Abstract
High-elevation valleys in dry areas of the Himalayas are among the most extreme, yet least explored environments on Earth. These barren, rocky valleys are subjected to year-round temperature fluctuations across the freezing point and very low availability of water and nutrients, causing previous workers to hypothesize that no photoautotrophic life (primary producers) exists in these locations. However, there has been no work using modern biogeochemical or culture-independent molecular methods to test the hypothesis that photoautotrophs are absent from high Himalayan soil systems. Here, we show that although microbial biomass levels are as low as those of the Dry Valleys of Antarctica, there are abundant microbial photoautotrophs, displaying unexpected phylogenetic diversity, in barren soils from just below the permanent ice line of the central Himalayas. Furthermore, we discovered that one of the dominant algal clades from the high Himalayas also contains the dominant algae in culture-independent surveys of both soil and ice samples from the Dry Valleys of Antarctica, revealing an unexpected link between these environmentally similar but geographically very distant systems. Phylogenetic and biogeographic analyses demonstrated that although this algal clade is globally distributed to other high-altitude and high-latitude soils, it shows significant genetic isolation by geographical distance patterns, indicating local adaptation and perhaps speciation in each region. Our results are the first to demonstrate the remarkable similarities of microbial life of arid soils of Antarctica and the high Himalayas. Our findings are a starting point for future comparative studies of the dry valleys of the Himalayas and Antarctica that will yield new insights into the cold and dry limits to life on Earth.
Keywords: cryophilic algae, cyanobacteria, cold deserts, dry valleys, subnival zone soils
1. Introduction
High-elevation ecosystems, above the highest reaches of vegetation, are among the most extreme terrestrial environments on Earth [1,2]. In fact, the high Himalayas have been called the ‘third pole’ because of the extreme physical environment coupled with the logistical difficulties involved in exploring these regions [3]. Both the North Pole and South Pole were reached four decades before the highest peak in the world, Mount Everest, was climbed in 1953. The highest soil ecosystems on Earth are characterized by low oxygen pressure, low levels of available water, high levels of radiation and extreme temperature cycling across the freezing point [2,4]. These physical extremes in turn lead high-elevation soils and sediments to be low in nutrients and ostensibly devoid of measurable life. However, recent work in the high Andes has shown greater than expected microbial biomass and biogeochemical cycling of nutrients even in undeveloped soils just below the permanent ice line [4,5]; but almost nothing is known about the responsible organisms or their global biogeographic distribution, especially in the highest mountains on Earth, the Himalayas.
Past researchers have hypothesized that life in the highest ice-free areas of the Himalayas is supported by Aeolian deposition of organic matter from lower elevations [2,6]. However, recent work has shown that photosynthetic microbes are abundant in similar barren and dry areas of the high Andes [4] and the Dry Valleys of Antarctica [7–9]. To date, no molecular work has been done to quantify or identify microbial phototrophs in barren, high-elevation areas of the Himalayas, although algae have been cultured from such soils [10]. Barren soils are very common above elevations of about 5000 m above sea level (m.a.s.l.) throughout the Himalayas and adjacent ranges of Asia [11], and are rapidly increasing in extent owing to glacial melting in the region [12,13]. These seemingly barren areas, above the extent of the highest vegetation, are here referred to as the ‘subnival zone’, following the precedent of Troll [11] and others [5]. Despite the extent and importance of subnival zone ecosystems in the Himalayas, there have been no modern studies of their biology or biogeochemistry. Therefore, in the autumn of 2008, we undertook an expedition to explore the microbial diversity and biogeochemistry of high-elevation ecosystems of the central Himalayas. The first goal of our study was to determine the presence and identity of the microbial phototrophs of several high and dry valleys of the central Himalayas. We carried out a broad study of soils in two valleys in the rain shadow of Dhaulagiri and the Annapurna Range in central Nepal, sampling soils up to the permanent ice line of each valley.
The second goal of our study was to test the hypothesis that photoautotrophic microbes of the high Himalayas are distributed throughout high-altitude and high-latitude ‘barren’ soils across the globe. Our preliminary work in the Himalayas and similar sites in other high mountain ranges with prominent subnival zones [5,14,15] indicated that these sites had many environmental and ecological attributes similar to those of the Dry Valleys of Antarctica. Yet there have been no comparative studies of these ecologically similar but geographically distant systems. Cold, barren ecosystems such as the subnival zone are ideal for testing biogeographic hypotheses concerning the global distribution and biogeography of microbes [16,17] because they are essentially isolated islands of the cryosphere surrounded by vast expanses of warmer ecosystems. This is especially true of the Himalayas, where the highest mountains on Earth rise out of a sea of tropical and subtropical biomes to the south. Here, we show for the first time that the dominant algal clade from culture-independent studies of the Dry Valleys of Antarctica is also abundant in the subnival zone of the high Himalayas, but that this clade shows significant genetic divergence in each of these and other extreme cold-soil ecosystems across the Earth.
2. Material and methods
(a). Sample collection
Soil samples were collected from 23 sites distributed in a nested sampling scheme, as has been applied in other barren high-elevation systems [5], across two high valleys in the rain shadow of Dhaulagiri and the Annapurna Range in the Manang and Mustang regions of north-central Nepal. All sites were above the highest extent of vegetation in each valley but below the permanent ice line (electronic supplementary material, figure S1). The highest sites sampled were located 1 km north of Thorong La (28°48′ N, 83°56′ E) on southeast facing slopes at elevations between 5486 and 5516 m.a.s.l. (electronic supplementary material, figure S1a). The medium and low sites (table 1 and electronic supplementary material, figure S1b,c) were located in the pristine and rarely visited Zun Tal Valley (28°43′ N, 83°54′ E), which is a hidden glacial valley bounded on the south by Peaks F and G and the north by Kangsartse and Lupratse [18]. All soil samples were frozen in the field and transported on ice to the laboratory for analysis within one week of sampling.
Table 1.
Biological and edaphic characteristics of the sampling sites in Nepal. The low and medium sites were in the Zun Tal high valley and the high sites were in the dry valley of the Mustang region north of Thorong La and the town of Muktinath. Microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) levels are among the lowest levels yet reported for soils and are approximately the same as levels measured in the Dry Valleys of Antarctica of 25.7 (s.e. = 2.2) and 4.4 (s.e. = 0.5) for MBC and MBN, respectively [24].
| sites | elevation (m.a.s.l.) | % water | MBC (µg g−1) | MBN (µg g−1) | photosynthetic filamentsa | photosynthetic ovoidsa |
|---|---|---|---|---|---|---|
| low | 5130 (5) | 1.1 (0.1) | 18.2 (7.2) | 0.6 (0.2) | 167 (80) | 14 (8) |
| n | 14 | 14 | 14 | 14 | 3 | 3 |
| medium | 5275 (11) | 1.5 (0.2) | 27.4 (11.5) | 1.0 (0.5) | n.d. | n.d. |
| n | 4 | 4 | 4 | 4 | ||
| high | 5501 (6) | 1.5 (0.6) | 18.5 (5.2) | 1.4 (0.5) | 78 (31) | 187 (74) |
| n | 5 | 5 | 5 | 5 | 3 | 3 |
aPhotosynthetic structures (figure 1) per gram of soil.
(b). Microbiological and molecular analyses
DNA was extracted from the soils using the MO BIO Power soil kit (MO BIO Laboratories, Inc., Carlsbad, CA, USA). Community small-subunit ribosomal DNA was amplified using the 18S primers 4Fa-short (5′-ATCCGGTTGATCCTGC-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′), and 16S primers 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1391R (5′-GACGGGCGGTGWGTRCA-3′) in separate reactions. PCR reactions were performed with 3.5 mM MgCl2, 0.25 mM each deoxynucleoside triphosphate, 0.4 µM each primer, 1 U Omni KlenTaq polymerase (MO BIO) and buffer supplied with the enzyme using a range of template concentrations. Thermal cycling was carried out for 25 cycles with an annealing temperature of 49 and 53°C for the 18S and 16S reactions, respectively. Amplicons from three different reactions were pooled and gel-purified using isolated bands from agarose gels and QIAquick gel purification columns (Qiagen, Valencia, CA, USA). Purified products were ligated into the pcr 2.1 vector (Invitrogen, Carlsbad, CA, USA) and transformed into Escherichia coli following the manufacturer's instructions. Using blue/white screening, transformants were inoculated into a 96-well deep-dish plate containing 1.5 ml of TB Dry nutrient broth (MO BIO). Cultures were shaken at 200 rpm for 16 h at 37°C and then centrifuged. Cell pellets were sent to Functional Biosciences (Madison, WI, USA) for plasmid extraction and were sequenced bi-directionally using sequencing primers T7 and M13R. Sequences were edited (vector-trimmed and assembled into contigs) using Sequencher 4.6 (Gene Codes Co., Ann Arbor, MI, USA).
Sequences were aligned using the web-based SINA aligner tool (http://www.arb-silva.de/documentation/background/) before being added into ARB (v. 9.4), with the most recent SILVA reference database as a means to classify broad phylotypes [19]. Alignments were then manually checked and adjusted before filtering out hyper-variable residues (less than 45% identity) for both algal and cyanobacterial sequences. After filtering and trimming, sequences of 1205 and 1650 bp were used to construct the cyanobacterial and algal phyolgenetic trees, respectively. For the larger bacterial libraries, when a group of sequences with 97 per cent identity or greater was found using DivergentSet, only one representative sequence was used for phylogenetic analysis [20]. For the algal 18S phylogeny, MrBayes (v. 3.1) was run to convergence with one million generations, a burn-in of 2500 and temperature setting of 0.02 [21]. MrBayes was also used for the cyanobacterial tree, with convergence reached after eight million generations, a burn-in of 20 000 and temperature of 0.015. For figure 1, the MEGA program was used to construct a neighbour-joining tree using the Kimura 2-parameter substitution model and calculate node support from 5000 bootstrap alignments [22]. The program Mothur v. 1.8.0 [23] was used to calculate the genetic distance matrices, and the Mantel tests were performed in R (http://pbil.univ-lyon1.fr/ade4html/mantel.rtest.html).
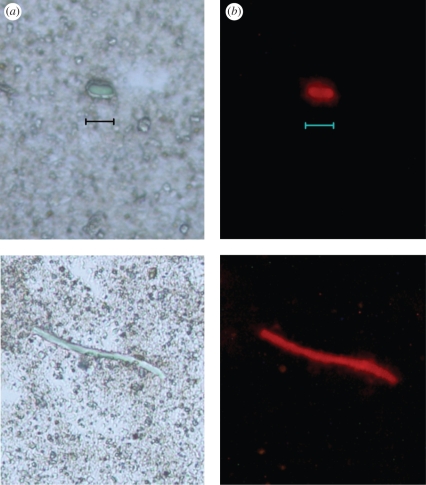
Figure 1.
Typical ovoid (top) and filamentous (bottom) cells from site 107 (5503 m.a.s.l.) at the high site in the Thorong La valley. The pictures are of the same field of view (magnification ×400) under (a) bright field microscopy and (b) epifluorescent microscopy. The red colour is due to the autofluorescence of chlorophyll. Scale bar, 10 µm.
Microbial biomass levels were measured using the chloroform fumigation method and other soil parameters were measured using standard methods as described elsewhere [5,14,15,24]. Algal and cyanobacterial counts were done using epifluorescence microscopy as described elsewhere [25].
3. Results and discussion
We carried out a broad study of soils in two dry valleys in the rain shadow of Dhaulagiri and the Annapurna Range in central Nepal, sampling soils up to the permanent ice line of each valley. Microbial biomass levels, measured using standard methods, were extremely low (table 1), slightly lower than levels (measured using the same technique) in soils of the Dry Valleys of Antarctica [24], and soil moisture levels were in the same range as those of the Dry Valleys of Antarctica [8,24], indicating that high Himalayan soils may be among the most extreme cold-soil environments on Earth.
Microscopic examination of soils from even our highest sites revealed the presence of ovoid and filamentous photosynthetic microbes exhibiting chlorophyll autofluorescence (figure 1 and table 1). Phylogenetic analysis of 18S and 16S rDNA clone libraries from these same soils revealed phototrophic microbial assemblages with high diversity (for full phylogenetic trees of see figures S2 and S3, electronic supplementary material). This was especially true for the cyanobacteria that included some undescribed and deeply divergent clades previously found in rock and barren soils of the Andes and Rocky Mountains [14,26,27]. Further phylogenetic analyses revealed that three phylotypes dominated the algal and cyanobacterial assemblages in these extreme soils. The algal libraries were dominated by well-supported clades in the Chlorophyceae and the Ulvophyceae (figure 2 and electronic supplementary material, figure S3), and the cyanobacterial community was dominated (54% of cyanobacterial phylotypes; electronic supplementary material, figure S2) by a well-supported, monophyletic clade equivalent to cultured and uncultured Microcoleus vaginatus [28,29].
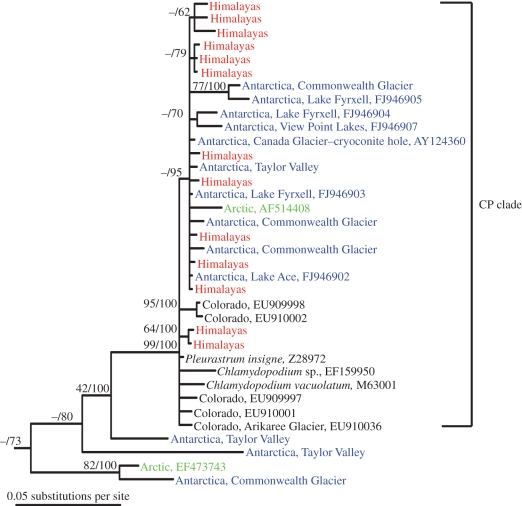
Figure 2.
Bayesian consensus tree of the dominant Chlorophyceae clade found in soils from the highest elevation sites in the Nepalese Himalayas and the closest BLAST matches from GenBank, as well as all cultured and environmental sequences from the high Arctic, Colorado Rocky Mountains and Dry Valleys of Antarctica. Support levels for nodes with values of more than 60 (neighbour-joining bootstrap support/Bayesian posterior probability) are shown. The CP clade (Chlamydopodium–Pleurastrum) was strongly supported by both methods and has been shown to be a monophyletic clade in previous research [31,32,34], although this is the first study to show their widespread distribution in cold-soil environments. Antarctic phylotypes without accession numbers are environmental sequences from Antarctic Dry Valley soils [8], and all branches labelled ‘Colorado’ are from soils near the Arikaree Glacier and the continental divide [14]. The only Arctic sequence (AF514408) in the CP clade was a ‘snow alga’ described and sequenced by Leya [33]. Antarctic sequences with appended accession numbers beginning with FJ are from algae isolated from lake samples [34] and the only alga sequence yet amplified from cryoconite-hole sediments (AY124360 [30]). Chlamydopodium vacuolatum (M63001) and Pleurastrum insigne (Z28972) are terrestrial algae [31,34], whereas Chlamydopodium sp. (EF159950) was isolated from a north temperate lake [45].
Given the similarity of high Himalayan soils to those of the Dry Valleys of Antarctica (table 1), we compared the dominant terrestrial algae of these two extreme environments using the culture-independent clone libraries. The Chlorophycean clade (CP clade, figure 2) that made up 36 per cent of 18S Himalayan clones also represented 50 per cent of the 18S algal clones from Antarctic Dry Valley soils [8]. Perhaps even more significantly, the CP clade contains the only algal sequence so far amplified and cloned from cryoconite-hole sediments of the McMurdo Dry Valleys [30]. This strongly supported monophyletic clade [31,32] also encompasses a terrestrial ‘snow’ alga isolated from the high (79° N) Arctic [33] and a dominant group of algae detected in clone libraries of barren soils from near the continental divide in Colorado, USA ([14]; figure 2). Taken together, these findings are the first molecular evidence of a globally dispersed, cryophillic clade of terrestrial algae and provide a unique opportunity to test whether extreme cryophiles are genetically the same across these geographically very distant sites.
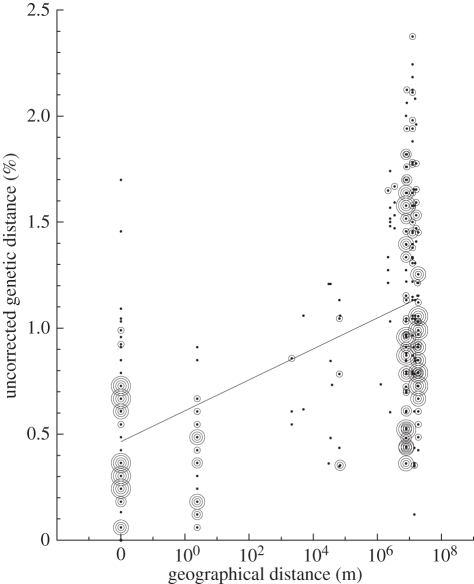
To perform such a test on the CP clade, we analysed pairwise comparisons of genetic distance versus geographical distance for all sequences from this clade. Despite the ubiquitous global distribution of the broader CP clade, it showed a highly significant isolation-by-distance pattern (p < 0.0002, Mantel test) driven by the genetic dissimilarity seen in pairwise comparisons at the intercontinental scale (figure 3). There was no significant (p = 0.1, Mantel test) geographical pattern at scales less than 50 km, indicating local adaptation in each region where we had enough small-scale replication (Himalayas, Rocky Mountains and Antarctica). Thus, we have shown for the first time the existence of a globally distributed terrestrial, cryophilic clade of algae that also shows evidence of geographical isolation at finer genetic scales. It is likely that this isolation-by-distance effect has resulted in endemic populations within the CP clade, as has been recently suggested for aquatic Antarctic algae [34].
Figure 3.
Genetic distance by geographical distance relationships for the dominant Chlorophyceae clade from the high Himalayas compared with all sequences (n = 406) in the same clade from the high Arctic, Antarctica and periglacial soils from the continental divide in Colorado. There was a significant (p < 0.0002, Mantel test) increase in genetic distance with geographical distance driven mostly by the differences at the intercontinental scale. The largest geographical distance in this study was between the Himalayan and Colorado sites (19 000 km). Circle size is proportional to the number of pairwise comparisons at each point on the graph, with bin sizes of 1, 2, 3, 4 and ≥5 for the smallest to largest circles.
The other dominant clade of algae (53% of algal phylotypes) in our Himalayan soils is apparently not so globally abundant as the CP clade. Members of this little-studied clade within the Ulvophyceae (electronic supplementary material, figure S3) have also been detected in one freshwater Antarctic sample (AM109906) and in early successional soils of northern Italy (Pseudendoclonium basiliense [35]), but their function in soil is completely unknown. However, fresh- and saltwater members of this clade (e.g. Gomontia polyrhiza) are known to bore into shells and wood [36,37]. Thus it is possible that these organisms survive endolithically in calcareous rocks found at our highest Himalayan sites, but much more work and data are needed to discern the function and biogeographic distribution of this mysterious group of algae.
The dominant cyanobacterial clade (M. vaginatus) in high Himalayan soils (54% of cyanobacterial phylotypes) was also the dominant clade in sites along the continental divide in Colorado (42% of cyanobacterial phylotypes), and is common in clone libraries of barren soils of the high Andes [4]. Furthermore, morphospecies of M. vaginatus have previously been reported to occur in Arctic, Alpine and Antarctic soils [38–40], but the present study is the first molecular phylogenetic evidence of their global pattern of distribution to barren high-elevation and high-latitude sites. Pairwise comparisons of genetic relatedness versus geographical distance for all high-altitude and high-latitude environmental sequences revealed much larger within-site genetic variation in M. vaginatus compared with the CP clade, but still showed a significant global isolation-by-distance pattern (p < 0.0003, Mantel test), indicating global distribution of this clade, but some local selection for some subtypes over others. Microcoleus vaginatus is eminently suited for existence in dry soils from extreme environments because it lives colonially in protective sheathes and emerges only when water is available [40,41] and can remain in a desiccated state for decades awaiting water pulses [40]. It remains to be seen whether the two dominant algal groups (the CP clade and the Ulvophyceae clade) discovered in the present study possess survival traits similar to those of the well-studied M. vaginatus.
The present study also raises the question of whether microbes in extreme environments are actually functioning there or are being constantly deposited from elsewhere (and thus end up being found in clone libraries and culturing efforts even if they cannot function in those environments [42]). At present, this remains an open question for soils of both the high Himalayas and the Dry Valleys of Antarctica, but there is accumulating evidence that photoautotrophs and chemoautotrophs can function in similar extreme soils of the high Andes and Rocky Mountains [4,14,43]. In addition, our phylogenetic analyses (e.g. figure 2) revealed that members of the CP clade are monophyletic and may comprise a family of organisms (as noted by the presence of sequenced representatives from two separate genera, Pleurastrum and Chlamydopodium, within the same polytomy). Future work using the ITS region may help resolve species-level relationships within this clade (cf. [44]). Additionally, members of the CP clade do show a unique and significant isolation-by-distance effect of their genetic signature by region (figure 3), indicating endemism. The M. vaginatus clade shows less genetic differentiation at distant sites compared with local sites (data not shown), indicating either the functioning of multiple and similar subclades at each site or constant global dispersal of dormant structures. Given the known distribution of M. vaginatus in the world's major deserts, it is reasonable to assume that its dormant dust-borne propagules are being constantly deposited in areas where they may not be able to function. By contrast, the CP clade is not a known desert dweller [45,46], nor are any dormant structures known for the few studied species in this clade [32,45].
Overall, our results demonstrate that previously unexplored periglacial soils of the high Himalayas contain microbial biomass levels and phototrophic microbes similar to the Dry Valleys of Antarctica. Perhaps the most important discovery of this study was the ubiquity of the algal CP clade in the high Himalayas, the high Arctic, the Dry Valleys of Antarctica and along the North American continental divide. This finding highlights the other similarities between these environments, and paves the way for future comparative studies of both the common organisms and environmental variables of these extreme systems. The ubiquity of the CP clade in these extreme environments also makes it an excellent candidate for future physiological and genomic studies aimed at understanding adaptations to, and primary production in, the extreme cryosphere. Such studies would illuminate the environmental limits for photosynthetic life on Earth, and perhaps elsewhere in the universe.
Acknowledgements
We thank Adina Racoviteanu, Bharat B. Shrestha, Nima Sherpa, Karma Gurung, Krishna K. Shrestha, Pat Kociolek, Evan Thomas and Diana R. Nemergut for helpful discussions or assistance in the field, and J. W. Fell and G. Scorzetti for providing DNA sequences from the Dry Valleys of Antarctica. This project was funded by the NSF Microbial Observatories Programme (MCB-0455606) and NSF grant DEB-0922267. Expedition funding was from a University of Colorado Faculty Fellowship and the National Geographic Committee for Research and Exploration. GenBank accession numbers for the new Himalayan sequences used in this study are HQ188942–HQ189117.
References
- 1.Costello E. K., Halloy S. R. P., Reed S. C., Sowell P., Schmidt S. K. 2009. Fumarole-supported islands of biodiversity within a hyperarid, high-elevation landscape on Socompa Volcano, Puna de Atacama, Andes. Appl. Environ. Microbiol. 75, 735–747 10.1128/AEM.01469-08 (doi:10.1128/AEM.01469-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swan L. W. 1992. The aeolian biome, ecosystems of the Earth's extremes. Bioscience 42, 262–270 10.2307/1311674 (doi:10.2307/1311674) [DOI] [Google Scholar]
- 3.Dyhrenfurth G. O. 1955. To the third pole: the history of the high Himalayas. London, UK: Werner Laurie [Google Scholar]
- 4.Schmidt S. K., Nemergut D. R., Miller A. E., Freeman K. R., King A. J., Seimon A. 2009. Microbial activity and diversity during extreme freeze–thaw cycles in periglacial soils, Cordillera Vilcanota, Perú. Extremophiles 13, 807–816 10.1007/s00792-009-0268-9 (doi:10.1007/s00792-009-0268-9) [DOI] [PubMed] [Google Scholar]
- 5.King A. J., Freeman K. R., McCormick K. F., Lynch R. C., Lozupone C., Knight R., Schmidt S. K. 2010. Biogeography and habitat modelling of high-alpine bacteria. Nat. Commun. 1, 53. 10.1038/ncomms1055 (doi:10.1038/ncomms1055) [DOI] [PubMed] [Google Scholar]
- 6.Edwards J. S. 1988. Life in the allobiosphere. Trends Ecol. Evol. 3, 111–114 10.1016/0169-5347(88)90118-8 (doi:10.1016/0169-5347(88)90118-8) [DOI] [PubMed] [Google Scholar]
- 7.Cary S. C., McDonald I. R., Barrett J. E., Cowan D. A. 2010. On the rocks: the microbiology of the Antarctic Dry Valley soils. Nat. Rev. Microbiol. 8, 129–138 10.1038/nrmicro2281 (doi:10.1038/nrmicro2281) [DOI] [PubMed] [Google Scholar]
- 8.Fell J. W., Scorzetti G., Connell L., Craig S. 2006. Biodiversity of micro-eukaryotes in Antarctic Dry Valley soils with <5% soil moisture. Soil Biol. Biochem. 38, 3107–3119 10.1016/j.soilbio.2006.01.014 (doi:10.1016/j.soilbio.2006.01.014) [DOI] [Google Scholar]
- 9.Nienow J. A., Friedmann E. I. 1993. Terrestrial lithophytic (rock) communities. In Antarctic microbiology (ed. Friedmann E. I.), pp. 343–412 New York, NY: Wiley-Liss [Google Scholar]
- 10.Reisigl H. 1969. Bodenalgen-studien II. Österr. Bot. Z. 116, 492–506 10.1007/BF01379645 (doi:10.1007/BF01379645) [DOI] [Google Scholar]
- 11.Troll C. 1973. High mountain belts between the polar caps and the equator: their definition and lower limit. Arctic Alpine Res. 5, 19–27 [Google Scholar]
- 12.Byers A. C. 2007. An assessment of contemporary glacier fluctuations in Nepal's Khumbu Himal using repeat photography. Himalayan J. Sci. 4, 21–26 [Google Scholar]
- 13.Ding Y., Shiyin S., Li J., Shangguan D. 2006. The retreat of glaciers in response to recent climate warming in western China. Ann. Glaciol. 43, 97–105 10.3189/172756406781812005 (doi:10.3189/172756406781812005) [DOI] [Google Scholar]
- 14.Freeman K. R., Pescador M. Y., Reed S. C., Costello E. K., Robeson M. S., Schmidt S. K. 2009. Soil CO2 flux and photoautotrophic community composition in high elevation, ‘barren’ soils. Environ. Microbiol. 11, 674–686 10.1111/j.1462-2920.2008.01844.x (doi:10.1111/j.1462-2920.2008.01844.x) [DOI] [PubMed] [Google Scholar]
- 15.Freeman K. R., Martin A. P., Karki D., Lynch R. C., Mitter M. S., Meyer A. F., Longcore J. E., Simmons D. R., Schmidt S. K. 2009. Evidence that chytrids dominate fungal communities in high elevation soils. Proc. Natl Acad. Sci. USA 106, 18 315–18 320 10.1073/pnas.0907303106 (doi:10.1073/pnas.0907303106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green J. L., Bohannan B. J. M., Whitaker R. J. 2008. Microbial biogeography: from taxonomy to traits. Science 320, 1039–1043 10.1126/science.1153475 (doi:10.1126/science.1153475) [DOI] [PubMed] [Google Scholar]
- 17.Martiny J. B. H., et al. 2006. Microbial biogeography: putting microorganisms on the map. Nat. Rev. Microbiol. 4, 102–112 10.1038/nrmicro1341 (doi:10.1038/nrmicro1341) [DOI] [PubMed] [Google Scholar]
- 18.Kuhle M. 1982. Der Dhaulagiri- und Annapurna-Himalaya. Ein Beitrag zur Geomorphologie extremer Hochgebirge. Zeitscrift Geomorph. 41(Suppl.), 1–229 [Google Scholar]
- 19.Ludwig W., et al. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32, 1363–1371 10.1093/nar/gkh293 (doi:10.1093/nar/gkh293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Widmann J., Hamady M., Knight R. 2006. DivergentSet, a tool for picking non-redundant sequences from large sequence collections. Mol. Cell Proteomics 5, 1520–1532 [DOI] [PubMed] [Google Scholar]
- 21.Huelsenbeck J. P., Ronquist F. 2001. MrBayes: Bayesian inference of phylogeny. Bioinformatics 17, 754–755 10.1093/bioinformatics/17.8.754 (doi:10.1093/bioinformatics/17.8.754) [DOI] [PubMed] [Google Scholar]
- 22.Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599 10.1093/molbev/msm092 (doi:10.1093/molbev/msm092) [DOI] [PubMed] [Google Scholar]
- 23.Schloss P. D., Handlesman J. 2006. Introducing SONS, a tool for operational taxonomic unit-based comparisons of microbial community memberships and structures. Appl. Environ. Microbiol. 72, 6773–6779 10.1128/AEM.00474-06 (doi:10.1128/AEM.00474-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ball B. A., Virginia R. A., Barrett J. E., Parsons A. N., Wall W. H. 2009. Interactions between physical and biotic factors influence CO2 flux in Antarctic Dry Valley soils. Soil Biol. Biochem. 41, 1510–1517 10.1016/j.soilbio.2009.04.011 (doi:10.1016/j.soilbio.2009.04.011) [DOI] [Google Scholar]
- 25.Kaštovská K., Elster J., Stibal M., Šantrůčková H. 2005. Microbial assemblages in soil microbial succession after glacial retreat in Svalbard (high Arctic). Microb. Ecol. 50, 396–407 10.1007/s00248-005-0246-4 (doi:10.1007/s00248-005-0246-4) [DOI] [PubMed] [Google Scholar]
- 26.Nemergut D. R., Anderson S. P., Cleveland C. C., Martin A. P., Miller A. E., Seimon A., Schmidt S. K. 2007. Microbial community succession in an unvegetated, recently deglaciated soil. Microb. Ecol. 53, 110–122 10.1007/s00248-006-9144-7 (doi:10.1007/s00248-006-9144-7) [DOI] [PubMed] [Google Scholar]
- 27.Walker J. J., Pace N. R. 2007. Phylogenetic composition of Rocky Mountain endolithic microbial ecosystems. Appl. Environ. Microbiol. 73, 3497–3504 10.1128/AEM.02656-06 (doi:10.1128/AEM.02656-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Pichel F., López-Cortéz A., Nübel U. 2001. Phylogenetic and morphological diversity of cyanobacteria in soil desert crusts from the Colorado Plateau. Appl. Environ. Microbiol. 67, 1902–1910 10.1128/AEM.67.4.1902-1910.2001 (doi:10.1128/AEM.67.4.1902-1910.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegesmund M. A., Johansen J. R., Karsten U., Friedl T. 2008. Coleofasciculus gen. nov. (Cyanobacteria): morphological and molecular criteria for revision of the genus Microcoleus Gomont. J. Phycol. 44, 1572–1585 10.1111/j.1529-8817.2008.00604.x (doi:10.1111/j.1529-8817.2008.00604.x) [DOI] [PubMed] [Google Scholar]
- 30.Christner B. C., Kvitko B. H., Reeve J. N. 2003. Molecular identification of Bacteria and Eukarya inhabiting an Antarctic cryoconite hole. Extremophiles 7, 177–183 [DOI] [PubMed] [Google Scholar]
- 31.Friedl T., Zeltner C. J. 1994. Assessing the relationships of some coccoid green lichen algae and the Microthamniales (Chlorophyta) with 18S ribosomal RNA gene sequence comparisons. J. Phycol. 30, 500–506 10.1111/j.0022-3646.1994.00500.x (doi:10.1111/j.0022-3646.1994.00500.x) [DOI] [Google Scholar]
- 32.Lewis L. A., Wilcox L. W., Fuerst P. A., Floyd G. L. 1992. Concordance of molecular and ultrastructural data in the study of zoosporic chlorococcalean green algae. J. Phycol. 28, 375–380 10.1111/j.0022-3646.1992.00375.x (doi:10.1111/j.0022-3646.1992.00375.x) [DOI] [Google Scholar]
- 33.Leya T. 2004. Field studies and genetic investigations of the cryophilic snow algae of northwest Spitzbergen. Thesis, Humboldt-Universitaet zu Berlin, Germany [Google Scholar]
- 34.De Wever A., Leliaert F., Verleyen E., Vanormelingen P., Van der Gucht K., Hodgson D. A., Sabbe K., Vyverman W. 2009. Hidden levels of phylodiversity in Antarctic green algae: further evidence for the existence of glacial refugia. Proc. R. Soc. B 276, 3591–3599 10.1098/rspb.2009.0994 (doi:10.1098/rspb.2009.0994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zancan S., Trevisan R., Paoletti M. G. 2006. Soil algae composition under different agro-ecosystems in north-eastern Italy. Agric. Ecosyst. Environ. 112, 1–12 10.1016/j.agee.2005.06.018 (doi:10.1016/j.agee.2005.06.018) [DOI] [Google Scholar]
- 36.Moore G. T. 1918. A wood-penetrating alga, Gomontia lignicola, n. sp. Ann. Mo. Bot. Gard. 5, 211. 10.2307/2990066 (doi:10.2307/2990066) [DOI] [Google Scholar]
- 37.O'Kelly C. J., Wysor B., Bellows W. K. 2004. Collinsiella (Ulvophyceae, Chlorophyta) and other ulotrichalean taxa with shell-boring sporophytes form a monophyletic clade. Phycologia 43, 41–49 10.2216/i0031-8884-43-1-41.1 (doi:10.2216/i0031-8884-43-1-41.1) [DOI] [Google Scholar]
- 38.Broady P. A. 1996. Diversity, distribution and dispersal of Antarctic terrestrial algae. Biodivers. Conserv. 5, 1307–1336 [Google Scholar]
- 39.Cowan D. A., Tow L. A. 2004. Endangered Antarctic environments. Annu. Rev. Microbiol. 58, 649–690 10.1146/annurev.micro.57.030502.090811 (doi:10.1146/annurev.micro.57.030502.090811) [DOI] [PubMed] [Google Scholar]
- 40.Drouet F. 1962. Gomont's ecophenes of the blue-green alga, Microcoleus vaginatus. Proc. Acad. Nat. Sci. Phila. 114, 191–205 [Google Scholar]
- 41.Garcia-Pichel F., Pringault O. 2001. Cyanobacteria track water in desert soils. Nature 413, 380–381 10.1038/35096640 (doi:10.1038/35096640) [DOI] [PubMed] [Google Scholar]
- 42.Hubert C., et al. 2009. A constant flux of diverse thermophilic bacteria into the cold Arctic seabed. Science 325, 1541–1544 10.1126/science.1174012 (doi:10.1126/science.1174012) [DOI] [PubMed] [Google Scholar]
- 43.Schmidt S. K., et al. 2008. The earliest stages of ecosystem succession in high-elevation (5000 metres above sea level), recently de-glaciated soils. Proc. R. Soc. B 275, 2793–2802 10.1098/rspb.2008.0808 (doi:10.1098/rspb.2008.0808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis L. A., Flechtner V. R. 2004. Cryptic species of Scenedesmus (Chlorophyta) from desert soil communities of Western North America. J. Phycol. 40, 1127–1137 10.1111/j.1529-8817.2004.03235.x (doi:10.1111/j.1529-8817.2004.03235.x) [DOI] [Google Scholar]
- 45.Gray D. W., Lewis L. A., Cardon Z. G. 2007. Photosynthetic recovery following desiccation of desert green algae (Chlorophyta) and their aquatic relatives. Plant Cell Environ. 30, 1240–1255 10.1111/j.1365-3040.2007.01704.x (doi:10.1111/j.1365-3040.2007.01704.x) [DOI] [PubMed] [Google Scholar]
- 46.Lewis L. A., Lewis P. O. 2005. Unearthing the molecular phylodiversity of desert soil green algae (Chlorophyta). Syst. Biol. 54, 936–947 10.1080/10635150500354852 (doi:10.1080/10635150500354852) [DOI] [PubMed] [Google Scholar]