Abstract
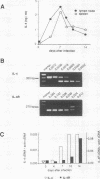
This study was performed to evaluate the soluble interleukin-4 receptor (sIL-4R) as a potential antagonist of interleukin-4 (IL-4) in an infectious disease. It is shown that antigen-triggered proliferation and cytokine secretion of Leishmania major-specific, cloned Th2 cells in vitro can be inhibited dose dependently by recombinant murine, but not control human, sIL-4R. In vivo, we found that endogenous synthesis of IL-4 mRNA is upregulated during the first week of infection, while an increase of IL-4R mRNA occurred later after infection of BALB/c mice with L. major. To interfere successfully with the IL-4 ligand-receptor interaction, we therefore chose to treat infected BALB/c mice with recombinant sIL-4R during the onset (e.g., days 0 to 7) of the immune response. Treatment with murine, but not with human, sIL-4R during the first week of infection rendered BALB/c mice clinically resistant to L. major, led to a 7- to 12-fold reduction of the parasite load in spleen and lymph nodes at 7 weeks of infection, shifted the pattern of cytokines towards a Th1 type, and provided durable resistance against reinfection. Thus, it could be demonstrated that the balance among sIL-4R, membrane-bound IL-4R, and their ligand IL-4 can be modulated in vivo, thereby modifying the antiparasitic immune response. These results suggest a therapeutic value of sIL-4R in diseases in which neutralization of IL-4 is desirable.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebischer T., Moody S. F., Handman E. Persistence of virulent Leishmania major in murine cutaneous leishmaniasis: a possible hazard for the host. Infect Immun. 1993 Jan;61(1):220–226. doi: 10.1128/iai.61.1.220-226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C., Gessner A., Röllinghoff M. Cytokines in leishmaniasis: a complex network of stimulatory and inhibitory interactions. Immunobiology. 1993 Nov;189(3-4):356–396. doi: 10.1016/S0171-2985(11)80366-9. [DOI] [PubMed] [Google Scholar]
- Chatelain R., Varkila K., Coffman R. L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992 Feb 15;148(4):1182–1187. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fanslow W. C., Clifford K. N., Park L. S., Rubin A. S., Voice R. F., Beckmann M. P., Widmer M. B. Regulation of alloreactivity in vivo by IL-4 and the soluble IL-4 receptor. J Immunol. 1991 Jul 15;147(2):535–540. [PubMed] [Google Scholar]
- Fanslow W. C., Clifford K., VandenBos T., Teel A., Armitage R. J., Beckmann M. P. A soluble form of the interleukin 4 receptor in biological fluids. Cytokine. 1990 Nov;2(6):398–401. doi: 10.1016/1043-4666(90)90047-w. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R., Vitetta E. S. A soluble, high-affinity, interleukin-4-binding protein is present in the biological fluids of mice. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4202–4206. doi: 10.1073/pnas.87.11.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrone P., Djossou O., Galizzi J. P., Banchereau J. A recombinant extracellular domain of the human interleukin 4 receptor inhibits the biological effects of interleukin 4 on T and B lymphocytes. Eur J Immunol. 1991 Jun;21(6):1365–1369. doi: 10.1002/eji.1830210606. [DOI] [PubMed] [Google Scholar]
- Gessner A., Blum H., Röllinghoff M. Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology. 1993 Dec;189(5):419–435. doi: 10.1016/S0171-2985(11)80414-6. [DOI] [PubMed] [Google Scholar]
- Gessner A., Moskophidis D., Lehmann-Grube F. Enumeration of single IFN-gamma-producing cells in mice during viral and bacterial infection. J Immunol. 1989 Feb 15;142(4):1293–1298. [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak-Frendscho M., Brown J. F., Iizawa Y., Wagner R. D., Czuprynski C. J. Administration of anti-IL-4 monoclonal antibody 11B11 increases the resistance of mice to Listeria monocytogenes infection. J Immunol. 1992 Jun 15;148(12):3978–3985. [PubMed] [Google Scholar]
- Harada N., Castle B. E., Gorman D. M., Itoh N., Schreurs J., Barrett R. L., Howard M., Miyajima A. Expression cloning of a cDNA encoding the murine interleukin 4 receptor based on ligand binding. Proc Natl Acad Sci U S A. 1990 Feb;87(3):857–861. doi: 10.1073/pnas.87.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Schoenhaut D. S., Rerko R. M., Rosser L. E., Gately M. K. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993 May 1;177(5):1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzerda R. L., March C. J., Mosley B., Lyman S. D., Vanden Bos T., Gimpel S. D., Din W. S., Grabstein K. H., Widmer M. B., Park L. S. Human interleukin 4 receptor confers biological responsiveness and defines a novel receptor superfamily. J Exp Med. 1990 Mar 1;171(3):861–873. doi: 10.1084/jem.171.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug M. S., Berger S. L. First-strand cDNA synthesis primed with oligo(dT). Methods Enzymol. 1987;152:316–325. doi: 10.1016/0076-6879(87)52036-5. [DOI] [PubMed] [Google Scholar]
- Lohoff M., Dirks M., Rohwer P., Röllinghoff M. Studies on the mechanism of polyclonal B cell stimulation by TH2 cells. Eur J Immunol. 1989 Jan;19(1):77–81. doi: 10.1002/eji.1830190113. [DOI] [PubMed] [Google Scholar]
- Maliszewski C. R., Sato T. A., Vanden Bos T., Waugh S., Dower S. K., Slack J., Beckmann M. P., Grabstein K. H. Cytokine receptors and B cell functions. I. Recombinant soluble receptors specifically inhibit IL-1- and IL-4-induced B cell activities in vitro. J Immunol. 1990 Apr 15;144(8):3028–3033. [PubMed] [Google Scholar]
- Mosley B., Beckmann M. P., March C. J., Idzerda R. L., Gimpel S. D., VandenBos T., Friend D., Alpert A., Anderson D., Jackson J. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989 Oct 20;59(2):335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Street N. F., Budd R., O'Garra A., Fong T. A., Bond M. W., Moore K. W., Sher A., Fiorentino D. F. Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol Rev. 1991 Oct;123:209–229. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Platzer C., Richter G., Uberla K., Hock H., Diamantstein T., Blankenstein T. Interleukin-4-mediated tumor suppression in nude mice involves interferon-gamma. Eur J Immunol. 1992 Jul;22(7):1729–1733. doi: 10.1002/eji.1830220710. [DOI] [PubMed] [Google Scholar]
- Reed S. G., Scott P. T-cell and cytokine responses in leishmaniasis. Curr Opin Immunol. 1993 Aug;5(4):524–531. doi: 10.1016/0952-7915(93)90033-o. [DOI] [PubMed] [Google Scholar]
- Renz H., Domenico J., Gelfand E. W. IL-4-dependent up-regulation of IL-4 receptor expression in murine T and B cells. J Immunol. 1991 May 1;146(9):3049–3055. [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991 Nov 1;147(9):3149–3155. [PubMed] [Google Scholar]
- Sher A., Coffman R. L. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- Solbach W., Forberg K., Röllinghoff M. Effect of T-lymphocyte suppression on the parasite burden in Leishmania major-infected, genetically susceptible BALB/c mice. Infect Immun. 1986 Dec;54(3):909–912. doi: 10.1128/iai.54.3.909-912.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypek J. P., Chung C. L., Mayor S. E., Subramanyam J. M., Goldman S. J., Sieburth D. S., Wolf S. F., Schaub R. G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993 Jun 1;177(6):1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Ceredig R., Cerottini J. C., Louis J. A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically-susceptible BALB/c mice. J Immunol. 1985 Sep;135(3):2108–2114. [PubMed] [Google Scholar]
- Titus R. G., Marchand M., Boon T., Louis J. A. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985 Sep;7(5):545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Zettlmeissl G., Gregersen J. P., Duport J. M., Mehdi S., Reiner G., Seed B. Expression and characterization of human CD4:immunoglobulin fusion proteins. DNA Cell Biol. 1990 Jun;9(5):347–353. doi: 10.1089/dna.1990.9.347. [DOI] [PubMed] [Google Scholar]