC, also known as the West Wind Drift), a strong, circumpolar current driven by prevailing westerly winds at subantarctic latitudes. Vast quantities of detached bull kelp have been observed floating in the path of the ACC [18], and apparently have potential to drift for long distances [11,18,21,28]. The holdfasts (basal attachment discs) of bull kelp are hollowed-out and occupied by diverse invertebrate taxa, which are consequently hypothesized to inherit some of the dispersal potential of their macroalgal host [5,18,29,30].
Six large specimens of detached southern bull kelp (Durvillaea antarctica), and their invertebrate passengers (molluscan, arthropod and echinoderm taxa), washed ashore on St Clair Beach in mainland New Zealand in 2009–2010 (with five arriving in February 2009, and one in May 2010). We conducted genetic analyses of these bull kelp specimens, and of one of the invertebrate taxa associated with them, to determine the source population/s of the rafted communities.
2. Methods
(a). Site and sample collection
Goose barnacles of the genus Lepas are pelagic, and only colonize floating objects at sea, such as driftwood or buoys [31,32]. Weekly inspection of beach-cast bull kelp (wrack) at St Clair Beach, South Island (New Zealand), typically reveals D. antarctica plants completely lacking or bearing only small goose barnacles (C. I. Fraser 2006–2010, personal observation; figure 1a), indicating a short duration at sea following local detachment [19]. By contrast, five complete specimens of beach-cast D. antarctica covered in notably large Lepas australis (figure 1b) were found at St Clair Beach on 26 February 2009, and a sixth such specimen was collected on 30 May 2010. Although numerous other bull kelp specimens were found on St Clair Beach on the sampling dates, only those with large (greater than 10 mm capitulum length) goose barnacles were collected.
Figure 1.
Bull kelp wrack at St Clair Beach, New Zealand. (a) Photograph of a detached, beach-cast bull kelp specimen at St Clair Beach in March 2009 (not one of the rafts that formed the focus of this study). Bull kelp specimens are frequently deposited on St Clair Beach in southeastern New Zealand, but generally show no signs of lengthy oceanic drifting. For example, this specimen is estimated to have spent only a short period adrift, owing to complete absence of goose barnacles (Lepas sp.) that rapidly colonize buoyant objects at sea. (b) One of the six bull kelp wrack specimens of subantarctic origin. Encrustations of Lepas sp. of various sizes are visible on the holdfast and stipes of the bull kelp. (Photograph taken at St Clair Beach on 26 February 2009.) Scale bars, (a) 500 mm and (b) 50 mm.
Pieces of bull kelp frond tissue were preserved in 95 per cent ethanol in the field. Most of the bull kelp wrack specimens had multiple stipes but conjoined holdfasts (figure 1), indicating that each ‘specimen’ in fact comprised multiple bull kelp individuals. Whereas only one tissue sample was taken from each specimen for DNA analysis from the samples collected in February 2009 (wracks nos 1–5), a sample was taken from each of the four individuals that made up the specimen that arrived in May 2010 (wrack no. 6). Each bull kelp holdfast was placed in a plastic bag in the field and taken to the laboratory for dissection. Holdfasts were dissected using a large knife, with each cut into slices of approximately 20 mm width. Holdfast slices were inspected carefully by eye, and all invertebrates were removed using forceps and preserved in 95 per cent ethanol.
(b). Genetic analyses
DNA was extracted and partial sequences obtained for two genes for bull kelp: mitochondrial cytochrome c oxidase subunit I (COI) and chloroplast ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (rbcL) following the protocols provided in Fraser et al. [33]. Partial mitochondrial COI sequences were obtained for Limnoria following the protocols provided in Nikula et al. [29]. Phylogenetic analyses of D. antarctica and Limnoria included previously published sequence data [28,29] as well as the wrack samples collected in 2009–2010. Sampling details of all attached bull kelp [28] and associated benthic invertebrate communities [29] are summarized in the electronic supplementary material, tables S1–S2. Published sequences of Fucus vesiculosus (GenBank accession no. AY494079) and congeneric taxa Durvillaea potatorum (FJ873092) and Durvillaea willana (EU918569) were included as outgroups in bull kelp analyses. Outgroup taxa used in the phylogenetic analysis of Limnoria were the isopods Ligia hawaiensis (GenBank accession no. AY051329) and Munna sp. (GenBank accession no. HQ161066). Maximum likelihood (ML) phylogenetic trees were constructed using PhyML [34], applying the best-fit substitution model as determined by Modeltest 3.06 [35] based on AIC (model parameters are given in electronic supplementary material, table S3). Robustness of the tree topologies was evaluated with 1000 replicate bootstrap analyses using PhyML [34], and with Bayesian posterior probability (PP) estimations in MrBayes 3.1.2 [36] that used four MCMC chains of 5 000 000 generations, sampled trees each 100 generations and discarded the first 10 000 trees as burn-in. All unique DNA sequences determined during this study were deposited with GenBank (electronic supplementary material, table S1).
(c). Goose barnacle analyses
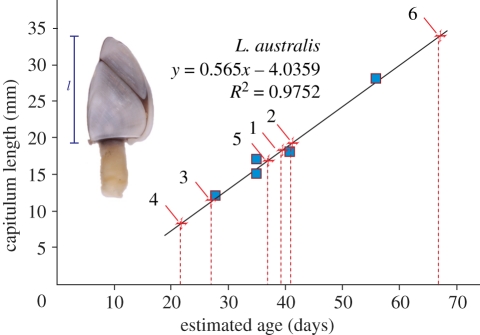
The duration of a macroalgal rafting event can potentially be estimated by analysis of the size of attached goose barnacles (Lepas sp.) that rapidly colonize flotsam in the marine environment [19,31,32,37]. For each bull kelp specimen, the 10 largest Lepas barnacles were removed and preserved in 95 per cent ethanol. Capitulum length of each Lepas was measured using digital vernier callipers following Hinojosa et al. [32]. The age of the largest barnacle from each bull kelp specimen—an indicator of minimum rafting duration—was estimated using published growth rates for L. australis in waters off New Zealand's South Island [31] (figure 2).
Figure 2.
Estimation of goose barnacle age. Blue squares: estimated age plotted against capitulum length for L. australis in southeastern New Zealand waters, compiled using published data [31]. ‘Estimated age’ refers to the length of time floating beacons had spent adrift before collection and measurement of barnacles. Red crosses: capitulum lengths (l) of the largest L. australis from each of the six bull kelp wrack samples plotted against ages derived from the regression equation. The estimated age (in days) for each wrack specimen is: 67.7, 40.7, 39.2, 37.2, 27.1, 22.4. Wrack specimen identification numbers are shown. Upper-left image overlay, L. australis from bull kelp wrack (length approximately scaled to the y-axis).
3. Results
(a). Genetic analyses
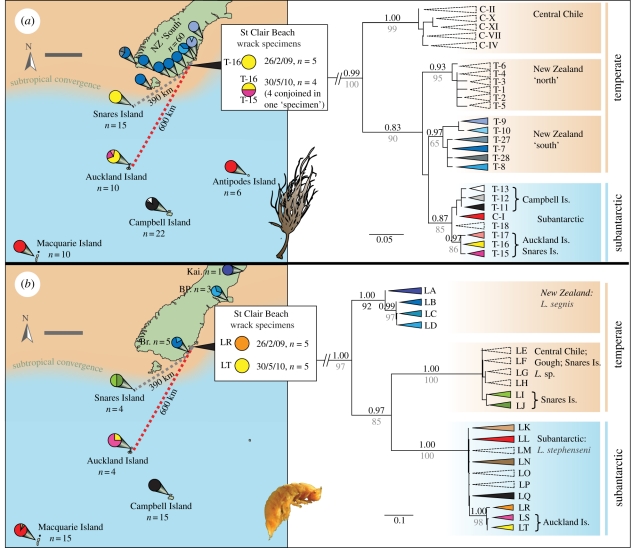
When compared with our Southern Hemisphere-wide genetic survey of D. antarctica [28], the mitochondrial (COI) and chloroplast (rbcL) DNA sequences of the beach-cast specimens show that the six bull kelp plants collected (those bearing large goose barnacles) all belong to an exclusively ‘subantarctic’ genetic lineage. This lineage is highly distinct from all mainland New Zealand lineages ([28,33]; figure 3 and electronic supplementary material, figure S1). More specifically, the COI haplotype shared by all six wrack specimens (haplotype T-16) has only been detected at two subantarctic island groups (Auckland Islands and Snares Islands [28]), and is distinct from haplotypes found at other subantarctic locations (including Campbell, Macquarie, Antipodes, Bounty, Kerguelen, Crozet, Marion, Gough and South Georgia islands). Of the four bull kelp individuals that comprised specimen no. 6 (collected in May 2010), two yielded the COI haplotype shared among the Snares and Auckland Islands, whereas two yielded a haplotype thus far only detected from the Auckland Islands (haplotype T-15 figure 3). Similar results were obtained for chloroplast DNA, with three rbcL haplotypes detected among the six wrack specimens. One rbcL haplotype has previously been found only on the Snares and Auckland Islands (haplotype T-R-6; wrack specimen nos 1, 2 and 5), another only on the Auckland Islands (haplotype T-R-7; wrack specimen no. 6, all four individuals) and the third (haplotype T-R-15; wrack specimen nos 3 and 4) has not been previously detected but is closely related to the other two (electronic supplementary material, table S1 and figure S1).
Figure 3.
Genetic (mtDNA) evidence of a subantarctic source for beach-cast bull kelp specimens found on mainland New Zealand. (a,b): Left panels: map of the southern New Zealand region including subantarctic islands. Haplotype distributions and proportions for COI for (a) bull kelp, D. antarctica, and (b) the isopod Limnoria are shown as pie charts emerging from each site (site codes for mainland New Zealand: Kai, Kaikoura; BP, Banks Peninsula; Br, Brighton). Minimum-distance rafting trajectories from the Snares and the Auckland islands to St Clair Beach, New Zealand, are shown by dashed lines, with distances indicated. The approximate location of the subtropical convergence is shown. Right panels: phylogeographic relationships based on COI data for (a) bull kelp, D. antarctica and (b) the isopod Limnoria. Node support values of the major, well-supported branches are shown, with Bayesian PP values above and ML bootstrap values (in grey) below. Outgroups have been trimmed for clarity. Scale bars, (a,b) 200 km.
In addition to information derived from the DNA analysis of the bull kelp wrack specimens themselves (above), their geographical origins may be elucidated by genetic characterization of associated epifaunal invertebrates. Several species from the isopod genus Limnoria are obligatorily algal-associated, and may spend their entire lives boring into macroalgal holdfasts [38–40]. Limnoria isopods secured from cavities of the wrack holdfasts (specimen nos 3, 5 and 6) were genetically highly divergent from all mainland New Zealand Limnoria haplotypes (Limnoria segnis), and instead grouped with the widespread subantarctic Limnoria stephenseni (figure 3b). In particular, the mtDNA haplotypes of the rafted Limnoria were either identical or closely related to haplotypes that have only been detected from the Auckland Islands. All specimens of Limnoria collected from D. antarctica holdfasts from the Snares Islands, on the other hand, form a highly divergent mtDNA clade with specimens from central Chile and Gough Island (electronic supplementary material, table S1 and figure 3b: haplotypes LE–LJ).
Aside from the epifaunal Limnoria specimens and the abundant Lepas barnacles that colonize detached macroalgae via a planktonic larval phase [32], the bull kelp wrack specimens housed nine other macroinvertebrate taxa that were still alive upon landfall in New Zealand: one amphipod species (Parawaldeckia karaka); a pycnogonid (Tanystylum antipodum); six molluscan taxa (the bivalve Kidderia sp.; the gastropods Cantharidus (‘Margarella’) rosea and Cellana strigilis; the chitons Onithochiton neglectus—a brooder—and Plaxiphora boydeni; and the nudibranch Fiona pinnata); and an echinoderm (the rocky-reef sea-star Stichaster australis) (for abundance data, see electronic supplementary material, table S4). All of these taxa have distributions encompassing both southern mainland New Zealand and some of the New Zealand southern/subantarctic islands. All are benthic, with the exceptions of L. australis and F. pinnata, which colonize floating pelagic substrata. Preliminary genetic data (not shown) suggest there is no diagnostic mtDNA differentiation across the New Zealand subantarctic and mainland ranges of C. rosea, O. neglectus and P. karaka, making these taxa uninformative in determining geographical origins of beach-cast bull kelp specimens. The limpet C. strigilis was apparently a juvenile (shell length less than 2 mm), and was identified by comparison of an mtDNA (COI) sequence with sequences available on GenBank. Furthermore, this individual shared an identical 16S haplotype with specimens recently detected within bull kelp holdfasts from Campbell Island and in holdfasts of a large composite (Macrocystis pyrifera and D. antarctica) kelp raft drifting 13 km off Campbell Island (data not shown).
(b). Goose barnacle analyses
On the basis of capitulum growth rates derived from surveys of L. australis off eastern New Zealand (approximately 0.5 mm per day [31]), and the maximum size observed for L. australis on the beach-cast D. antarctica specimens, we infer that the five D. antarctica plants with the largest goose barnacles collected in February 2009 had spent a minimum of 20–40 days at sea before washing ashore, while the specimen collected in May 2010 had been at sea for at least 65 days. These estimates assume that colonization of drifting bull kelp by Lepas is as rapid on macroalgae as it was observed to be on the steel/wood beacons used in Skerman's [31] experimental study. Goose barnacles (Lepas sp.) are purely pelagic, colonizing drifting objects at sea [32], and have never, to our knowledge, been observed growing on attached bull kelp.
4. Discussion
(a). Geographical origins of the rafts
Our genetic analyses of beach-cast bull kelp (D. antarctica) and their isopod (L. stephenseni) passengers show that the wrack specimens recovered from St Clair Beach in February 2009 and May 2010 were undoubtedly of subantarctic origin, and had drifted at least 390 km (from the Snares Islands) or 600 km (from the Auckland Islands). The strong buoyancy of D. antarctica means that wind, in addition to water movement, is likely to play a major role in determining the geographical fate of drifting specimens [41–43]. Although southern New Zealand is broadly dominated by westerly winds [44], summer months can be marked by prevailing easterlies that periodically deposit macroalgae onto the eastern shores of the South Island [45], as during mid to late February 2009 (electronic supplementary material, figure S2). Indeed, a recent oceanographic modelling study based on empirical data from satellite drifters [46] showed that buoyant objects originating from the Auckland and Snares Islands are likely to be driven northwards to arrive on New Zealand mainland shores. By contrast, the same study showed that drifters from other subantarctic sources (e.g. Campbell, Antipodes, Bounty and Chatham islands) tend to be driven away from New Zealand [46]. Genetic data from bull kelp specimen no. 6 strongly suggest the Auckland Islands as its source (two COI and four rbcL haplotypes from specimen no. 6 were previously detected only from the Auckland Islands). Furthermore, data from L. stephenseni collected from three of the beach-cast specimens (nos 3, 5 and 6) also promote the Auckland Islands as the most likely source population. Bull kelp holdfasts around New Zealand's South Island are inhabited by L. segnis, whereas subantarctic bull kelp populations primarily host L. stephenseni [39,29]; the former species has been recorded only from New Zealand, and the latter only from the subantarctic region [38]. Despite being common across the subantarctic, L. stephenseni has never been recorded from the Snares Islands, although several other species of algal-associated Limnoria have [38]. The epifaunal genetic data are therefore compatible with wrack specimens nos 3, 5 and 6 originating from the Auckland Islands. In contrast, the sea-star S. australis has previously been recorded from the Snares Islands [47], but not yet from the Auckland Islands. At least one of the wrack specimens may, therefore, have originated from the Snares Islands. Buoyant material has a tendency to accumulate in ‘windrows’ (along the lines formed by near-surface oceanographic features called Langmuir cells [48]) and drifting macroalgae from different origins may therefore meet at sea and become entangled, continuing the journey together to arrive at a common destination. We propose that the beach-cast bull kelp specimens that form the focus of this study originated from both the Snares and Auckland Islands, and were driven by wind and oceanographic features to arrive at St Clair Beach roughly simultaneously.
(b). Speed of travel
The maximum sizes of goose barnacles (L. australis) attached to the bull kelp wrack indicate a drifting duration of at least 20–40 days for the specimens collected in February 2009, and at least 65 days for the specimens collected in May 2010. If the bull kelp wrack specimens carrying the smaller Lepas (estimated to be about 20 days old) originated from the closest possible source (the Snares Islands), and if they travelled in a direct line from origin to destination, they must have drifted at an average speed of about 0.2 m s−1. Alternatively, if these bull kelp specimens originated from the Auckland Islands, their average speed must have exceeded 0.3 m s−1. With neither currents nor wind likely to have driven them directly from origin to destination, the drifting bull kelp would almost certainly have travelled considerably greater distances than the minimum, straight-line trajectories shown in figure 3, and at much greater speeds than those estimated above. Regardless, the speeds and durations of these inferred rafting events are consistent with a recent oceanographic modelling study which indicates that passively drifting objects originating from the Auckland Islands can reach southeastern South Island within 30–50 days of departure [46]. A study by Coombs and Landis [49] showed that pumice from a volcanic eruption in the South Sandwich Islands (east of the southern tip of South America) began to wash up on New Zealand shores 530 days after the eruption, and must therefore have travelled at a speed of around 0.3 m s−1. Similarly, a review of records of drifting objects at sea by Thiel and Gutow [42] concluded that most travel at an average speed of approximately 0.1–0.3 m s−1. The longest distance between any two land masses with D. antarctica populations in the path of the ACC is approximately 8000 km, from New Zealand to the southern tip of South America. With drifting D. antarctica clearly able to travel at a comparable speed to the pumice noted by Coombs and Landis [49], detached bull kelp could potentially traverse the distance between, for example, New Zealand and South America in a little under a year, or the distance between Gough Island (Atlantic Ocean) and the Kerguelen Islands (Indian Ocean) in three or four months. Such durations may well be within the lifespans of their invertebrate passengers, and/or subsequent generations of those that brood their offspring within the bull kelp holdfasts (e.g. Limnoria [40]).
(c). Rafting realized
The ecological and evolutionary importance of macroalgal rafting as a dispersal mechanism has long remained uncertain, largely owing to lack of knowledge regarding the capacity of fauna to: (i) survive long voyages at sea and (ii) successfully make landfall and establish [5,18]. Recent research has demonstrated that, in spite of faunal abundance dropping sharply in macroalgal holdfasts within minutes of detachment, there is little change in the number of passengers during subsequent hours, indicating a high rafting potential of those that remain [50,51]. The level of epifaunal diversity we detected in the bull kelp wrack specimens parallels that observed in an experimental epifaunal study of artificially detached, moored D. antarctica [30], and shows that successful long-distance transportation is indeed possible for a broad suite of algal-associated marine invertebrate taxa.
Our results demonstrate survival of numerous taxa during the rafting journey and following landfall, but successful colonization of a new territory also relies on establishment of immigrants in their new environment. Negligible mtDNA differentiation between subantarctic and mainland populations of the bull kelp-associated taxa C. rosea, O. neglectus and P. karaka (of which the latter two are brooders) suggests that rafting may indeed facilitate dispersal of these taxa. Similarly, the wide (subantarctic and mainland) distribution of the brooding pycnogonid T. antipodum [52] and of the chiton P. boydeni [53] suggests these species may also use bull kelp for long-distance dispersal and migration. A recent phylogeographic study of the limpet C. strigilis showed low mtDNA diversity among sites in southern New Zealand and the New Zealand subantarctic [54]. Our discovery of a juvenile C. strigilis on wrack specimen no. 6, as well as numerous juveniles on bull kelp drifting offshore close to Campbell Island, and within bull kelp holdfasts at Campbell Island, suggests that drifting bull kelp may facilitate population connectivity of this species. Conversely, the absence of subantarctic Limnoria and Durvillaea genetic lineages around mainland New Zealand implies that establishment of new immigrants of these taxa can be limited by extrinsic factors, such as temperature, or by the presence of pre-existing conspecific or congeneric populations [28]. Success also necessarily depends on landfall being made at a suitable location; invertebrates from the six rafts studied here, for example, would have been unlikely to survive, as they were deposited on a sandy beach rather than rocky shore habitat. Broadly, however, our study demonstrates an important ecological mechanism that is likely to have facilitated the post-glacial recolonization of subantarctic coasts following the Last Glacial Maximum [28,29].
Acknowledgements
We thank all those who assisted with algal and invertebrate collections that formed the basis of the previously published work this article builds on. Thanks to H. Haazen (Waterline Yachts) and the crew of the Tiama for facilitating many subantarctic collections; the New Zealand Department of Conservation for permits to collect from the New Zealand subantarctic islands; A. Paradkar for field assistance and advice; R. Cumming for field assistance and collections from Puysegur Point; T. King for laboratory assistance; M. Kennedy for analytical advice; J. Lawn for collection and sequencing of some Limnoria samples from Brighton; B. Marshall, R. Willan, K. Donald and H. Spencer for mollusc identification; C. Arango for identifying the pycnogonid; D. Craw for examining clasts from holdfasts; N. Kilgallen for examining amphipods; M. Thiel, C. Collins and S. Smith for insights during our research; O. Errington for technical help with figure production; and K. Miller for Lepas photography. This work was funded by Marsden contract 07-UOO-099, Department of Zoology and University of Otago Research grants to J.M.W. and a postdoctoral fellowship for C.I.F. from the Allan Wilson Center for Molecular Ecology and Evolution.
References
- 1.Darwin C. R. 1872. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life, 6th edn. London, UK: John Murray; [PMC free article] [PubMed] [Google Scholar]
- 2.Kinlan B. P., Gaines S. D. 2003. Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 84, 2007–2020 10.1890/01-0622 (doi:10.1890/01-0622) [DOI] [Google Scholar]
- 3.Whittaker R. J., Fernàndez-Palacios J. M. 2007. Island biogeography: ecology, evolution, and conservation. Oxford, UK: Oxford University Press [Google Scholar]
- 4.Bellemain E., Ricklefs R. E. 2008. Are islands the end of the colonization road? Trends Ecol. Evol. 23, 461–468 10.1016/j.tree.2008.05.001 (doi:10.1016/j.tree.2008.05.001) [DOI] [PubMed] [Google Scholar]
- 5.Thiel M., Haye P. A. 2006. The ecology of rafting in the marine environment. III. Biogeographical and evolutionary consequences. Oceanogr. Mar. Biol. Annu. Rev. 44, 323–429 [Google Scholar]
- 6.Wheeler W. M. 1916. Ants carried in a floating log from the Brazilian coast to San Sebastian Island. Psyche 28, 180–183 [Google Scholar]
- 7.Heatwole H., Levins R. 1972. Biogeography of the Puerto Rican Bank—flotsam transport of terrestrial animals. Ecology 53, 112–117 10.2307/1935715 (doi:10.2307/1935715) [DOI] [Google Scholar]
- 8.Helmuth B., Veit R. R., Holberton R. 1994. Long-distance dispersal of a subantarctic brooding bivalve (Gaimardia trapesina) by kelp rafting. Mar. Biol. 120, 421–426 10.1007/BF00680216 (doi:10.1007/BF00680216) [DOI] [Google Scholar]
- 9.Censky E. J., Hodge K., Dudley J. 1998. Over-water dispersal of lizards due to hurricanes. Nature 395, 556–556 10.1038/26886 (doi:10.1038/26886) [DOI] [Google Scholar]
- 10.Highsmith R. C. 1985. Floating and algal rafting as potential dispersal mechanisms in brooding invertebrates. Mar. Ecol. Prog. Ser. 25, 169–179 10.3354/meps025169 (doi:10.3354/meps025169) [DOI] [Google Scholar]
- 11.Edgar G. J. 1987. Dispersal of faunal and floral propagules associated with drifting Macrocystis pyrifera plants. Mar. Biol. 95, 599–610 10.1007/BF00393104 (doi:10.1007/BF00393104) [DOI] [Google Scholar]
- 12.Davenport J., Rees E. I. S. 1993. Observations on neuston and floating weed patches in the Irish Sea. Estuar. Coast. Shelf Sci. 36, 395–411 10.1006/ecss.1993.1024 (doi:10.1006/ecss.1993.1024) [DOI] [Google Scholar]
- 13.Holmquist J. G. 1994. Benthic macroalgae as a dispersal mechanism for fauna—influence of a marine tumbleweed. J. Exp. Mar. Biol. Ecol. 180, 235–251 10.1016/0022-0981(94)90069-8 (doi:10.1016/0022-0981(94)90069-8) [DOI] [Google Scholar]
- 14.Ingólfsson A. 1995. Floating clumps of seaweed around Iceland—natural microcosms and a means of dispersal for shore fauna. Mar. Biol. 122, 13–21 10.1007/BF00349273 (doi:10.1007/BF00349273) [DOI] [Google Scholar]
- 15.Ingólfsson A. 1998. Dynamics of macrofaunal communities of floating seaweed clumps off western Iceland: a study of patches on the surface of the sea. J. Exp. Mar. Biol. Ecol. 231, 119–137 10.1016/S0022-0981(98)00089-6 (doi:10.1016/S0022-0981(98)00089-6) [DOI] [Google Scholar]
- 16.Hobday A. J. 2000. Persistence and transport of fauna on drifting kelp (Macrocystis pyrifera (L.) C. Agardh) rafts in the Southern California Bight. J. Exp. Mar. Biol. Ecol. 253, 75–96 10.1016/S0022-0981(00)00250-1 (doi:10.1016/S0022-0981(00)00250-1) [DOI] [PubMed] [Google Scholar]
- 17.Ólafsson E., Ingólfsson A., Steinarsdóttir M. B. 2001. Harpacticoid copepod communities of floating seaweed: controlling factors and implications for dispersal. Hydrobiologia 453, 189–200 [Google Scholar]
- 18.Smith S. D. A. 2002. Kelp rafts in the Southern Ocean. Glob. Ecol. Biogeogr. 11, 67–69 10.1046/j.1466-822X.2001.00259.x (doi:10.1046/j.1466-822X.2001.00259.x) [DOI] [Google Scholar]
- 19.Macaya E. C., et al. 2005. Presence of sporophylls in floating kelp rafts of Macrocystis spp. (Phaeophyceae) along the Chilean Pacific coast. J. Phycol. 41, 913–922 10.1111/j.1529-8817.2005.00118.x (doi:10.1111/j.1529-8817.2005.00118.x) [DOI] [Google Scholar]
- 20.Hernandez-Carmona G., Hughes B., Graham M. H. 2006. Reproductive longevity of drifting kelp Macrocystis pyrifera (Phaeophyceae) in Monterey Bay, USA. J. Phycol. 42, 1199–1207 10.1111/j.1529-8817.2006.00290.x (doi:10.1111/j.1529-8817.2006.00290.x) [DOI] [Google Scholar]
- 21.Moore L. B., Cribb A. B. 1952. The brown alga Durvillea antarctica in Australian waters. Nature 169, 1100–1101 10.1038/1691100a0 (doi:10.1038/1691100a0) [DOI] [PubMed] [Google Scholar]
- 22.McKenzie P. F., Bellgrove A. 2008. Dispersal of Hormosira banksii (Phaeophyceae) via detached fragments: reproductive viability and longevity. J. Phycol. 44, 1108–1115 10.1111/j.1529-8817.2008.00563.x (doi:10.1111/j.1529-8817.2008.00563.x) [DOI] [PubMed] [Google Scholar]
- 23.Johannesson K. 1988. The paradox of Rockall—why is a brooding gastropod (Littorina saxatilis) more widespread than one having a planktonic larval dispersal stage (L. littorea)? Mar. Biol. 99, 507–513 10.1007/BF00392558 (doi:10.1007/BF00392558) [DOI] [Google Scholar]
- 24.Parker T., Tunnicliffe V. 1994. Dispersal strategies of the biota on an oceanic seamount—implications for ecology and biogeography. Biol. Bull. USA 187, 336–345 10.2307/1542290 (doi:10.2307/1542290) [DOI] [PubMed] [Google Scholar]
- 25.Hurr K. A., Lockhart P. J., Heenan P. B., Penny D. 1999. Evidence for the recent dispersal of Sophora (Leguminosae) around the Southern Oceans: molecular data. J. Biogeogr. 26, 565–577 10.1046/j.1365-2699.1999.00302.x (doi:10.1046/j.1365-2699.1999.00302.x) [DOI] [Google Scholar]
- 26.Waters J. M., Roy M. S. 2004. Out of Africa: the slow train to Australasia. Syst. Biol. 53, 18–24 10.1080/10635150490264671 (doi:10.1080/10635150490264671) [DOI] [PubMed] [Google Scholar]
- 27.Donald K. M., Kennedy M., Spencer H. G. 2005. Cladogenesis as the result of long-distance rafting events in South Pacific topshells (Gastropoda, Trochidae). Evolution 59, 1701–1711 [PubMed] [Google Scholar]
- 28.Fraser C. I., Nikula R., Spencer H. G., Waters J. M. 2009. Kelp genes reveal effects of subantarctic sea ice during the Last Glacial Maximum. Proc. Natl Acad. Sci. USA 106, 3249–3253 10.1073/pnas.0810635106 (doi:10.1073/pnas.0810635106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikula R., Fraser C. I., Spencer H. G., Waters J. M. 2010. Circumpolar dispersal by rafting in two subantarctic kelp-dwelling crustaceans. Mar. Ecol. Prog. Ser. 405, 221–230 10.3354/meps08523 (doi:10.3354/meps08523) [DOI] [Google Scholar]
- 30.Edgar G. J., Burton H. R. 2000. The biogeography of shallow-water macrofauna at Heard Island. Pap. Proc. R. Soc. Tasman. 133, 23–26 [Google Scholar]
- 31.Skerman T. M. 1958. Rates of growth in two species of Lepas (Cirripedia). NZ J. Sci. 1, 402–411 [Google Scholar]
- 32.Hinojosa I., Boltana S., Lancellotti D., Macaya E., Ugalde P., Valdivia N., Vasquez N., Newman W. A., Thiel M. 2006. Geographic distribution and description of four pelagic barnacles along the south east Pacific coast of Chile—a zoogeographical approximation. Rev. Chil. Hist. Nat. 79, 13–27 [Google Scholar]
- 33.Fraser C. I., Hay C. H., Spencer H. G., Waters J. M. 2009. Genetic and morphological analyses of the southern bull kelp Durvillaea antarctica (Phaeophyceae: Durvillaeales) in New Zealand reveal cryptic species. J. Phycol. 45, 436–443 10.1111/j.1529-8817.2009.00658.x (doi:10.1111/j.1529-8817.2009.00658.x) [DOI] [PubMed] [Google Scholar]
- 34.Guindon S., Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704 10.1080/10635150390235520 (doi:10.1080/10635150390235520) [DOI] [PubMed] [Google Scholar]
- 35.Posada D., Crandall K. A. 1998. Modeltest: testing the model of DNA substitution. Bioinformatics 14, 817–818 10.1093/bioinformatics/14.9.817 (doi:10.1093/bioinformatics/14.9.817) [DOI] [PubMed] [Google Scholar]
- 36.Ronquist F., Huelsenbeck J. P. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 10.1093/bioinformatics/btg180 (doi:10.1093/bioinformatics/btg180) [DOI] [PubMed] [Google Scholar]
- 37.Thiel M., Gutow L. 2005. The ecology of rafting in the marine environment. II. The rafting organisms and community. Oceanogr. Mar. Biol. Annu. Rev. 43, 279–418 [Google Scholar]
- 38.Cookson L. J. 1991. Australasian species of Limnoriidae (Crustacea: Isopoda). Mem. Mus. Vic. 52, 137–262 [Google Scholar]
- 39.Smith S. D. A., Simpson R. D. 2002. Spatial variation in the community structure of intertidal habitats at Macquarie Island (sub-Antarctic). Antarct. Sci. 14, 374–384 10.1017/S0954102002000160 (doi:10.1017/S0954102002000160) [DOI] [Google Scholar]
- 40.Thiel M. 2003. Reproductive biology of Limnoria chilensis: another boring peracarid species with extended parental care. J. Nat. Hist. 37, 1713–1726 [Google Scholar]
- 41.Deysher L., Norton T. A. 1981. Dispersal and colonization in Sargassum muticum (Yendo) Fensholt. J. Exp. Mar. Biol. Ecol. 56, 179–195 10.1016/0022-0981(81)90188-X (doi:10.1016/0022-0981(81)90188-X) [DOI] [Google Scholar]
- 42.Thiel M., Gutow L. 2005. The ecology of rafting in the marine environment. I. The floating substrata. Oceanogr. Mar. Biol. Annu. Rev. 42, 181–263 10.1201/9780203507810.ch6 (doi:10.1201/9780203507810.ch6) [DOI] [Google Scholar]
- 43.Hinojosa I. A., Rivadeneira M. M., Thiel M. In press Temporal and spatial distribution of floating objects in coastal waters of central-southern Chile and Patagonian fjords. Cont. Shelf Res. (doi:10.1016/j.csr.2010.04.013) [Google Scholar]
- 44.McGlone M. S. 2002. The late quaternary peat, vegetation and climate history of the southern oceanic islands of New Zealand. Quat. Sci. Rev. 21, 683–707 10.1016/S0277-3791(01)00044-0 (doi:10.1016/S0277-3791(01)00044-0) [DOI] [Google Scholar]
- 45.Zemke-White W. L., Speed S. R., McClary D. J. 2005. Beach-cast seaweed: a review. In New Zealand fisheries assessment report 2005/144, vol. 2005. Wellington, New Zealand: Ministry of Fisheries [Google Scholar]
- 46.Chiswell S. M. 2009. Colonisation and connectivity by intertidal limpets among New Zealand, Chatham and Sub-Antarctic Islands. II. Oceanographic connections. Mar. Ecol.-Prog. Ser. 388, 121–135 10.3354/meps08167 (doi:10.3354/meps08167) [DOI] [Google Scholar]
- 47.Pawson D. L. 1965. New records of echinoderms from the Snares Islands to the south of New Zealand. Trans. R. Soc. NZ Zool. 6, 253–260 [Google Scholar]
- 48.Langmuir I. 1938. Surface motion of water induced by wind. Science 87, 119–123 10.1126/science.87.2250.119 (doi:10.1126/science.87.2250.119) [DOI] [PubMed] [Google Scholar]
- 49.Coombs D. S., Landis C. A. 1966. Pumice from South Sandwich eruption of March 1962 reaches New Zealand. Nature 209, 289–290 10.1038/209289b0 (doi:10.1038/209289b0) [DOI] [Google Scholar]
- 50.Miranda L., Thiel M. 2008. Active and passive migration in boring isopods Limnoria spp. (Crustacea, Peracarida) from kelp holdfasts. J. Sea Res. 60, 176–183 10.1016/j.seares.2008.06.002 (doi:10.1016/j.seares.2008.06.002) [DOI] [Google Scholar]
- 51.Gutow L., Gimenez L., Boos K., Saborowski R. 2009. Rapid changes in the epifaunal community after detachment of buoyant benthic macroalgae. J. Mar. Biol. Assoc. UK 89, 323–328 [Google Scholar]
- 52.Child C. A. 1998. The marine fauna of New Zealand: Pycnogonida (sea spiders). NIWA Biodiv. Mem. 109, 1–71 [Google Scholar]
- 53.Murdoch R. C. 1982. A new species of Plaxiphora (Mollusca, Polyplacophora) from Southern New Zealand. NZ J. Mar. Freshwat. Res. 16, 175–178 10.1080/00288330.1982.9515960 (doi:10.1080/00288330.1982.9515960) [DOI] [Google Scholar]
- 54.Goldstien S. J., Gemmell N. J., Schiel D. R. 2009. Colonisation and connectivity by intertidal limpets among New Zealand, Chatham and sub-Antarctic Islands. I. Genetic connections. Mar. Ecol.-Prog. Ser. 388, 111–119 10.3354/meps08046 (doi:10.3354/meps08046) [DOI] [Google Scholar]