Abstract
Polyphenisms—the expression of discrete phenotypic morphs in response to environmental variation—are examples of phenotypic plasticity that may potentially be adaptive in the face of predictable environmental heterogeneity. In the butterfly Bicyclus anynana, we examine the hormonal regulation of phenotypic plasticity that involves divergent developmental trajectories into distinct adult morphs for a suite of traits as an adaptation to contrasting seasonal environments. This polyphenism is induced by temperature during development and mediated by ecdysteroid hormones. We reared larvae at separate temperatures spanning the natural range of seasonal environments and measured reaction norms for ecdysteroids, juvenile hormones (JHs) and adult fitness traits. Timing of peak ecdysteroid, but not JH titres, showed a binary response to the linear temperature gradient. Several adult traits (e.g. relative abdomen mass) responded in a similar, dimorphic manner, while others (e.g. wing pattern) showed a linear response. This study demonstrates that hormone dynamics can translate a linear environmental gradient into a discrete signal and, thus, that polyphenic differences between adult morphs can already be programmed at the stage of hormone signalling during development. The range of phenotypic responses observed within the suite of traits indicates both shared regulation and independent, trait-specific sensitivity to the hormone signal.
Keywords: phenotypic plasticity, hormonal regulation, ecdysone, life history, reaction norm, seasonal polyphenism
1. Introduction
Phenotypic plasticity is the ability of individual genotypes to produce different phenotypes when exposed to environmental variation [1,2]. Potentially, it allows organisms to persist in variable environments, and it is therefore of major evolutionary significance. Furthermore, phenotypic plasticity reveals how the developmental mechanisms that translate genotypes into phenotypes can be modulated by the environment and how sensitivity to the environment can be a source of phenotypic variation [3–5]. The reaction norm concept describes phenotypic variation as a function of the environment and provides an experimental framework for studying developmental sensitivity to the environment [6,7]. A flat reaction norm represents a canalized phenotype, whereas a steep reaction norm represents a plastic phenotype. Polyphenisms can be seen as an extreme case of phenotypic plasticity, where alternative discrete phenotypes develop in response to environmental variation [1,4,8].
Hormones play crucial regulatory roles in coordinating the expression of physiological, behavioural and morphological traits into an integrated life history [9–11]. The two major classes of insect hormones, ecdysteroids and juvenile hormones (JHs), have been implicated in many cases of insect polyphenisms, such as horned beetles, butterflies, social insects and sand crickets [12–15]. While various studies have measured reaction norms across an environmental gradient for phenotypic traits (e.g. [16,17]), and others have measured differences in hormone dynamics between morphs at the extreme ends of a reaction norm (e.g. [18]), these approaches have rarely been combined (but see [19]). It is therefore unknown whether discrete differences between adult morphs are already present at the endocrine level during development.
A polyphenism typically involves a suite of morphological, physiological and life-history traits that may respond to the same environmental signal (e.g. [20,21]). The central regulation of systemic hormone titres enables integration of traits at the organismal level, but can thereby potentially constrain their independent evolution [22–24]. On the other hand, sensitivity of the local tissue determines the response to the hormone, indicating scope for differentiated regulation of the traits comprising the polyphenism [9]. In contrast with theoretical advances (e.g. [24]), there is little empirical knowledge on the extent to which suites of traits regulated by the same hormone constitute integrated phenotypes across environmental gradients, or can respond independently.
With the present study, we aim to understand how hormonal mechanisms regulate a suite of fitness traits involved in the phenotypic plasticity in Bicyclus anynana. This afrotropical butterfly has evolved developmental plasticity as an adaptation to its seasonal environment [25,26]. In the warm wet season, butterflies have large, prominent eyespots on the ventral surface of their wings, which are probably involved in the deflection of predatory attacks [27]. Butterflies of the cool dry season express a cryptic wing pattern with small to virtually absent eyespots. In the dry season in the field there is strong natural selection against conspicuous eyespots [13]. Furthermore, these butterflies express an alternative physiology and life-history strategy that allows them to bridge the period of (nutritional) stress that the dry season represents [28]. During the dry season, adults have altered metabolic rate, and accumulate more mass and higher fat content during the larval stage, important fitness traits associated with adult starvation resistance [20,29–31]. Finally, reproduction is delayed until the end of the dry season [25,28,32]. The seasonal adult morphs are induced in response to temperature during a critical period of pre-adult development [25,26]. Analyses of the reaction norm for wing pattern have revealed a linear response to developmental temperature [25,33], but it is unknown how the life-history traits respond to a gradient in environmental temperature.
Ecdysteroids have been found to be involved in regulating wing-pattern plasticity in B. anynana [18,23,34], but it is unknown whether these hormones have a role in regulating the full suite of traits involved in the seasonal adaptation. Furthermore, it is unknown how ecdysteroid titres change along a continuous gradient in environmental temperature and how this response relates to those of the phenotypic traits. In this study, we apply the reaction norm concept to the hormone dynamics underlying the phenotypic response. The extension of the use of the reaction norm perspective to developmental and molecular processes regulating the phenotype promises to be a useful tool in the integrative study of phenotypic plasticity [35].
We manipulated the developmental environment by rearing cohorts of larvae under five different temperatures spanning the natural range of seasonal environments, with the lowest temperature corresponding to the dry-season environment and the highest to the wet-season environment. We measured the reaction norms for ecdysteroids and JHs during the critical pupal stage, as well as for size at maturity, relative abdomen to total body mass (as a measure of allocation of resources to early life reproduction versus flight ability), metabolic rate, fat reserves and ventral wing pattern—key fitness traits involved in the seasonal polyphenism.
2. Material and methods
(a). Experimental design
Cohorts of B. anynana used in this experiment were derived from an outbred wild-type population established in the laboratory in 1988. The experiment was carried out in two phases, one for the measurement of phenotypic traits and the other for measurement of hormone titres. In each phase, 2000 larvae were reared from egg to adult (n = 400 per temperature treatment). Eggs were collected from the wild-type population on a single day and kept at 23.5°C until hatching. Larvae were reared on maize (Zea mays) in climate-controlled chambers at 70 per cent relative humidity (RH) with a 12 L : 12 D light/dark cycle. After hatching, larvae were randomly divided over each of five climate-controlled chambers (19°C, 21°C, 23°C, 25°C and 27°C, ±0.5°C) representing five temperature treatments, with a different allocation of temperature treatments to chambers in the two phases of the experiment. The lowest temperature corresponds to dry-season conditions in the field and the highest temperature to wet-season conditions [25]. Temperature and RH were logged throughout the rearing process using data loggers (±0.2°C) to ensure stability of environmental conditions.
(b). Life-history traits
For each individual, we recorded development time as the number of days between hatching of the egg and eclosion of the butterfly. Pupae were weighed within 36 h after pupation to the nearest 0.1 mg. One day after eclosion, 100 males and 100 females per temperature treatment were haphazardly selected for resting metabolic rate (RMR) measurements. Fifty butterflies per rearing temperature per sex were measured at 19°C, and 50 at 27°C, in a climate-controlled chamber during the dark phase of the diurnal cycle. RMR was measured as the individual rate of CO2 respiration (millilitre per hour) over a period of 20 min, following [20]. Following RMR measurements, wings were cut off after which the butterflies were dried for 48 h at 55°C and weighed to the nearest 0.01 mg. Total fat (triglyceride and free fatty acids) was extracted by incubating the dried butterflies at room temperature in 2 : 1 (v/v) dichloromethane : methanol for 96 h, followed by drying and weighing, yielding fat-free dry weight. Fat content was calculated by subtracting the fat-free dry weight from the initial dry mass. In order to estimate allocation of resources to different parts of the body, thorax and abdomen were dried and weighed separately.
(c). Wing pattern
The ventral surface of one hindwing of each individual was photographed using a digital still camera connected to a binocular microscope (Leica). The images were analysed with ImagePro 6.0 software to measure the following wing pattern elements: (i) distance between the first and the fifth eyespot; (ii) radius of the inner black disc of the fifth eyespot; (iii) radius of the white centre of the fifth eyespot; and (iv) width of the median band (after [36]).
(d). Hormone titres
For female pupae of each temperature treatment, we measured ecdysone (Ecd), 20-hydroxyecdysone (20E), and JH-I, JH-II and JH-III titres at 11 time points throughout the earlier 55 per cent of the pupal stage, with five replicate pupae per time point. To correct for the direct effect of temperature on pupal development time, we scaled the time points for each temperature treatment separately to the total average duration of the pupal stage. For each temperature treatment, we chose 11 time points after pupation, corresponding to approximately 5 to 55 per cent of total pupal developmental time, spanning the relevant time window for ecdysteroid dynamics [23]. We took 20 µl haemolymph samples from individual pupae, sampling each pupa only once. Hormone titres were measured from haemolymph by liquid chromatography–mass spectrometry (LC-MS), using the method developed by Westerlund & Hoffmann [37] and Westerlund [38], with minor modifications to the protocol (for details see electronic supplementary material). This method allows for simultaneous quantification of all hormones from the same sample.
(e). Statistical analyses
(i). Life-history traits and wing pattern
Data were analysed using two-way analysis of variance (ANOVA) for each trait separately, with temperature treatment and sex as fixed effects. RMR and fat content were first analysed with dry weight as the only independent variable, of which the residuals were used as the dependent variables in the ANOVAs. Likewise, for relative abdomen mass we used the residuals of the model with abdomen dry weight as dependent, and total dry weight as independent variable. Finally, the four wing-pattern measurements were reduced using a principal component analysis (PCA; cf. [36]), pooling data across the sexes. The first principal component (PC1) explained 50.5 per cent of the total variation and was associated with the traits that are indicative of seasonality (radius of the inner black disc of the fifth eyespot, radius of the white centre of the fifth eyespot and width of the median band). PC2 explained 30.3 per cent of the total variation and was associated with the distance between the first and the fifth eyespot, an index of size rather than seasonality. Thus, only PC1 was further analysed. Post hoc comparisons between specific levels of a factor were performed using Tukey's honest significant differences (HSD) tests.
(ii). Hormone titres
Previous work on B. anynana has shown that ecdysteroid titres peak at around 20 to 40 per cent of pupal development (hours after pupation as percentage of total pupal development time), with lower titres before and after. Titres of the two seasonal morphs have similarly shaped curves and similar absolute values, but show a difference in timing of peak titres [18,23,34]. To compare hormone dynamics across temperature treatments, we estimated the timing of the peaks for Ecd and 20E by fitting, for each hormone separately, the function
to the time series of each temperature, where Y is the hormone concentration (picograms per microlitre) at time t (relative time after pupation as fraction of total pupal time), and a and b are parameters determining the height and timing of the peak.
For each treatment, and for Ecd and 20E separately, we randomly drew one data point for each time point, using the five replicate pupae per time point, yielding five replicate time series per treatment per hormone. Through each time series, we fitted the function with parameter values minimizing residual sum of squares. We thus obtained, per temperature treatment per hormone, five independent estimates of the two parameter values based on the five replicate pupae per time point. For this function, the timing of the peak tpeak is given by b/2a (calculated by setting the first derivative of the function to 0), yielding five independent estimates of tpeak. This tpeak was subsequently used as dependent variable in a one-way ANOVA with temperature treatment as fixed factor. Post hoc comparisons between specific treatment levels were performed using Tukey's HSD tests. As we had no a priori expectations regarding JH-III concentration dynamics during the pupal stage, we used JH-III concentration as the dependent variable in a linear model with temperature treatment as fixed factor and relative time after pupation (as fraction of total pupal time) as covariate.
To estimate the potential effect of diurnal cycle on hormone concentrations (cf. [39]), we used, for each hormone separately, one-way ANOVA with hour of day at which a pupa was sampled as fixed factor and hormone concentration as dependent variable, followed by Tukey's HSD tests.
3. Results
(a). Reaction norms of phenotypic traits
All phenotypic traits involved in the seasonal variation showed a significant response to the gradient of developmental temperature. However, the precise shape of each reaction norm differed across traits. Some traits changed gradually and linearly along the temperature gradient, while other traits showed a discontinuous change at intermediate temperatures. Furthermore, for some traits there were marked differences between males and females in their response, while for other traits no such sex specificity was found.
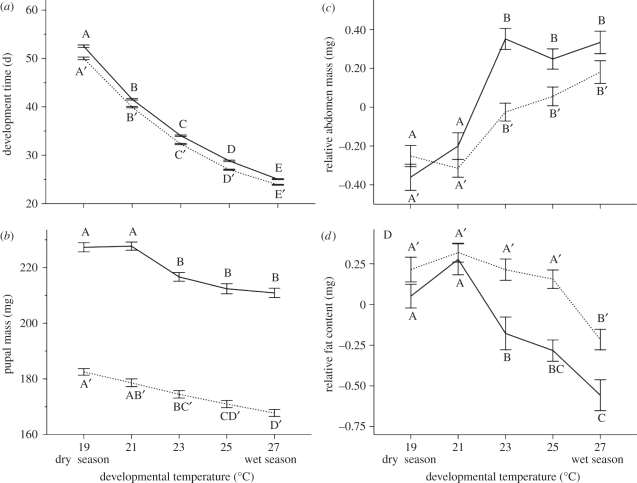
In both sexes, development time decreased continuously with increasing developmental temperature; larvae developed faster under wet-season conditions. Though males developed faster than females (p < 0.001), the shape of the reaction norm was virtually identical between the sexes (figure 1a).
Figure 1.
Effects of developmental temperature on (a) development time, (b) pupal mass, (c) relative abdomen mass (residuals from regression of abdomen dry mass on total dry mass) and (d) relative fat content (residuals from regression of fat content on dry mass). Females and males are represented by the solid and dotted lines, respectively. Error bars represent ±1 s.e. with 50 < n < 150. Significant differences across the temperature treatments (Tukey's HSD, p < 0.05) are indicated by different letters, coding for females and males separately.
Across the temperature gradient, pupal mass was lower for males than for females (p < 0.0001), and in both sexes pupae were larger when reared at lower temperatures, corresponding to dry-season conditions. However, the shape of the reaction norm differed between the sexes. In males, pupal mass decreased in a continuous, linear manner with increasing developmental temperature, with intermediately sized pupae at intermediate temperatures. By contrast, in females, pupal mass did not change within the lower or higher ends of the reaction norm but showed a significant decrease between 21°C and 23°C (figure 1b).
Relative abdomen mass, as a measure of relative allocation to reproduction versus flight, was higher in adult females than in males, but only when they had developed at the three higher temperatures (i.e. wet-season conditions; p < 0.01). At lower temperatures, males did not differ from females. In females, the response to developmental temperature was discontinuous between the two lower and three higher temperatures, with a significant increase between 21°C and 23°C. For males, the pattern was qualitatively similar (i.e. a relatively larger abdomen under wet-season compared with dry-season conditions), but the overall difference between the highest and the lowest temperature was smaller than for females (figure 1c).
At the three higher temperatures, females had lower adult relative fat content than males (p < 0.01), while at 19°C and 21°C males did not differ from females. Male relative fat content did not change along the temperature gradient, with the exception of 27°C, where it was lowest when compared with the other temperatures. In females, relative fat content decreased discontinuously with increasing developmental temperature (i.e. females developed highest fat content under dry-season conditions; figure 1d).
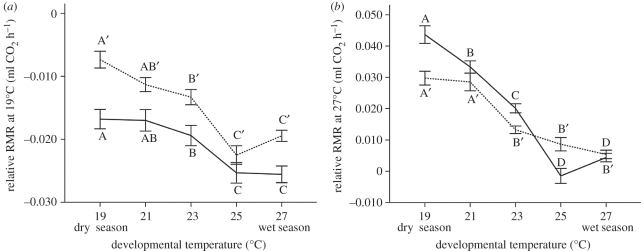
For both sexes and all developmental temperatures, adult RMR (the rate of CO2 respiration at rest) was lower when measured at 19°C than when measured at 27°C (p < 0.0001; compare figure 2a with 2b). RMR at 19°C was higher for males when compared with females across the developmental temperature gradient (p < 0.0001). In both sexes, RMR measured at 19°C showed a discontinuous shift along the developmental temperature gradient; adults developed at lower temperature (dry-season conditions) had higher RMR at 19°C than those developed at higher temperature (figure 2a). In both sexes, RMR measured at 27°C showed a similar decrease with increasing developmental temperature (i.e. butterflies developed highest RMR when reared under dry-season conditions). In males, the response was discontinuous while in females it was almost linear, with the exception of the highest developmental temperature. Furthermore, at the lowest developmental temperature, RMR measured at 27°C was lower in males than in females, while this was not the case at the higher temperatures (figure 2b).
Figure 2.
Effects of developmental temperature on adult relative RMR (residuals from regression of RMR on mass) measured at (a) 19°C and (b) 27°C. Note the difference in scale. Females and males are represented by the solid and dotted lines, respectively. Error bars represent ±1 s.e. with n = 50. Significant differences across the temperature treatments (Tukey's HSD, p < 0.05) are indicated by different letters, coding for males and females separately.
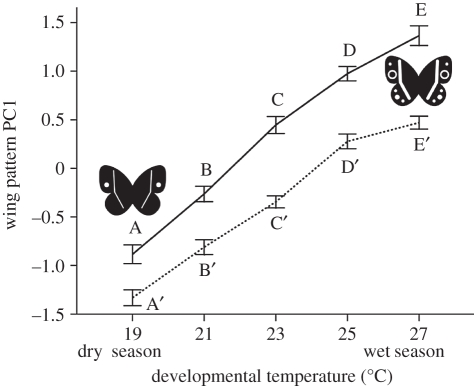
Finally, PC1 of wing pattern changed linearly along the temperature axis in both males and females. Butterflies developed larger eyespots when reared under wet-season conditions, and the reaction norms differed in elevation between the sexes (p < 0.001; figure 3).
Figure 3.
Effects of developmental temperature on the first principal component (PC1) of wing pattern, explaining 50.5% of variation in eyespot and band size. Females and males are represented by the solid and dotted lines, respectively. Error bars represent ±1 s.e. with n = 50. Significant differences across the temperature treatments (Tukey's HSD, p < 0.05) are indicated by different letters, coding for females and males separately.
(b). Dynamics and reaction norms of female hormone concentrations during pupal stage
(i). Lack of diurnal cycle in hormone concentrations
Careful visual inspection of Ecd, 20E and JH-III concentrations plotted against time of day at which a sample was taken revealed no indication for a diurnal cycle for any of these hormones (cf. [39]). For Ecd and JH-III, no effect of time of day on concentration was found (p > 0.1). For 20E, there was a small but significant effect of time of day on concentration (p = 0.05), but post hoc comparisons between specific levels (i.e. hours) were not significant (p > 0.1).
(ii). Ecdysone and 20-hydroxyecdysone
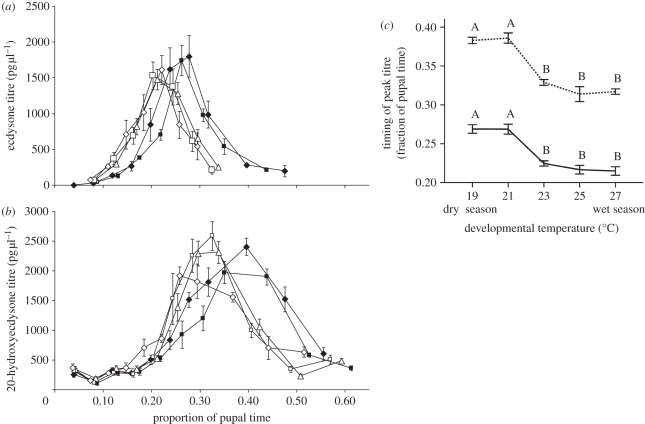
Both ecdysteroids showed qualitatively similar dynamics during the pupal stage, with low early concentrations, peak concentrations between 20 to 40 per cent of pupal development (hours after pupation as percentage of total pupal development time), and low late concentrations (figure 4). For both hormones, concentrations were in a similar range across all temperature treatments (Ecd: approx. 30–1800 pg µl−1; 20E: approx. 100–2600 pg µl−1), but varied with time after pupation. Ecd concentrations were below detection levels very early and very late in the pupal stage.
Figure 4.
Effects of developmental temperature on ecdysone (Ecd) and 20-hydroxyecdysone (20E) dynamics during the pupal stage. Titres (±s.e.) throughout the pupal stage (fraction of total pupal development time) across the five temperature treatments (filled diamonds, 19°C; filled squares, 21°C; open triangles, 23°C; open squares, 25°C; open diamonds, 27°C) of (a) Ecd and (b) 20E. (c) Reaction norms of estimated time of peak (±s.e.) Ecd (solid line) and 20E (dashed line) titre for developmental temperature.
While the absolute concentrations were similar across temperature treatments, the timing of increase, peak and decrease of hormone concentration showed a marked shift between the temperature treatments. We formally compared hormone dynamics throughout the pupal stage across temperature treatments by constructing, for each ecdysteroid separately, nonlinear regression models with hormone concentration as dependent variable and relative time after pupation (as fraction of total pupal time) as independent variable (see §2). All models were significant (95% confidence interval (CI) for p: 0.0002–0.0040) and captured most of the variation (95% CI for R2: 0.79–0.86). Using the estimated parameters for each model, we calculated peak concentrations and their timings.
Peak Ecd concentrations did not differ across temperature treatments (p > 0.7). However, there was a significant shift in the timing of peak concentrations with increasing developmental temperature. Concentrations peaked late at lower temperatures (dry-season conditions) and early at higher temperatures (wet-season conditions; p < 0.0001), with a discontinuous shift between these two types of dynamics occurring between 21°C and 23°C, and no changes between the two lower or among the three higher temperatures (figure 4a,c).
Similarly, peak 20E concentrations did not differ across temperature treatments (p > 0.1), but there was a significant shift in the timing of peak concentrations with increasing developmental temperature. At lower temperatures (dry-season conditions), 20E concentrations peaked late when compared with the higher temperatures (wet-season conditions; p < 0.0001), with a discontinuous shift occurring between 21°C and 23°C. Again, this shift was the only change in timing along the temperature gradient and no intermediate types of dynamics were observed at intermediate temperatures (figure 4b,c).
Ecd concentrations peaked earlier than those of 20E, with a time lag of approximately 10 per cent of pupal time (hours after pupation as percentage of total pupal development time), which was constant along the temperature gradient (figure 4c).
(iii). Juvenile hormones
JH-I was only detected in haemolymph of approximately 14 per cent of all pupae, with concentrations ranging from 1 to 35 pg µl−1 and no effect of developmental temperature. JH-II was below detection level in all analysed pupae. JH-III was detected in haemolymph of pupae of all developmental stages across all developmental temperatures, in a comparable range of concentrations (30–100 pg µl−1; figure 5). There was no effect of relative time after pupation (as fraction of total pupal time) on JH-III titres (p > 0.1), but there was a small but significant effect of developmental temperature (p < 0.0001), with pupae developed at 19°C having highest, and pupae developed at 21°C lowest, JH-III concentrations.
Figure 5.
Dynamics during pupal stage of juvenile hormone (JH)-III titres (±s.e.) across the five temperature treatments. JH-I was only detected in approximately 14 per cent of all pupae, and JH-II was detected in none of the pupae. Filled diamonds, 19°C; filled squares, 21°C; open triangles, 23°C; open squares, 25°C; open diamonds, 27°C.
4. Discussion
(a). Hormone dynamics
The discontinuous expression of a polyphenic trait across an environmental gradient requires some form of a switch mechanism between alternative developmental trajectories. This developmental switch could arise by a change in: (i) the hormone titre; (ii) the sensitivity to the hormone; (iii) the hormone timing; and (iv) the window of sensitivity [12]. Experimental studies have linked each of these scenarios to polyphenisms, either by direct measurement of titres, titre regulators or sensitivity, or indirectly by hormone manipulation. Examples include Ecd titre changes linked to horn length in beetles [40]; morph-associated differences in JH dynamics in a wing-polymorphic crickets [39]; and differences in the timing of Ecd release between the two wing-pattern morphs of butterflies [18]. However, these studies typically concern the hormonal dynamics under only two environmental conditions, which makes it impossible to discern whether these hormonal changes are continuous or discrete. Analysing the precise shape of the reaction norm at the hormone level reveals how a continuous environmental trajectory is translated into discrete alternative developmental trajectories (e.g. [19]).
For the first time, we show that the dichotomy between adult phenotypic morphs can already be programmed at the stage of hormone signalling during development. Our results reveal a discontinuous shift in the timing of peak Ecd and 20E titres in response to a linear gradient of developmental temperatures (figure 4c). Both hormones show this shift in timing between 21°C and 23°C, while there are no significant differences in timing between the two lower temperatures (dry-season conditions), nor among the three higher temperatures (wet-season conditions). None of the measured JHs show a clear response to the temperature gradient (figure 5) and all are, therefore, unlikely to be involved in regulating the polyphenism. Our results indicate that a discontinuous response of ecdysteroid dynamics to the continuous environmental gradient underlies the polyphenism in B. anynana.
(b). Phenotypic responses
In females, but not in males, the response of pupal mass to developmental temperature was discontinuous. Thus, despite large, continuous changes in development time in response to the temperature gradient (figure 1a), female larvae ultimately develop a discontinuous pattern in pupal mass (figure 1b). The correspondence between the responses of female pupal mass and ecdysteroid dynamics (figure 4) to the temperature gradient suggests that these traits share upstream regulators.
In adults, the relative contribution of the abdomen to total body mass showed a clear discontinuous response to developmental temperature (figure 1c), which was particularly pronounced in females. These results indicate a higher relative allocation to flight during the harsh dry season and to reproduction during the favourable wet season, especially in females. Adult fat content in males remained fairly constant across most developmental temperatures, with the exception of the highest, whereas the response was more discontinuous in females (figure 1d). The response of RMR ranged from clearly discontinuous to more linear (figure 2). Overall, the responses of a number of adult traits to the temperature gradient are strikingly similar to the discontinuous response of pupal ecdysteroid dynamics (figure 4), indicating a regulatory role for ecdysteroid signalling during the pupal stage in shaping adult physiological and allocation traits.
In accordance with earlier results [25,33], we found a linear response of ventral wing pattern to the temperature gradient (figure 3), contrasting with the discontinuous reaction norm for pupal ecdysteroid dynamics (figure 4). Previous studies, using hormone manipulation and artificial selection experiments, have demonstrated a functional role of pupal ecdysteroids in the regulation of wing pattern polyphenism, as well as genetic correlations between dynamics of ecdysteroid titres and wing pattern [18,23,34]. Combined, these findings strongly imply an additional level of regulation between the hormone signal and the response of the developmental pathways producing the wing pattern. This regulation is likely to involve changes in the window of hormone sensitivity (for example by altered timing of Ecd receptor expression [12,18]).
5. Conclusions
By applying a continuous temperature gradient to developing larvae and pupae, we showed that a discontinuous ecdysteroid signal during the pupal stage underlies the seasonal polyphenism in B. anynana. Furthermore, several fitness traits (such as relative abdomen mass, RMR, female pupal mass and fat content) displayed a similar, dimorphic response, indicating shared regulation of these traits. In contrast, ventral wing pattern, known to be regulated by ecdysteroids [23], responded in a linear manner. Taken together, our findings suggest that the diversity in shapes of reaction norms of the traits involved in the phenotypic plasticity stems from variation in how each trait responds to the ecdysteroid dynamics.
In view of our results and of earlier findings that revealed a short window of sensitivity of the ventral wing pattern to 20E injections [34] and a complete lack of sensitivity of the dorsal wing pattern [18], we propose that variation across traits in windows of sensitivity to the systemic hormone signal can be a general mechanism underlying both linear and discrete responses to environmental gradients within suites of traits that share a hormonal regulator. This diversity in responses allows for both flexibility and integration of traits underlying adaptations to divergent environments.
Acknowledgements
We thank Niels Wurzer, Mariël Lavrijsen and David Hallesleben for the plant rearing, Joost van den Heuvel for advice on the statistical analyses, and two anonymous reviewers for comments on the manuscript. This work was supported by the EU-funded Network of Excellence LifeSpan (FP6 036894), and the Earth and Life Sciences programme of the Netherlands Organization for Scientific Research (grant no. 814.01.012).
References
- 1.Stearns S. C. 1989. The evolutionary significance of phenotypic plasticity. Phenotypic sources of variation among organisms can be described by developmental switches and reaction norms. Bioscience 39, 436–445 10.2307/1311135 (doi:10.2307/1311135) [DOI] [Google Scholar]
- 2.Schlichting C., Pigliucci M. 1998. Phenotypic evolution: a reaction norm perspective. Sunderland, MA: Sinauer [Google Scholar]
- 3.Brakefield P. M., French V., Zwaan B. J. 2003. Development and the genetics of evolutionary change within insect species. Annu. Rev. Ecol. Evol. Syst. 34, 633–660 10.1146/annurev.ecolsys.34.011802.132425 (doi:10.1146/annurev.ecolsys.34.011802.132425) [DOI] [Google Scholar]
- 4.West-Eberhard M. J. 2003. Developmental plasticity and evolution. New York, NY: Oxford University Press, Inc [Google Scholar]
- 5.Gilbert S. F., Epel D. 2008. Ecological developmental biology: integrating epigenetics, medicine, and evolution. Sunderland, MA: Sinauer [Google Scholar]
- 6.Debat V., David P. 2001. Mapping phenotypes: canalization, plasticity and developmental stability. Trends Ecol. Evol. 16, 555–561 10.1016/S0169-5347(01)02266-2 (doi:10.1016/S0169-5347(01)02266-2) [DOI] [Google Scholar]
- 7.Sultan S. E. 2007. Development in context: the timely emergence of eco-devo. Trends Ecol. Evol. 22, 575–582 10.1016/j.tree.2007.06.014 (doi:10.1016/j.tree.2007.06.014) [DOI] [PubMed] [Google Scholar]
- 8.Shapiro A. M. 1976. Seasonal polyphenism. Evol. Biol. 9, 259–333 [Google Scholar]
- 9.Nijhout H. F. 1994. Insect hormones. Princeton, NJ: Princeton University Press [Google Scholar]
- 10.Zera A. J., Harshman L. G., Williams T. D. 2007. Evolutionary endocrinology: the developing synthesis between endocrinology and evolutionary genetics. Annu. Rev. Ecol. Evol. Syst. 38, 793–817 10.1146/annurev.ecolsys.38.091206.095615 (doi:10.1146/annurev.ecolsys.38.091206.095615) [DOI] [Google Scholar]
- 11.Ketterson E. D., Atwell J. W., McGlothlin J. W. 2009. Phenotypic integration and independence: hormones, performance, and response to environmental change. Integr. Comp. Biol. 49, 365–379 10.1093/icb/icp057 (doi:10.1093/icb/icp057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nijhout H. F. 2003. Development and evolution of adaptive polyphenisms. Evol. Dev. 5, 9–18 10.1046/j.1525-142X.2003.03003.x (doi:10.1046/j.1525-142X.2003.03003.x) [DOI] [PubMed] [Google Scholar]
- 13.Brakefield P. M., Frankino W. A. 2009. Polyphenisms in Lepidoptera: multidisciplinary approaches to studies of evolution. In Phenotypic plasticity in insects: mechanisms and consequences (eds Whitman D. W., Ananthakrishnan T. N.), pp. 121–152 Plymouth, UK: Science Publishers, Inc [Google Scholar]
- 14.Zera A. J. 2007. Wing polymorphism in Gryllus (Orthoptera : Gryllidae): endocrine, energetic and biochemical bases of morph specializations for flight vs. reproduction. In Phenotypic plasticity in insects: mechanisms and consequences (eds Whitman D. W., Ananthakrishnan T. N.), pp. 547–590 Plymouth, UK: Science Publishers, Inc [Google Scholar]
- 15.Smith C. R., Toth A. L., Suarez A. V., Robinson G. E. 2008. Genetic and genomic analyses of the division of labour in insect societies. Nat. Rev. Genet. 9, 735–748 10.1038/nrg2429 (doi:10.1038/nrg2429) [DOI] [PubMed] [Google Scholar]
- 16.Trotta V., Calboli C. F., Ziosi M., Guerra D., Pezzoli M. C., David J. R., Cavicchi S. 2006. Thermal plasticity in Drosophila melanogaster: a comparison of geographic populations. BMC Evol. Biol. 6, 67. 10.1186/1471-2148-6-67 (doi:10.1186/1471-2148-6-67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liefting M., Hoffmann A. A., Ellers J. 2009. Plasticity versus environmental canalization: population differences in thermal responses along a latitudinal gradient in Drosophila serrata. Evolution 63, 1954–1963 10.1111/j.1558-5646.2009.00683.x (doi:10.1111/j.1558-5646.2009.00683.x) [DOI] [PubMed] [Google Scholar]
- 18.Brakefield P. M., Kesbeke F., Koch P. B. 1998. The regulation of phenotypic plasticity of eyespots in the butterfly Bicyclus anynana. Am. Nat. 152, 853–860 10.1086/286213 (doi:10.1086/286213) [DOI] [PubMed] [Google Scholar]
- 19.Anstey M. L., Rogers S. M., Ott S. R., Burrows M., Simpson S. J. 2009. Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science 323, 627–630 10.1126/science.1165939 (doi:10.1126/science.1165939) [DOI] [PubMed] [Google Scholar]
- 20.Pijpe J., Brakefield P. M., Zwaan B. J. 2007. Phenotypic plasticity of starvation resistance in the butterfly Bicyclus anynana. Evol. Ecol. 21, 589–600 10.1007/s10682-006-9137-5 (doi:10.1007/s10682-006-9137-5) [DOI] [Google Scholar]
- 21.Brisson J. A. 2010. Aphid wing dimorphisms: linking environmental and genetic control of trait variation. Phil. Trans. R. Soc. B 365, 605–616 10.1098/rstb.2009.0255 (doi:10.1098/rstb.2009.0255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ketterson E. D., Val Nolan J. 1999. Adaptation, exaptation, and constraint: a hormonal perspective. Am. Nat. 154, S4–S25 10.1086/303280 (doi:10.1086/303280) [DOI] [PubMed] [Google Scholar]
- 23.Zijlstra W. G., Steigenga M. J., Koch P. B., Zwaan B. J., Brakefield P. M. 2004. Butterfly selected lines explore the hormonal basis of interactions between life histories and morphology. Am. Nat. 163, E76–E87 10.1086/383595 (doi:10.1086/383595) [DOI] [PubMed] [Google Scholar]
- 24.McGlothlin J. W., Ketterson E. D. 2008. Hormone-mediated suites as adaptations and evolutionary constraints. Phil. Trans. R. Soc. B 363, 1611–1620 10.1098/rstb.2007.0002 (doi:10.1098/rstb.2007.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brakefield P. M., Reitsma N. 1991. Phenotypic plasticity, seasonal climate and the population biology of Bicyclus butterflies (Satyridae) in Malawi. Ecol. Entomol. 16, 291–303 10.1111/j.1365-2311.1991.tb00220.x (doi:10.1111/j.1365-2311.1991.tb00220.x) [DOI] [Google Scholar]
- 26.Brakefield P. M., Gates J., Keys D., Kesbeke F., Wijngaarden P. J., Monteiro A., French V., Carroll S. B. 1996. Development, plasticity and evolution of butterfly eyespot patterns. Nature 384, 236–242 10.1038/384236a0 (doi:10.1038/384236a0) [DOI] [PubMed] [Google Scholar]
- 27.Lyytinen A., Brakefield P. M., Lindstrom L., Mappes J. 2004. Does predation maintain eyespot plasticity in Bicyclus anynana? Proc. R. Soc. Lond. B 271, 279–283 10.1098/rspb.2003.2571 (doi:10.1098/rspb.2003.2571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brakefield P. M., Pijpe J., Zwaan B. J. 2007. Developmental plasticity and acclimation both contribute to adaptive responses to alternating seasons of plenty and of stress in Bicyclus butterflies. J. Biosci. 32, 465–475 10.1007/s12038-007-0046-8 (doi:10.1007/s12038-007-0046-8) [DOI] [PubMed] [Google Scholar]
- 29.de Jong M. A., Kesbeke F. M., Brakefield P. M., Zwaan B. J. 2010. Geographic variation in thermal plasticity of life-history and wing pattern in Bicyclus anynana. Clim. Res. 43, 91–102 10.3354/cr00881 (doi:10.3354/cr00881) [DOI] [Google Scholar]
- 30.Zwaan B. J., Bijlsma R., Hoekstra R. F. 1991. On the developmental theory of ageing. I. Starvation resistance and longevity in Drosophila melanogaster in relation to preadult breeding conditions. Heredity 68, 123–130 10.1038/hdy.1992.19 (doi:10.1038/hdy.1992.19) [DOI] [PubMed] [Google Scholar]
- 31.Chippindale A. K., Chu T., Rose M. R. 1996. Complex trade-offs and the evolution of starvation resistance in Drosophila melanogaster. Evolution 50, 753–766 10.2307/2410848 (doi:10.2307/2410848) [DOI] [PubMed] [Google Scholar]
- 32.Fischer K., Eenhoorn E., Bot A. N., Brakefield P. M., Zwaan B. J. 2003. Cooler butterflies lay larger eggs: developmental plasticity versus acclimation. Proc. R. Soc. Lond. B 270, 2051–2056 10.1098/rspb.2003.2470 (doi:10.1098/rspb.2003.2470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wijngaarden P. J., Koch P. B., Brakefield P. M. 2002. Artificial selection on the shape of reaction norms for eyespot size in the butterfly Bicyclus anynana: direct and correlated responses. J. Evol. Biol. 15, 290–300 10.1046/j.1420-9101.2002.00380.x (doi:10.1046/j.1420-9101.2002.00380.x) [DOI] [Google Scholar]
- 34.Koch P. B., Brakefield P. M., Kesbeke F. 1996. Ecdysteroids control eyespot size and wing color pattern in the polyphenic butterfly Bicyclus anynana (Lepidoptera: Satyridae). J. Insect Physiol. 42, 223–230 10.1016/0022-1910(95)00103-4 (doi:10.1016/0022-1910(95)00103-4) [DOI] [Google Scholar]
- 35.Aubin-Horth N., Renn S. C. P. 2009. Genomic reaction norms: using integrative biology to understand molecular mechanisms of phenotypic plasticity. Mol. Ecol. 18, 3763–3780 10.1111/j.1365-294X.2009.04313.x (doi:10.1111/j.1365-294X.2009.04313.x) [DOI] [PubMed] [Google Scholar]
- 36.Wijngaarden P. J., Brakefield P. M. 2001. Lack of response to artificial selection on the slope of reaction norms for seasonal polyphenism in the butterfly Bicyclus anynana. Heredity 87, 410–420 10.1046/j.1365-2540.2001.00933.x (doi:10.1046/j.1365-2540.2001.00933.x) [DOI] [PubMed] [Google Scholar]
- 37.Westerlund S. A., Hoffmann K. H. 2004. Rapid quantification of juvenile hormones and their metabolites in insect haemolymph by liquid chromatography-mass spectrometry (LC-MS). Anal. Bioanal. Chem. 379, 540–543 10.1007/s00216-004-2598-x (doi:10.1007/s00216-004-2598-x) [DOI] [PubMed] [Google Scholar]
- 38.Westerlund S. A. 2004. Measuring juvenile hormone and ecdysteroid titres in insect haemolymph simultaneously by LC-MS: the basis for determining the effectiveness of plant-derived alkaloids as insect growth regulators. PhD thesis. Universität Bayreuth, Bayreuth, Germany: See http://opus.ub.uni-bayreuth.de/volltexte/2004/106/index.html [Google Scholar]
- 39.Zhao Z. W., Zera A. J. 2004. The hemolymph JH titre exhibits a large-amplitude, morph-dependent, diurnal cycle in the wing-polymorphic cricket, Gryllus firmus. J. Insect Physiol. 50, 93–102 10.1016/j.jinsphys.2003.10.003 (doi:10.1016/j.jinsphys.2003.10.003) [DOI] [PubMed] [Google Scholar]
- 40.Emlen D. J., Nijhout H. F. 1999. Hormonal control of male horn length dimorphism in the dung beetle Onthophagus taurus (Coleoptera: Scarabaeidae). J. Insect Physiol. 45, 45–53 10.1016/S0022-1910(98)00096-1 (doi:10.1016/S0022-1910(98)00096-1) [DOI] [PubMed] [Google Scholar]