Abstract
Light is the principal cue that entrains the circadian timing system, but the threshold of entrainment and the relative contributions of the retinal photoreceptors—rods, cones and intrinsically photosensitive retinal ganglion cells—are not known. We measured thresholds of entrainment of wheel-running rhythms at three wavelengths, and compared these to thresholds of two other non-image-forming visual system functions: masking and the pupillary light reflex (PLR). At the entrainment threshold, the relative spectral sensitivity and absolute photon flux suggest that this threshold is determined by rods. Dim light that entrained mice failed to elicit either masking or PLR; in general, circadian entrainment is more sensitive by 1–2 log units than other measures of the non-image-forming visual system. Importantly, the results indicate that dim light can entrain circadian rhythms even when it fails to produce more easily measurable acute responses to light such as phase shifting and melatonin suppression. Photosensitivity to one response, therefore, cannot be generalized to other non-image-forming functions. These results also impact practical problems in selecting appropriate lighting in laboratory animal husbandry.
Keywords: circadian, suprachiasmatic nucleus, retina, photoreceptor, wavelength
1. Introduction
The circadian system plays a critical role in regulating rhythms in physiology and behaviour, and in keeping them synchronized to 24 h rhythms in the environment. The light–dark cycle is the principal cue that entrains the circadian clock in the suprachiasmatic nucleus (SCN) of the hypothalamus. While threshold and relative spectral sensitivity to light have been studied for some indices of circadian function (e.g. phase shifts, melatonin suppression: [1–3]), these have not been described for entrainment. Whether threshold determinations based on responses to acute light pulses extrapolate to entrainment is not known. As a consequence, the photoreceptors that mediate entrainment, and the limits of sensitivity to light of this fundamental circadian function remain unknown.
The circadian clock in the SCN is one component of the broader non-image-forming visual system that also includes control of pupil size and the acute modulation of activity by light [4]. Mammalian photoreceptors include rods, cones and melanopsin-containing intrinsically photosensitive retinal ganglion cells (ipRGC) [5]. The relative contributions of the three photoreceptors to a given physiological or behavioural response depend on wavelength, intensity and duration of light.
The intensity in the diurnal portion of the light : dark cycle (LD) affects how animals entrain. The dimmer the light, the earlier locomotor activity and melatonin secretion begin [6,7]. Dim light in the nocturnal portion of the LD cycle also affects phase angle of entrainment and the rate of resynchronization in jetlag models [8]. Furthermore, some mice switch from nocturnal to diurnal behaviour in dim light [9]. Despite these effects of dim light, the few studies that have addressed entrainment thresholds have employed white light and have studied retinally degenerate and mutant mice, thus precluding both the description of spectral sensitivity of entrainment, and the responsible photoreceptors, in animals with normal retinae [10–15].
In this study, we assessed wheel-running rhythms in mice in diminishing irradiance steps in three wavelengths to identify the irradiance threshold and the photoreceptors that mediate entrainment. These thresholds were compared with those for masking, the pupillary light reflex (PLR), as well as threshold values in the literature for these and other measures of the non-image-forming visual system. The results have practical significance for the types of light used in the laboratory animal husbandry. More importantly, the results show that dim light can entrain circadian rhythms even though it may be too dim to elicit other circadian responses.
2. Methods
(a). Animals and housing
Male C57Bl/6 mice (Charles River Laboratories, Wilmington, MA, USA) were housed individually in clear polycarbonate cages (32 × 14 × 13 cm) on pine shavings, with food (LabDiet 5001, PMI Nutrition, Brentwood, MO, USA) and water available ad libitum. The light cycle was 12 h light and 12 h dark (12 L : 12 D) except during masking when animals were housed in 3.5 L : 3.5 D (DT17C, Intermatic, Spring Grove, IL or XT Table Top Timer, Chrontrol, San Diego, CA, USA). Each cage was equipped with a running wheel (11 cm diameter). Environmental noise was masked by white noise (76 dB SPL).
(b). Light
Light-emitting diodes (LEDs) were mounted in an array on the ceiling of each shelf to evenly illuminate the cages, 15 cm below, with blue, green or red light. The inter-bulb distance was 8–9 cm for blue and green LEDs and 4–5 cm for red LEDs. Effective wavelengths (λe) were obtained from the product of the LED emission spectra and each photoreceptor's sensitivity at each wavelength according to Govardovskii et al. [16] and are used throughout (blue: λpeak ± spectral half width (mean λe): 464 ± 12 (465 nm); green: 524 ± 23 (518 nm); red: λpeak = 639 ± 9 (635 nm); details in the electronic supplementary material, table S1). For testing PLR, the eye was illuminated with a single LED (blue: 470 ± 11 (470 nm); green: 520 ± 18 (517 nm); red: 632 ± 10 (626 nm); electronic supplementary material, table S1). Irradiance and photopic illuminance were measured 4 cm from the cage floor (IL1700, International Light Technologies, Peabody, MA, USA). Irradiance was converted and is reported as log photon flux (log(photons cm−2 s−1)) unless otherwise noted. One group of mice was tested for entrainment thresholds in red and blue light, PLR in red and blue light and masking in blue light. A second group of mice was tested for entrainment in green light, PLR in green light and masking in green and red light.
(c). Responses measured
To measure entrainment, wheel-running activity was monitored remotely by Vitalview (Minimitter, Bend, OR, USA), with counts collected in 10 min bins, and plotted in actograms. Daily onset of activity bouts was calculated by ClockLab (Actimetrics, Wilmette, IL, USA), and adjusted when necessary by eye. Entrainment was defined as rhythmic activity with a stable phase angle of entrainment relative to lights-off and a period of 24 h. To determine entrainment thresholds (the minimum irradiance necessary to entrain mice), the irradiance of the 12 L : 12 D cycle was decreased every two to four weeks, together with a 2 h advance of the LD cycle. Entrainment to the new photocycle was identified by a transient interval of rhythmic activity with period less than 24 h followed by a 24 h rhythm with stable phase angle of entrainment. One mouse switched from a nocturnal to a diurnal pattern of entrainment; its data are not included in the analysis.
Masking was calculated as the per cent of total activity occurring during the light with mice housed in a 3.5 L : 3.5 D cycle, to which they cannot entrain [17].
PLR was tested in unanaesthetized mice, dark-adapted for at least 1 h, 6–10 h after lights-on [18]. Mice were exposed to a 10 s pulse of light from an LED placed 10 cm from the eye, and pupil size was recorded under infrared light (DCR-DVD610, Sony, San Diego, CA, USA with a macro converter lens, DVS-WA45-30M, B&H, New York, NY, USA). Pupil size was measured in ImageJ just before and after the 10 s light exposure, and is reported as per cent of the initial dark-adapted pupil size. Unlike the consensual response, this method overestimates retinal irradiance at the bright end of the irradiance–response curve but not at the dim end. Corneal irradiance is reported throughout.
(d). Data analysis
Half-maximal response irradiances (I50) were determined from the best-fit four parameter logistic function (see the electronic supplementary material). The data were fit to Vitamin-A1 spectral sensitivity functions [16] by the method of least squares. A Monte-Carlo simulation was used to generate confidence intervals for the best-fit photopigment templates (see the electronic supplementary material). Differences in response to wavelength were assessed with ANOVA, and pairwise differences assessed with the post hoc Tukey test. First responses to light were assessed by one-sample tests for deviation from 50 per cent (masking), and by one-tailed paired t-tests to compare initial and final pupil size (PLR). Individual differences were assessed by Pearson correlation. These tests were all performed using Statview (SAS Institute, Inc., Cary, NC, USA). Significance was set at the 0.05 level.
3. Results
(a). Entrainment
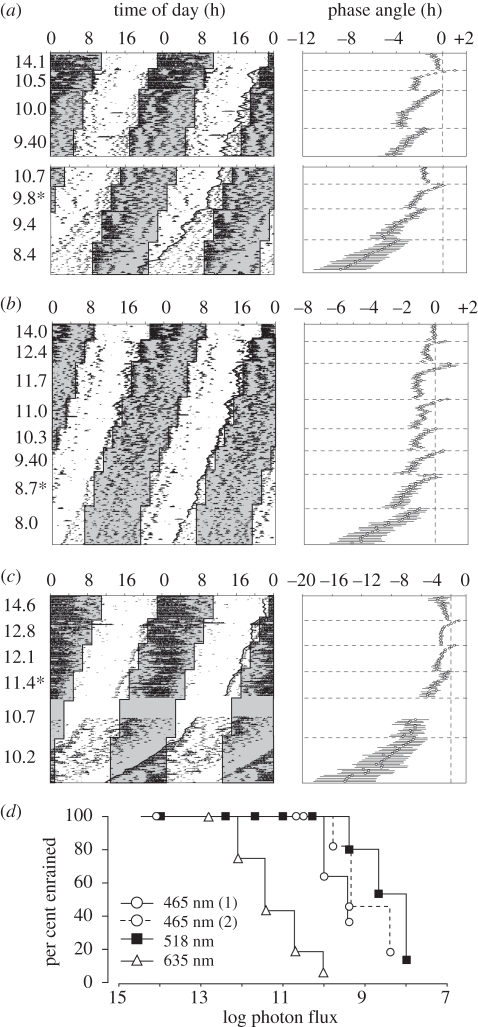
At each phase shift and step down of irradiance, we evaluated whether mice free-ran or entrained to the new photocycle. Entrainment is manifest here as a transient interval with period less than 24 h followed by a period of 24 h and phase angle of entrainment of 0.5–3 h. This can be seen in the individual activity records and in the plots of mean phase angle relative to lights-off (figure 1a–c). At irradiances that entrain all mice, mean phase angle gets earlier during the re-entrainment interval and stabilizes at a constant phase angle (e.g. green 11.7, figure 1b). At irradiances that entrain only a subset of mice, the contributions of mice that re-entrain and those that continue to free-run are observed in the change in slope of onset time over days (e.g. during intervals at 9.8 (465 nm) and 9.4 (518 nm), figure 1a,b). The per cent of animals entrained at each step is shown in figure 1d.
Figure 1.
Representative double-plotted activity records (left panels) and phase angle (group data, right panels) in blue (a) (465 nm, n = 11), green (b) (518 nm, n = 15), and red light (c) (635 nm, n = 16). The log photon flux at each intensity step is marked to the left. In the left panels, lights-off is in grey and the LD transitions marked by lines. Wheel running is indicated by black hatch marks; consecutive days proceed from top to bottom. Onsets on the right side of the actograms are connected for ease of visualization. Last intensity to which these mice entrain (records in (a) and (c) are from the same mouse). Owing to a light pulse during the blue activity recording around day 70, mice were re-entrained to a photon flux of 10.7, before proceeding. Owing to equipment failure, 13 days of activity data were not recorded in 635 nm. In the right panels, the mean (±s.e.) phase angle of entrainment are plotted for all mice. Lights-off is set at 0, and irradiance changes occur at the horizontal dashed lines. (d) Survival curves showing the percentage of animals entrained at each step; the two 465 nm traces correspond to the two runs of decreasing irradiance (top and bottom parts of (a)).
Table 1 shows the mean minimum light intensities to which mice entrained. Sensitivity to green and blue light was similar, with both thresholds significantly lower than for red (ANOVA, F2,39 = 52.3, p < 0.001; Tukey test, p < 0.001). Similar results were obtained when the I50/entrainment was estimated from the per cent of mice entrained at each irradiance step (electronic supplementary material, figure S1). The mean thresholds were used in the studies of masking and PLR described next.
Table 1.
Irradiance and photopic illuminance at entrainment thresholds.
| λ (nm) | log photon flux (log photons cm−2 s−1) | irradiance (nW cm−2) | illuminance (lux) |
|---|---|---|---|
| 465 | 9.46 ± 0.19 | 1.24 | 0.001 |
| 518 | 9.13 ± 0.20 | 0.52 | 0.0005 |
| 635 | 11.87 ± 0.19 | 228 | 0.4 |
(b). Masking
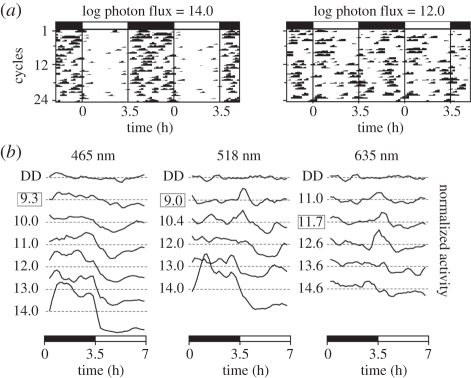
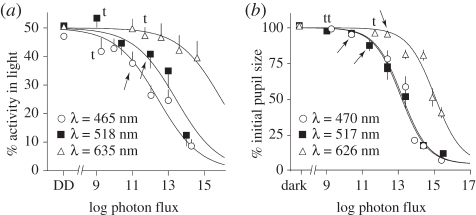
Masking is a graded response: bright light suppresses wheel running, while dim light has little effect (figure 2). Negative masking is more pronounced at short wavelengths, and positive masking is more pronounced at longer wavelengths (figure 2b). I50/mask (log photon flux) was 12.4 ± 0.2 for blue, 13.4 ± 0.3 for green and 16.0 ± 0.4 for red (figure 3a; logistic function coefficients a = min = 0%, b = max = 50%, c = slope = −0.37). There was a significant effect of photon flux on the degree of masking in both blue (repeated measures ANOVA, F6,78 = 27.8, p < 0.001) and green light (F5,50 = 11.8, p < 0.001). At the threshold of entrainment, there was no significant negative masking by light of any wavelength (one-sample test, p > 0.05). The dimmest intensities at which a significant effect of light could be detected were 11.0 and 12.0 (log photon flux) in blue and green, respectively (arrows in figure 3a, one-sample test, p < 0.05). There was no significant effect of light intensity on masking in red light (F5,50 = 1.5, p = 0.2). In a few mice, negative masking could be observed at irradiances too dim to cause entrainment (mouse in 465 and 635 nm, figure 1).
Figure 2.
Masking behaviour. (a) Representative actograms showing negative masking in bright 518 nm light (left) or no masking in dim (right). The LD cycle is indicated by the bars at the top. (b) Mean activity profiles (3 point running means) are shown for 6 or 7 irradiance steps. Activity profiles for each mouse were first normalized to 1; traces are separated vertically for ease of visualization. The dotted lines indicate mean activity level for each trace, and the distance between dotted lines is equal to 1. Log photon flux is marked at the left of each trace; the box indicates the entrainment threshold. Positive masking is observed in dimmer light, especially during the first 30 min of light exposure (518 nm at 9 and 10.4; 635 nm at 11.7 and 12.6). Positive masking is never observed at 465 nm; negative masking is never observed at 635 nm.
Figure 3.
Irradiance–response curves for (a) masking and (b) PLR. Arrows indicate the first irradiance at which significant masking or pupil constriction is observed (one-sample test or one-tailed paired t-test, p < 0.05). t: threshold entrainment irradiance for each wavelength.
(c). PLR
Light at the threshold irradiance for entrainment failed to constrict the pupil (figure 3b, one-tailed paired t-test, p > 0.2). Significant constriction as assessed by t-test was detected first at log photon fluxes of 10.4 (470 nm), 11.4 (517 nm, but note that p = 0.08 at 10.4) and 12.4 (626 nm). PLR is a graded response over approximately five orders of magnitude (figure 3b; repeated measures ANOVA, 470 nm: F7,49 = 138; 517 nm: F7,28 = 29; 626 nm: F6,78 = 86; all p < 0.001). I50/PLR (log photon flux) was 13.2 ± 0.1 for blue, 13.1 ± 0.1 for green and 15.0 ± 0.1 for red (a = 5%, b = 100%, d = −0.59).
(d). Spectral sensitivity of non-image-forming visual functions
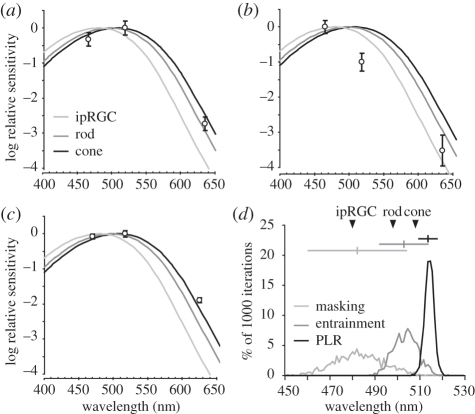
Mean entrainment thresholds, I50/masking and I50/PLR were plotted against Govardovskii Vitamin-A1 templates for the murine rod (λmax = 498 nm), mid-wavelength cone (508 nm) and ipRGC (480 nm; figure 4a–c; [16,19–21]). The ultraviolet cone is minimally sensitive to these wavelengths and is not included [22]. Raw entrainment threshold data were best fit by an A1 template with a peak at 503 nm. A Monte-Carlo simulation based on the means and standard deviation for entrainment, or the I50 and standard deviation for masking and PLR, were used to generate 95% confidence intervals (CI) for the best-fit A1 templates. With 1000 iterations, the simulation produced distributions showing the likelihood of finding a best-fit template with given λmax (figure 4d). The simulation yielded a mean λmax of 503 nm for entrainment (CI, 492–514 nm), 482 nm for masking (CI, 460–504 nm) and 514 nm for PLR (CI, 510–518 nm).
Figure 4.
Spectral sensitivity of (a) entrainment, (b) masking and (c) PLR. Relative thresholds (○) are plotted against A1 templates for rod (λmax = 498 nm), mid-wavelength cone (λmax = 508 nm) and ipRGC (λmax = 480 nm). These data were used in Monte-Carlo simulations to estimate confidence intervals for λmax for each measure, resulting in a λmax histogram (d). The horizontal lines and vertical hatch mark at the top show the 95% confidence interval and mean for each measure. λmax for each photoreceptor is marked (▾).
(e). Comparison of sensitivity thresholds
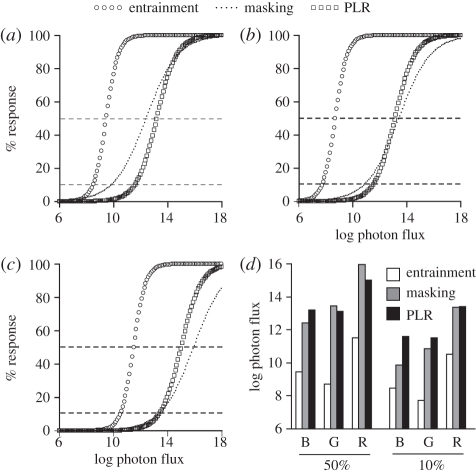
To understand relative sensitivity among the three measures, the irradiance–response curves calculated above were re-plotted by wavelength (figure 5a–c). As measured at both 50 per cent and 10 per cent maximal response, entrainment is more sensitive than masking and PLR (figure 5d). A survey of the literature on photosensitivity indicates that the entrainment I50 is 1–2 log units lower than half-maximal values for other measures of the non-image-forming visual system in general and of the circadian system in particular (electronic supplementary material, table S2).
Figure 5.
Comparison of photosensitivity. The irradiance–response curves from figure S1 and figure 3 are plotted together by colour (a, blue; b, green; c, red). All curves were transformed to a common scale. y-axis: 0% response is defined by no entrainment, negative masking, or pupil constriction; 100% response is defined by 100% of animals entrained, and maximum negative masking pupil constriction. The 50% and 10% response levels are marked by dashed lines and plotted for blue, green and red light in (d).
(f). Individual differences
We reasoned that if sensitivity to light is determined by retinal factors alone, then individuals most sensitive in one measure should be most sensitive in the others. Nevertheless, we found no significant correlations between thresholds for entrainment, masking and PLR (blue entrainment: versus masking, R = −0.41, or versus PLR, R = −0.10; green entrainment: versus masking, R = −0.12, or versus PLR, R = 0.25; red entrainment: versus PLR, R = 0.15; all p > 0.2). Neither were there significant correlations between I50 calculated for masking and for PLR in blue (R = 0.37, p = 0.23) or green light (R = 0.37, p = 0.21). There was a significant correlation between wavelengths for masking and PLR (masking: green versus red: R = 0.72, n = 9, p < 0.05; PLR: red versus blue: R = 0.67, n = 11, p < 0.05; entrainment: blue versus red: R = 0.45, n = 11, p = 0.17).
4. Discussion
This report shows that mice entrain to dim light via classical photoreceptors, and describes for the first time, to our knowledge, a threshold and a spectral sensitivity function for circadian entrainment. Within the non-image-forming visual system, entrainment is more sensitive than any other response described. Full entrainment is observed at dim light that elicits negligible masking and PLR. Red light can entrain circadian rhythms at intensities typically thought to have no effect on the circadian system of mice.
The present objective was to identify the photoreceptors mediating entrainment in mice with normal retinae; previous experiments in dim light entrainment with rodless–coneless mice demonstrated a role for these outer layer photoreceptors, but used white light and did not separate the role of rods and cones ([10,12]; but see [11]). The distinct roles of rods and cones are unclear. Two studies point to rods as mediating entrainment in dim light: mice whose photoreception is limited to cones show deficits in entrainment, and mice whose photoreception is limited to rods remain capable of entraining to dim light [14,15]. These two studies also showed that the lengthening of free-running period in constant light, another measure of circadian photoreception, does not require cones or melanopsin. By contrast, mice that have rods but lack the mid-wavelength cone do not entrain to LD cycles at 1 lux while controls do entrain, implicating the cone as the limiting photoreceptor [13]. These studies all employed mutant mice in addressing entrainment sensitivity, which may partly explain the discrepancies.
The present data, obtained in wild-type mice with intact retinae, clarify the roles of rods and cones. Consistent with both rods and cones, the relative spectral sensitivity of entrainment in our mice was best fit by an A1 template with a 95% CI that overlapped with λmax for both rods and cones. Thus, spectral data do not differentiate between these photoreceptors. The photon flux at threshold, however, suggests a rod-mediated mechanism. At the threshold of entrainment in 518 nm light, the estimated incident photon flux on the retina is 5E + 8 photons cm−2 s−1 (corneal photon flux of 1.4E + 9 photons cm−2 s−1; dark adapted pupil size of 4.9 mm2; retina size of 14.5 mm2; [23]). This falls in the dynamic range of rods [24], and is too dim to excite cones [25] or ipRGCs [26]. These data, therefore, strongly suggest that rods define the lower limit of entrainment.
Compared with the photoreceptors mediating entrainment, much more is known about photoreceptors mediating PLR. Work in mutant and wild-type mice demonstrates that PLR is mediated primarily by cones [15,18]. The present results confirm that PLR is cone-mediated.
Negative masking is mediated by ipRGC photoreception, whereas rods and possibly cones, contribute to both positive and negative masking depending on light intensity [17,27,28]. Our masking data support this (figure 2b): negative masking is most pronounced and positive masking is undetectable in blue light that maximally excites ipRGCs, whereas negative masking is absent and positive masking is greatest in red light that has a greater relative effect on rods and cones compared with ipRGCs. The λmax confidence interval for the negative masking spectral sensitivity function was wide and overlapped with both ipRGCs and rods. This is consistent with the strong negative masking in bright light at shorter wavelengths, and also with the low amplitude and transient masking in light too dim to excite ipRGCs. Also, despite large differences in I50, there is some overlap in the dynamic ranges between masking and entrainment (figures 1 and 5).
These data have important practical and basic biological implications. On the practical side, they help define appropriate background lighting in laboratory animal husbandry. Dim red light (typically reported as less than 1 lux or less than 0.1 lux) is routinely used to aid behavioural observations and animal care, and constant dim red light is often substituted for constant darkness in circadian studies for the same reason. The justifications include the relatively low sensitivity of rhodopsin to red light, and the inability of dim red light to phase-shift circadian rhythms [2,29]. Nevertheless, the present report shows that dim red light on the order of 0.1–1 lux is an effective entraining signal. Dim red light and constant darkness are not equivalent (e.g. [30]).
With regard to basic biology, the results indicate a range of photic sensitivities across different measures and have implications for our understanding of photoreception. Entrainment is more sensitive to light than are either masking or PLR. The entrainment I50 is also lower than half-maximal irradiances reported in the literature for other indices of circadian function, including phase shifting and melatonin suppression (electronic supplementary material, table S2). Even though a given light may be too dim to elicit an acute response from the circadian system, it may nevertheless entrain the circadian system. This is important for understanding the non-image-forming visual system, as phase shifting and melatonin suppression are often used as indices for light's effect on the circadian system, in both human and animal studies [31,32].
Dim light presented for long durations and detected by rods can entrain circadian rhythms in normal mice with intact retinae. This confirms the sufficiency of rods as mediators of entrainment in dim light [14,15]. The striking difference in photosensitivity among physiological measures shows that photosensitivity of a given measure cannot be easily inferred from surrogate indices. The actions of long-duration dim light are not well understood beyond the behavioural level. Determining the mechanisms by which light detected by rods and cones is integrated remains to be explored.
Acknowledgements
All procedures were approved by the Columbia University Animal Care and Use Committee.
This work was supported by grants MH075045, NS37919 (to R.S.) and T32DK07328 (to M.P.B.). We thank Dr Joseph LeSauter and Zi Lin for technical assistance, and Drs Paul Witkovsky, Howard Cooper, Russell Foster and Stuart Peirson for their helpful comments on an early draft of this manuscript.
References
- 1.Cardinali D. P., Larin F., Wurtman R. J. 1972. Control of the rat pineal gland by light spectra. Proc. Natl Acad. Sci. USA 69, 2003–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi J. S., DeCoursey P. J., Bauman L., Menaker M. 1984. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature 308, 186–188 10.1038/308186a0 (doi:10.1038/308186a0) [DOI] [PubMed] [Google Scholar]
- 3.Nelson D. E., Takahashi J. S. 1991. Comparison of visual sensitivity for suppression of pineal melatonin and circadian phase-shifting in the golden hamster. Brain Res. 554, 272–277 10.1016/0006-8993(91)90200-F (doi:10.1016/0006-8993(91)90200-F) [DOI] [PubMed] [Google Scholar]
- 4.Foster R. G. 2002. Keeping an eye on the time: the Cogan Lecture. Invest. Ophthalmol. Vis. Sci. 43, 1286–1298 [PubMed] [Google Scholar]
- 5.Hattar S., et al. 2003. Melanopsin and rod–cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424, 76–81 10.1038/nature01761 (doi:10.1038/nature01761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pohl H. 1976. Proportional effect of light on entrained circadian rhythms of birds and mammals. J. Comp. Physiol. 112, 103–108 10.1007/BF00612678 (doi:10.1007/BF00612678) [DOI] [Google Scholar]
- 7.Wright K. P., Jr, Gronfier C., Duffy J. F., Czeisler C. A. 2005. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J. Biol. Rhyth. 20, 168–177 10.1177/0748730404274265 (doi:10.1177/0748730404274265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans J. A., Elliott J. A., Gorman M. R. 2009. Dim nighttime illumination accelerates adjustment to timezone travel in an animal model. Curr. Biol. 19, R156–R157 10.1016/j.cub.2009.01.023 (doi:10.1016/j.cub.2009.01.023) [DOI] [PubMed] [Google Scholar]
- 9.Doyle S. E., Yoshikawa T., Hillson H., Menaker M. 2008. Retinal pathways influence temporal niche. Proc. Natl Acad. Sci. USA 105, 13 133–13 138 10.1073/pnas.0801728105 (doi:10.1073/pnas.0801728105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebihara S., Tsuji K. 1980. Entrainment of the circadian activity rhythm to the light cycle: effective light intensity for a zeitgeber in the retinal degenerate C3H mouse and the normal C57BL mouse. Physiol. Behav. 24, 523–527 10.1016/0031-9384(80)90246-2 (doi:10.1016/0031-9384(80)90246-2) [DOI] [PubMed] [Google Scholar]
- 11.Foster R. G., Helfrich-Forster C. 2001. The regulation of circadian clocks by light in fruitflies and mice. Phil. Trans. R. Soc. Lond. B 356, 1779–1789 10.1098/rstb.2001.0962 (doi:10.1098/rstb.2001.0962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mrosovsky N. 2003. Contribution of classic photoreceptors to entrainment. J. Comp. Physiol. A 189, 69–73 10.1007/s00359-002-0378-7 (doi:10.1007/s00359-002-0378-7) [DOI] [PubMed] [Google Scholar]
- 13.Dkhissi-Benyahya O., Gronfier C., De Vanssay W., Flamant F., Cooper H. M. 2007. Modeling the role of mid-wavelength cones in circadian responses to light. Neuron 53, 677–687 10.1016/j.neuron.2007.02.005 (doi:10.1016/j.neuron.2007.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altimus C. M., Guler A. D., Alam N. M., Arman A. C., Prusky G. T., Sampath A. P., Hattar S. 2010. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat. Neurosci. 13, 1107–1112 10.1038/nn.2617 (doi:10.1038/nn.2617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lall G. S., et al. 2010. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron 66, 417–428 10.1016/j.neuron.2010.04.037 (doi:10.1016/j.neuron.2010.04.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govardovskii V. I., Fyhrquist N., Reuter T., Kuzmin D. G., Donner K. 2000. In search of the visual pigment template. Vis. Neurosci. 17, 509–528 10.1017/S0952523800174036 (doi:10.1017/S0952523800174036) [DOI] [PubMed] [Google Scholar]
- 17.Mrosovsky N., Hattar S. 2003. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol. Int. 20, 989–999 10.1081/CBI-120026043 (doi:10.1081/CBI-120026043) [DOI] [PubMed] [Google Scholar]
- 18.Lucas R. J., Douglas R. H., Foster R. G. 2001. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat. Neurosci. 4, 621–626 10.1038/88443 (doi:10.1038/88443) [DOI] [PubMed] [Google Scholar]
- 19.Bowes C., Li T., Danciger M., Baxter L. C., Applebury M. L., Farber D. B. 1990. Retinal degeneration in the rd mouse is caused by a defect in the β subunit of rod cGMP-phosphodiesterase. Nature 347, 677–680 10.1038/347677a0 (doi:10.1038/347677a0) [DOI] [PubMed] [Google Scholar]
- 20.Sun H., Macke J. P., Nathans J. 1997. Mechanisms of spectral tuning in the mouse green cone pigment. Proc. Natl Acad. Sci. USA 94, 8860–8865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panda S., Nayak S. K., Campo B., Walker J. R., Hogenesch J. B., Jegla T. 2005. Illumination of the melanopsin signaling pathway. Science 307, 600–604 10.1126/science.1105121 (doi:10.1126/science.1105121) [DOI] [PubMed] [Google Scholar]
- 22.Jacobs G. H., Neitz J., Deegan J. F., II 1991. Retinal receptors in rodents maximally sensitive to ultraviolet light. Nature 353, 655–656 10.1038/353655a0 (doi:10.1038/353655a0) [DOI] [PubMed] [Google Scholar]
- 23.Jeon C. J., Strettoi E., Masland R. H. 1998. The major cell populations of the mouse retina. J. Neurosci. 18, 8936–8946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakatani K., Tamura T., Yau K. W. 1991. Light adaptation in retinal rods of the rabbit and two other nonprimate mammals. J. Gen. Physiol. 97, 413–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikonov S. S., Kholodenko R., Lem J., Pugh E. N., Jr 2006. Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J. Gen. Physiol. 127, 359–374 10.1085/jgp.200609490 (doi:10.1085/jgp.200609490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Do M. T., Kang S. H., Xue T., Zhong H., Liao H. W., Bergles D. E., Yau K. W. 2009. Photon capture and signalling by melanopsin retinal ganglion cells. Nature 457, 281–287 10.1038/nature07682 (doi:10.1038/nature07682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mrosovsky N., Foster R. G., Salmon P. A. 1999. Thresholds for masking responses to light in three strains of retinally degenerate mice. J. Comp. Physiol. A 184, 423–428 10.1007/s003590050341 (doi:10.1007/s003590050341) [DOI] [PubMed] [Google Scholar]
- 28.Thompson S., Foster R. G., Stone E. M., Sheffield V. C., Mrosovsky N. 2008. Classical and melanopsin photoreception in irradiance detection: negative masking of locomotor activity by light. Eur. J. Neurosci. 27, 1973–1979 10.1111/j.1460-9568.2008.06168.x (doi:10.1111/j.1460-9568.2008.06168.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wald G., Brown P. K. 1956. Synthesis and bleaching of rhodopsin. Nature 177, 174–176 10.1038/177174a0 (doi:10.1038/177174a0) [DOI] [PubMed] [Google Scholar]
- 30.McCormack C. E., Sontag C. R. 1980. Entrainment by red light of running activity and ovulation rhythms of rats. Am. J. Physiol. 239, R450–R453 [DOI] [PubMed] [Google Scholar]
- 31.Brainard G. C., Hanifin J. P., Greeson J. M., Byrne B., Glickman G., Gerner E., Rollag M. D. 2001. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J. Neurosci. 21, 6405–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gooley J. J., Rajaratnam S. M., Brainard G. C., Kronauer R. E., Czeisler C. A., Lockley S. W. 2010. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci. Transl. Med. 2, 31ra33. 10.1126/scitranslmed.3000741 (doi:10.1126/scitranslmed.3000741) [DOI] [PMC free article] [PubMed] [Google Scholar]