Abstract
Indirect competition is often mediated by plant responses to herbivore feeding damage and is common among phytophagous insect species. Plant-mediated responses may be altered by abiotic conditions such as nutrient supply, which can affect plant growth, morphology, and the concentration of primary and secondary metabolites. Nutrient supply can be manipulated by the type and amount of fertilizer applied to a plant. Brassica oleracea plants were grown in several types of fertilizer, including those commonly used in sustainable and conventional agricultural systems. The occurrence of indirect competition between two phytophagous species from different feeding guilds (a phloem-feeder and leaf-chewer) was assessed. The leaf-chewer reduced aphid populations on plants growing in most fertilizer treatments, but not on those in the ammonium nitrate fertilizer treatment, which caused the highest concentration of foliar nitrogen. The potential consequences of our findings are discussed for phytophagous species in conventional and sustainable agricultural systems.
Keywords: Brevicoryne brassicae, glucosinolate, induced defence, nitrogen, plant-mediated competition, Plutella xylostella
1. Introduction
Competition between phytophagous species sharing a host plant has been considered to play a structuring role in insect communities by some ecologists (e.g. [1]), whereas others have argued that food resources for phytophages are plentiful, and thus competition is rare (e.g. [2]; see references in [3] for full discussion). A recent meta-analysis found that competition among phytophagous insects occurred in 62 per cent of cases in which two species shared a host plant [3] and an earlier review found that 76 per cent of potential pairwise interactions were competitive [4].
Most phytophagous interactions examined involve indirect competition, whereby two species are spatially or temporally separated on the same host plant but affect each other through changes to host plant morphology or chemistry, often by inducing plant defences [3]. For example, root herbivores can alter the performance and abundance of above-ground phytophages through changes to host plant chemistry [5–7]. Kaplan & Denno [3] concluded that indirect plant-mediated mechanisms are likely to affect entire phytophage communities as well as pairwise interactions, and that their prevalence and importance may have previously been underestimated. The response of host plants to insect herbivore feeding damage may therefore play a key role in structuring communities of phytophages and the predators and parasitoids that feed on them [8–10].
Plant responses to feeding damage often depend on the abiotic conditions in which a plant grows [11]. For example, Hawkes & Sullivan [12] found that resource availability affected plant regrowth following damage by herbivores, and increasing nutrient availability to woody plants altered their induced response to artificial damage intended to mimic herbivory [13–15]. Higher demand for sustainable agriculture and horticulture has decreased the use of high-input mineral fertilizers and increased the use of green manures and animal fertilizers that provide slow-release forms of nitrogen [16]. Organically fertilized plants may contain a higher concentration of secondary metabolites than those grown in conventional mineral fertilizers [17–19]. If fertilizer type also alters the capacity of plants to produce induced defences, it could alter host-mediated interactions between phytophages in sustainable and high-input agricultural systems.
We grew Brassica oleracea plants in several types of fertilizers and assessed competition between a sap-feeder (Brevicoryne brassicae L.; Sternorrhyncha: Homoptera) and a leaf-chewer (Plutella xylostella L.; Lepidoptera: Plutellidae). Brevicoryne brassicae feeds predominantly on the plant apex and young foliage [20], whereas P. xylostella larvae feed mainly on older leaves (V. Chadfield & J. T. Staley 2009, unpublished data). The two species co-occur on crucifers during the spring and early summer in the UK [21], but are unlikely to compete through interference owing to their different feeding modes and sites.
Brassica-constitutive glucosinolates respond to both fertilizer concentration and type [21–24]. The effect of fertilizer type on induced concentrations of glucosinolates has not been previously investigated. Fertilizers used in the current study included a mineral source of nitrogen (ammonium nitrate) and two fertilizers that contained nitrogen derived from animal sources: organic chicken manure, which is regularly used in organic horticulture [25,26]; and an intermediate fertilizer that contains animal-derived nitrogen (hoof and horn) and mineral-derived potassium (John Innes base fertilizer). Potassium availability can affect nitrogen uptake by plants, which has the potential to alter plant–phytophage interactions [27]. Foliar nitrogen and glucosinolate concentrations were measured to determine whether fertilizer treatments altered these key determinants of Brassica plant quality for phytophages [28,29].
2. Material and methods
(a). Experimental design and plant cultivation
The experimental design consisted of fertilizer and insect-competition treatments imposed in a fully factorial design. Four resource treatments were applied: three fertilizer types (details below) and an unfertilized treatment. The insect treatments consisted of: a Brevicoryne brassicae L. (Sternorrhyncha: Homoptera) population (no interspecific competition; treatment abbreviation = B); a Plutella xylostella L. (Lepidoptera: Plutellidae) population (=P); or populations of both herbivore species feeding on a plant in interspecific competition (=B + P). Eight plants (replicates) were used for each of the 12 combinations of the two treatment factors.
Brassica oleracea var. capitata cv Derby Day seeds (Tozer Seeds, UK) were planted in 22 mm diameter × 50 mm peat plugs (Jiffy 7 pellets, LBS Horticulture, UK) in a greenhouse. Minimum temperature was 20°C during the day (16 h) and 14°C at night (8 h). Screened vents opened at temperatures of 3°C above the minimum temperature. Overhead lighting (mercury halide and sodium bulbs) was supplied during the day to ensure a minimum light intensity of 300 W m−2.
Seedlings were transplanted into compost consisting of 33 per cent peat, 33 per cent loam, 22 per cent sand and 12 per cent grit by volume (Monro Horticulture, UK) in 13 cm diameter × 12 cm tall pots two weeks after germination. The fertilizer treatments consisted of the addition of 9.28 g ammonium nitrate fertilizer (Nitram, AN), 62.8 g John Innes fertilizer (JI; Monro Horticulture, UK), 74.5 g chicken manure (CM; Greenvale Farms Ltd, UK) or no fertilizer (NF) to 10 l of potting compost prior to transplanting the seedlings. The AN fertilizer consists of 34.5 per cent N; chicken manure of 4.5 per cent N, 2.5 per cent P, 2.5 per cent K; and the JI fertilizer of 5.1 per cent N, 7.2 per cent P and 10 per cent K. Our treatments provided 0.32 g of total nitrogen per litre of potting compost for each fertilizer, 0.18 g phosphorus and potassium per litre of fertilizer for plants growing in chicken manure, and 0.45 g phosphorus and 0.63 g potassium per litre for plants in JI fertilizer. Plants were grown in compost for 4 weeks before being used for the experiment.
(b). Herbivore performance under competition
The two insect species were caged on host plants either as a single herbivore species (no interspecific competition) or together (interspecific competition). Five apterous B. brassicae adults were placed on the fifth leaf of 16 plants from each fertilizer treatment in a controlled environment room at 20°C (±1°C), 60 to 80 per cent relative humidity and 16 L : 8 D h photoperiod. To contain the insects, each plant was enclosed in a transparent plastic bag (24 cm diameter, 65 cm height) with perforated holes that allowed air circulation. After 48 h, groups of 10 second instar P. xylostella were weighed (Sartorius MP3 micro-balance, UK) and placed on each of eight plants already infested with B. brassicae and eight uninfested plants from each fertilizer treatment. Prior to the experiment, UK populations of B. brassicae and P. xylostella had been cultured separately on Chinese cabbage (Brassica chinensis L. var. pekinensis cv Wong Bok) for several generations under the same environmental conditions as detailed above [30]. The infestation sequence (B. brassicae before P. xylostella) was chosen to mimic the order of arrival of these species on UK Brassica plants [21].
Insect performance was assessed for both species. Four days after their introduction, the P. xylostella larvae were removed and weighed again to assess their relative growth rate, before being reintroduced to the same plant. Brevicoryne brassicae populations were counted on each plant 7 and 14 days after they were first introduced. Plants were checked daily, and as each P. xylostella pupated, it was removed and weighed again, and the date was recorded. In total, P. xylostella removed approximately 10 per cent of the foliage from each plant during their development.
(c). Plant biomass and chemistry
A separate batch of six plants was allocated to each of the 12 treatment combinations for foliar chemical analysis. Plants were grown and infested with herbivores as described above. Ten days after infestation with B. brassicae (approximately halfway through the experiment) the plants were harvested for analysis. Above-ground biomass was recorded. Samples were stored at −20°C prior to being freeze-dried and milled through a 1 mm diameter mesh. Total foliar nitrogen concentration was determined using an oxidative combustion method [31] in a FlashEA 1112 analyser (ThermoScientific, USA).
Foliar glucosinolates were separated and individual compounds were identified and quantified using the methods described by Heaney et al. [32]. Desulphoglucosinolates were extracted as detailed by Kazana et al. [33]. Samples were analysed by high-performance liquid chromatography on an Agilent 1200 series instrument equipped with a Phenomenex Luna 3 micron C18(2) (150 × 2 mm) reverse-phase column. Desulphoglucosinolates were separated using a water–acetonitrile gradient (solvent A water, solvent B acetonitrile; 0–15 min, 25% B; 15–17 min, 70% B) at a flow rate of 0.2 ml min−1. Retention times of known standards were used to identify desulphoglucosinolates; identification was confirmed by liquid chromatography–mass spectrometry. Glucosinolates were grouped into indole and aliphatic glucosinolates [29].
(d). Statistical analysis
Our statistical analyses tested the effects of fertilizer and insect competition treatments on the abundance of B. brassicae, the performance of P. xylostella, and plant biomass and chemistry. The effects of fertilizer treatment, competition with P. xylostella and time on the number of B. brassicae were analysed using a generalized linear mixed model (GLMM) with a quasi-Poisson distribution [34]. Likelihood-ratio tests were used to test the null hypotheses [34]. Mean relative growth rate, development time and pupal weight were calculated for the 10 P. xylostella feeding on each plant (to avoid pseudoreplication), and these means were used in statistical analyses. Factorial two-way analyses of variance (ANOVAs) were used to test the effects of fertilizer and herbivore treatments on the relative growth rate, development time and pupal masses of P. xylostella, and the biomass, nitrogen, carbon and glucosinolate content of B. oleracea foliage. Larval relative growth rate was calculated as (larval mass after 96 h of feeding − initial larval mass)/initial larval mass [35]. Relative growth rate of Plutella xylostella and the concentration of indole and aliphatic glucosinolates were ln-transformed prior to analysis to meet the assumptions of ANOVA. Following a significant ANOVA result, Tukey HSD post hoc tests were conducted to determine which factor level means differed significantly [36]. All analyses were conducted using R v. 2.7.2 [37].
3. Results
(a). Herbivore performance under competition
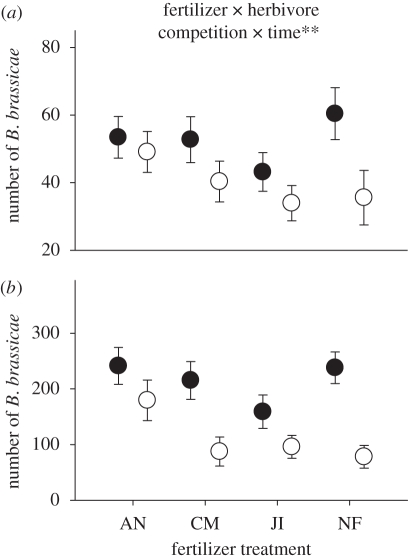
There was a significant interaction between fertilizer treatment, the presence of P. xylostella and time on the number of B. brassicae (GLMM likelihood-ratio test: fertilizer × herbivore competition × time: 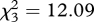 , p = 0.00708). Brevicoryne brassicae populations were significantly smaller on plants also containing P. xylostella compared with those plants containing only aphids on two of the four fertilizer treatments halfway through the experiment (7 days; figure 1a). By the end of the experiment (14 days), the presence of P. xylostella reduced B. brassicae populations on plants growing in all fertilizer treatments, except those in ammonium nitrate (figure 1b).
, p = 0.00708). Brevicoryne brassicae populations were significantly smaller on plants also containing P. xylostella compared with those plants containing only aphids on two of the four fertilizer treatments halfway through the experiment (7 days; figure 1a). By the end of the experiment (14 days), the presence of P. xylostella reduced B. brassicae populations on plants growing in all fertilizer treatments, except those in ammonium nitrate (figure 1b).
Figure 1.
Mean (±s.e.) Brevicoryne brassicae with (B + P, open circles) or without (B, filled circles) Plutella xylostella per plant growing in ammonium nitrate (AN), chicken manure (CM) or John Innes (JI) fertilizer, or unfertilized compost (NF) at (a) 7 days and (b) 14 days after infestation. Significant generalized linear mixed model parameters: **p < 0.01.
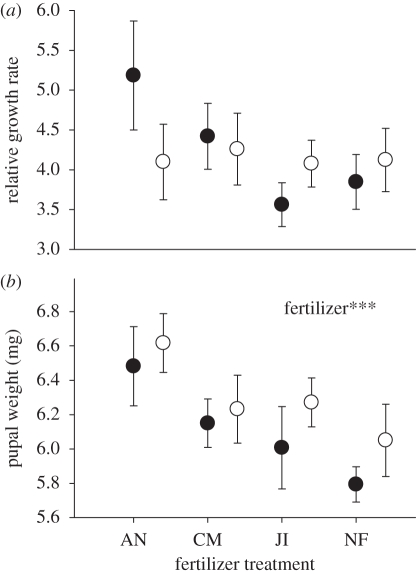
The performance of P. xylostella was not affected by competition with B. brassicae, but was altered by fertilizer treatment. Competition with B. brassicae did not affect the relative growth rate (F1,74 = 0.78, p > 0.05; figure 2a), larval development time (F1,74 = 0.53, p > 0.05) or pupal mass (F1,74 = 0.71, p > 0.05; figure 2b) of P. xylostella. Plutella xylostella pupae were significantly heavier on plants fertilized with ammonium nitrate than on unfertilized plants, and intermediate on plants growing in the other two fertilizers (F3,74 = 4.24, p < 0.001; figure 2b). Fertilizer treatment had no effect on either relative growth rate (F3,74 = 1.73, p > 0.05) or development time (F3,74 = 2.41, p > 0.05) of P. xylostella.
Figure 2.
Mean (±s.e.) Plutella xylostella (a) relative growth rate and (b) pupal weight with (B + P, open circles) or without (P, filled circles) B. brassicae on plants growing in ammonium nitrate (AN), chicken manure (CM) or John Innes (JI) fertilizer, or unfertilized compost (NF). Significant ANOVA factors: ***p < 0.001.
(b). Plant biomass and chemistry
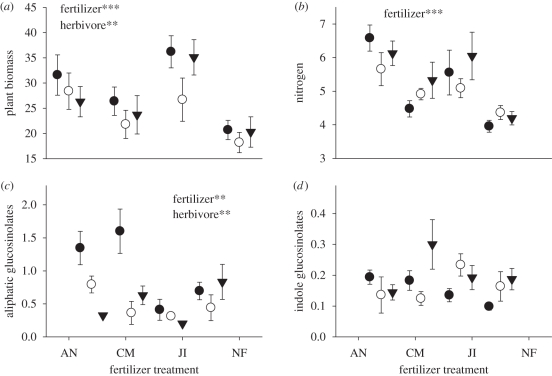
Plant biomass was affected both by fertilizer treatment and by which herbivore species fed on them. Plants grown in ammonium nitrate or JI fertilizer were significantly larger than those grown in CM or unfertilized compost (table 1; Tukey HSD post hoc tests all p < 0.05). Plants with only B. brassicae were also larger than those with only P. xylostella or both herbivores (table 1; Tukey HSD post hoc tests all p < 0.05). Fertilizer and herbivore competition treatments did not have interacting effects on plant size (figure 3a and table 1).
Table 1.
Analysis of variance results for the effects of herbivore treatment and fertilizer type on B. oleracea biomass, foliar nitrogen, carbon, total aliphatic glucosinolate and total indole glucosinolate content.
| fertilizer | herbivore competition | fertilizer × herbivore competition | |
|---|---|---|---|
| plant biomass | F3,53 = 19.02*** | F2,53 = 6.31** | F6,53 = 0.89 |
| nitrogen | F3,53 = 13.95 *** | F2,53 = 1.03 | F6,53 = 1.07 |
| carbon | F3,53 = 0.39 | F2,53 = 1.23 | F6,53 = 0.08 |
| aliphatic glucosinolates | F3,53 = 4.96 ** | F2,53 = 5.96 ** | F6,53 = 2.14 |
| indole glucosinolates | F3,53 = 1.42 | F2,53 = 1.66 | F6,53 = 2.13 |
***p < 0.001.
**p < 0.01.
Figure 3.
Mean (±s.e.) of (a) fresh biomass, (b) nitrogen, (c) total aliphatic glucosinolate and (d) total indole glucosinolate content (% dry weight) of Brassica oleracea foliage growing in ammonium nitrate (AN), chicken manure (CM) or John Innes (JI) fertilizer, or no fertilized compost (NF) with Brevicoryne brassicae (B, filled circles), Plutella xylostella (P, open circles) or both herbivores (B + P, filled inverted triangles). Significant ANOVA factors: ***p < 0.001; **p < 0.01.
Foliar nitrogen content was altered by the fertilizer treatment but not by the insect competition treatment (figure 3b and table 1). Foliar nitrogen was significantly more concentrated in plants fertilized with AN than those fertilized with CM or unfertilized plants (table 1; Tukey HSD post hoc tests all p < 0.05). Nitrogen content was intermediate in plants fertilized with JI fertilizer, but not significantly differently from those fertilized with AN.
Aliphatic glucosinolate concentration was affected both by the fertilizer and the herbivore treatments (figure 3c and table 1). Aliphatic glucosinolates were more concentrated in plants grown in AN or CM than those grown in JI fertilizer (Tukey HSD post hoc tests p < 0.05), and in intermediate levels in the unfertilized plants. Aliphatic glucosinolate concentration was also greater in plants with just B. brassicae than in plants with both B. brassicae and P. xylostella, or P. xylostella alone (Tukey HSD post hoc tests p < 0.05; figure 3c). Foliar carbon and indole glucosinolate contents were not affected by either the fertilizer or the herbivore treatments (figure 3d and table 1).
4. Discussion
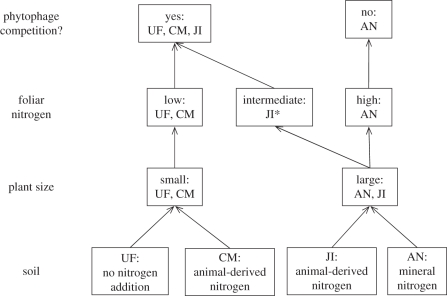
Asymmetric competition occurred between P. xylostella and B. brassicae, as P. xylostella reduced populations of B. brassicae but there was no reciprocal effect, in common with most studies of interactions between phytophagous insect species [3]. The interaction was altered by the type of fertilizer available to the plant as competition occurred under three treatments but not when AN was supplied (figure 4). The effect of fertilizer on the interaction is not explained by the quantity of plant biomass available to the phytophages, as plants grown in AN and JI fertilizers had more biomass than those grown in the other two treatments, but B. brassicae populations did not differ significantly between the four fertilizer treatments.
Figure 4.
Summary of fertilizer treatment (UF, unfertilized; CM, addition of organic chicken manure; JI, John Innes fertilizer; AN, ammonium nitrate) effects on Brassica plant size and foliar nitrogen concentration and competition between a leaf-chewing and sap-feeding phytophage. Asterisk (*) denotes no significant difference in foliar nitrogen concentration of plants growing in JI compared with the other three fertilizer treatments (all other differences are statistically significant).
The same total amount of nitrogen was applied to each plant for three of the four fertilizer treatments; however, the higher foliar nitrogen concentration in plants grown in AN indicates higher uptake for this treatment when compared with the other two fertilizers. Application of the AN treatment also resulted in no effect of P. xylostella on B. brassicae population size and maximal pupal size for P. xylostella. Nitrogen is a key limit on herbivore population growth for a range of feeding guilds [28,38,39]. No changes in glucosinolate concentration were detected in response to P. xylostella feeding damage in the current study, but the concentrations of other secondary compounds or of specific amino acids essential for B. brassicae population growth may have been altered under the P. xylostella treatment. The greater uptake of nitrogen by AN-fertilized plants may have allowed B. brassicae to overcome any negative effects of an induced plant response to P. xylostella feeding.
Two studies support the hypothesis that herbivory is less likely to induce production of secondary chemicals under high resource availability, reducing the incidence of plant-mediated competition. Bryant et al. [13] found that complete artificial defoliation of birch (Betula resinifera) increased tannin concentrations in plants in a low-nutrient treatment, but had no effect on those under high nutrients. Previous damage by gypsy moth populations increased phenolic concentration in unfertilized Quercus pinus foliage, but not in fertilized trees [40].
Brevicoryne brassicae increased the concentration of aliphatic glucosinolates, but this had no effect on P. xylostella performance, whereas P. xylostella feeding did not induce increased glucosinolate concentration. When both species fed on the same plant, the presence of P. xylostella prevented the increase in aliphatic glucosinolates found on plants with B. brassicae feeding on their own. Feeding by several Lepidoptera species on B. oleracae var alba induces the expression of LOX2, a gene that plays a key role in inducing the signalling compound jasmonic acid, which mobilizes diverse plant defences [41]. Feeding by P. xylostella did not induce LOX2 gene expression, so Poelman et al. [41] suggested that P. xylostella has a mechanism for suppressing plant defences in response to its own feeding. The suppression by P. xylostella of a defence normally induced by a second herbivore species has not previously been shown, and may allow P. xylostella to avoid potentially detrimental competitive interactions.
We found that the occurrence of competition between two phytophagous species was altered by the type of fertilizer supplied to the host plant, perhaps mediated by foliar nitrogen concentration. Whether competition structures phytophagous insect communities in natural and semi-natural habitats has been much debated (e.g. [1–4]), but the potential for the occurrence of competition to vary with the availability of key resources, such as nitrogen, has not been considered. Nitrogen concentration did not differ greatly between the four fertilizer treatments, with means between 4 and 6.5 per cent dry weight, yet the occurrence of competition differed. Angiosperm foliar nitrogen content typically ranges from 1.5 to 7 per cent dry weight [28]. Competition may therefore play an even stronger role in structuring insect herbivore communities in environments with limited resources than in resource-rich environments.
The move to sustainable agricultural and horticultural practices in Europe and North America includes a reduced use of chemical-based fertilizers, and an increased application of slow-release sources of nutrition based on animal or green manures [16]. Sustainable agricultural practices may have a greater role in ensuring global food security under altered climatic conditions [42]. Our findings suggest that phytophagous species may be released from competition using synthetic fertilizers, which could allow the build-up of pest populations. Proponents of organic agriculture have claimed that organically fertilized plants are ‘better defended’ against insect pests [17–19]. Our results provide little support for a consistent direct effect of fertilizer type on herbivore populations (see also [21]), but a release from competition on plants supplied with synthetic fertilizers might indirectly allow greater population growth under conventional agricultural practices.
Kaplan & Denno's [3] finding that indirect competition occurs more often than exploitation or interference competition in communities of phytophagous insects demonstrates the importance of induced plant responses to herbivory. Historically, most of the focus on competition between insect phytophages has come from entomologists, and the roles of plant chemistry and physiology under different abiotic conditions have been largely ignored. Future studies on competition between phytophages may benefit from the inclusion of abiotic treatments, such as the source of nutrients available to plants.
Acknowledgements
Thanks to Frank Wright for assistance with culturing insects, and to Professor van Emden for useful discussions. Funding was provided by BBSRC (grant BB/D01154x/1).
References
- 1.Connell J. H. 1983. On the prevalence and relative importance of interspecific competition: evidence from field experiments. Am. Nat. 122, 661–696 [Google Scholar]
- 2.Hairston N. G., Smith F. E., Slobodkin L. B. 1960. Community structure, population control and competition. Am. Nat. 94, 421–425 10.1086/282146 (doi:10.1086/282146) [DOI] [Google Scholar]
- 3.Kaplan I., Denno R. F. 2007. Interspecific interactions in phytophagous insects revisited: a quantitative assessment of competition theory. Ecol. Lett. 10, 977–994 10.1111/j.1461-0248.2007.01093.x (doi:10.1111/j.1461-0248.2007.01093.x) [DOI] [PubMed] [Google Scholar]
- 4.Denno R. F., McClure M. S., Ott J. R. 1995. Interspecific interactions in phytophagous insects: competition reexamined and resurrected. Ann. Rev. Entomol. 40, 297–331 10.1146/annurev.en.40.010195.001501 (doi:10.1146/annurev.en.40.010195.001501) [DOI] [Google Scholar]
- 5.Wackers F. L., Bezemer T. M. 2003. Root herbivory induces an above-ground indirect defence. Ecol. Lett. 6, 9–12 [Google Scholar]
- 6.Soler R., Bezemer T. M., Van der Putten W. H., Vet L. E. M., Harvey J. A. 2005. Root herbivore effects on above-ground herbivore, parasitoid and hyperparasitoid performance via changes in plant quality. J. Anim. Ecol. 74, 1121–1130 10.1111/j.1365-2656.2005.01006.x (doi:10.1111/j.1365-2656.2005.01006.x) [DOI] [Google Scholar]
- 7.Staley J. T., Mortimer S. R., Morecroft M. D., Brown V. K., Masters G. J. 2007. Summer drought alters plant-mediated competition between foliar- and root-feeding insects. Glob. Change Biol. 13, 866–877 [Google Scholar]
- 8.Kessler A., Halitschke R. 2007. Specificity and complexity: the impact of herbivore-induced plant responses on arthropod community structure. Curr. Opin. Plant Biol. 10, 409–414 10.1016/j.pbi.2007.06.001 (doi:10.1016/j.pbi.2007.06.001) [DOI] [PubMed] [Google Scholar]
- 9.Ohgushi T. 2008. Herbivore-induced indirect interaction webs on terrestrial plants: the importance of non-trophic, indirect, and facilitative interactions. Entomol. Experimental. Appl. 128, 217–229 10.1111/j.1570-7458.2008.00705.x (doi:10.1111/j.1570-7458.2008.00705.x) [DOI] [Google Scholar]
- 10.Poelman E. H., van Loon J. J. A., Dicke M. 2008. Consequences of variation in plant defense for biodiversity at higher trophic levels. Trends Plant Sci. 13, 534–541 10.1016/j.tplants.2008.08.003 (doi:10.1016/j.tplants.2008.08.003) [DOI] [PubMed] [Google Scholar]
- 11.Coley P. D., Bryant J. P., Chapin F. S. 1985. Resource availability and plant antiherbivore defense. Science 230, 895–899 10.1126/science.230.4728.895 (doi:10.1126/science.230.4728.895) [DOI] [PubMed] [Google Scholar]
- 12.Hawkes C. V., Sullivan J. J. 2001. The impact of herbivory on plants in different resource conditions: a meta-analysis. Ecology 82, 2045–2058 10.1890/0012-9658(2001)082[2045:TIOHOP]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[2045:TIOHOP]2.0.CO;2) [DOI] [Google Scholar]
- 13.Bryant J. P., Reichardt P. B., Clausen T. P., Werner R. A. 1993. Effects of mineral nutrition on delayed inducible resistance in Alaska paper birch. Ecology 74, 2072–2084 10.2307/1940853 (doi:10.2307/1940853) [DOI] [Google Scholar]
- 14.Mutikainen P., Walls M., Ovaska J., Keinanen M., Julkunen-Tiitto R., Vapaavuori E. 2000. Herbivore resistance in Betula pendula: effect of fertilization, defoliation, and plant genotype. Ecology 81, 49–65 [Google Scholar]
- 15.Cipollini D. F., Bergelson J. 2001. Plant density and nutrient availability constrain constitutive and wound-induced expression of trypsin inhibitors in Brassica napus. J. Chem. Ecol. 27, 593–610 10.1023/A:1010384805014 (doi:10.1023/A:1010384805014) [DOI] [PubMed] [Google Scholar]
- 16.Letourneau D. K., Bothwell S. G. 2008. Comparison of organic and conventional farms: challenging ecologists to make biodiversity functional. Front. Ecol. Environ. 6, 430–438 10.1890/070081 (doi:10.1890/070081) [DOI] [Google Scholar]
- 17.Phelan P. L., Mason J. F., Stinner B. R. 1995. Soil-fertility management and host preference by European corn borer, Ostrinia nubilalis (Hubner), on Zea mays L: a comparison of organic and conventional chemical farming. Agric., Ecosyst. Environ. 56, 1–8 10.1016/0167-8809(95)00640-0 (doi:10.1016/0167-8809(95)00640-0) [DOI] [Google Scholar]
- 18.Phelan P. L., Norris K. H., Mason J. F. 1996. Soil-management history and host preference by Ostrinia nubilalis: evidence for plant mineral balance mediating insect–plant interactions. Environ. Entomol. 25, 1329–1336 [Google Scholar]
- 19.Alyokhin A., Porter G., Groden E., Drummond F. 2005. Colorado potato beetle response to soil amendments: a case in support of the mineral balance hypothesis? Agric. Ecosyst. Environ. 109, 234–244 10.1016/j.agee.2005.03.005 (doi:10.1016/j.agee.2005.03.005) [DOI] [Google Scholar]
- 20.Costello M. J., Altieri M. A. 1995. Abundance, growth rate and parasitism of Brevicoryne brassicae and Myzus persicae (Homoptera, Aphididae) on broccoli grown in living mulches. Agric. Ecosyst. Environ. 52, 187–196 10.1016/0167-8809(94)00535-M (doi:10.1016/0167-8809(94)00535-M) [DOI] [Google Scholar]
- 21.Staley J. T., Stewart-Jones A., Pope T. W., Wright D. J., Leather S. R., Hadley P., Rossiter J. T., Van Emden H., Poppy G. M. 2010. Varying responses of insect herbivores to altered plant chemistry under organic and conventional treatments. Proc. R. Soc. B 277, 779–786 10.1098/rspb.2009.1631 (doi:10.1098/rspb.2009.1631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer J. 1992. The influence of different nitrogen and potassium fertilization on the chemical flavour composition of kohlrabi (Brassica oleracea var gongylodes L.). J. Sci. Food Agric. 60, 465–470 10.1002/jsfa.2740600410 (doi:10.1002/jsfa.2740600410) [DOI] [Google Scholar]
- 23.Chen Y. Z., Lin L., Wang C. W., Yeh C. C., Hwang S. Y. 2004. Response of two Pieris (Lepidoptera: Pieridae) species to fertilization of a host plant. Zool. Stud. 43, 778–786 [Google Scholar]
- 24.Rosen C. J., Fritz V. A., Gardner G. M., Hecht S. S., Carmella S. G., Kenney P. M. 2005. Cabbage yield and glucosinolate concentrations as affected by nitrogen and sulfur fertility. Hortscience 40, 1493–1498 [Google Scholar]
- 25.Kramer S. B., Reganold J. P., Glover J. D., Bohannan B. J. M., Mooney H. A. 2006. Reduced nitrate leaching and enhanced denitrifier activity and efficiency in organically fertilized soils. Proc. Natl Acad. Sci. USA 103, 4522–4527 10.1073/pnas.0600359103 (doi:10.1073/pnas.0600359103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghorbani R., Koocheki A., Jahan M., Asadi G. A. 2008. Impact of organic amendments and compost extracts on tomato production and storability in agroecological systems. Agron. Sustain. Dev. 28, 307–311 10.1051/agro:2008003 (doi:10.1051/agro:2008003) [DOI] [Google Scholar]
- 27.van Emden H. F. 1966. Studies on the relations of insects and host plant. III. A comparison of the reproduction of Brevicoryne brassicae and Myzus persicae (Hemiptera: Aphididae) on brussels sprout plants supplied with different amounts of nitrogen and potassium. Entomol. Experimental. Appl. 9, 444 [Google Scholar]
- 28.Mattson W. J. 1980. Herbivory in relation to plant nitrogen content. Ann. Rev. Ecol. Syst. 11, 119–161 10.1146/annurev.es.11.110180.001003 (doi:10.1146/annurev.es.11.110180.001003) [DOI] [Google Scholar]
- 29.Hopkins R. J., van Dam N. M., van Loon J. J. A. 2009. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Ann. Rev. Entomol. 54, 57–83 10.1146/annurev.ento.54.110807.090623 (doi:10.1146/annurev.ento.54.110807.090623) [DOI] [PubMed] [Google Scholar]
- 30.Staley J. T., Stewart-Jones A., Poppy G. M., Leather S. R., Wright D. J. 2009. Fertiliser affects the behaviour and performance of Plutella xylostella on Brassicas. Agric. Forest Entomol. 11, 275–282 10.1111/j.1461-9563.2009.00432.x (doi:10.1111/j.1461-9563.2009.00432.x) [DOI] [Google Scholar]
- 31.Wilson P. R. 1990. A new instrument concept for nitrogen/protein analysis; a challenge to the Kjeldahl method. Aspects Appl. Biol. 25, 443–446 [Google Scholar]
- 32.Heaney R. K., Spinks E. A., Hanley B., Fenwick G. R. 1986. Technical bulletin: analysis of glucosinolates in rapeseed. AFRC, Food Research Institute, Norwich, UK [Google Scholar]
- 33.Kazana E., Pope T. W., Tibbles L., Bridges M., Pickett J. A., Bones A. M., Powell G., Rossiter J. T. 2007. The cabbage aphid: a walking mustard oil bomb. Proc. R. Soc. B 274, 2271–2277 10.1098/rspb.2007.0237 (doi:10.1098/rspb.2007.0237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faraway J. J. 2005. Extending the linear model with R: generalised linear, mixed effects and nonparametric regression models. Boca Raton, FL: Chapman and Hall [Google Scholar]
- 35.Waldbauer G. P. 1968. The consumption and utilization of food by insects. Adv. Insect Physiol. 5, 229–288 10.1016/S0065-2806(08)60230-1 (doi:10.1016/S0065-2806(08)60230-1) [DOI] [Google Scholar]
- 36.Sokal R. R., Rohlf F. J. 1995. Biometry: the principles and practice of statistics in biological research. New York, USA: W. H. Freeman and Company [Google Scholar]
- 37.R Team 2006. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 38.White T. C. R. 1984. The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia 63, 90–105 10.1007/BF00379790 (doi:10.1007/BF00379790) [DOI] [PubMed] [Google Scholar]
- 39.Slansky F., Scriber J. M. 1985. Food consumption and utilization. In Comprehensive insect physiology, biochemistry and pharmacology, vol. 4 (eds Kerkut G. A., Gilbot L. I.), pp. 87–163 Oxford, UK: Pergamon Press [Google Scholar]
- 40.Hunter M. D., Schultz J. C. 1995. Fertilization mitigates chemical induction and herbivore responses within damaged oak trees. Ecology 76, 1226–1232 10.2307/1940929 (doi:10.2307/1940929) [DOI] [Google Scholar]
- 41.Poelman E. H., Broekgaarden C., Van Loon J. J. A., Dicke M. 2008. Early season herbivore differentially affects plant defence responses to subsequently colonizing herbivores and their abundance in the field. Mol. Ecol. 17, 3352–3365 10.1111/j.1365-294X.2008.03838.x (doi:10.1111/j.1365-294X.2008.03838.x) [DOI] [PubMed] [Google Scholar]
- 42.FAO. Declaration of the high-level conference on world food security: the challenges of climate change and bioenergy. Food and Agriculture Organization of the United Nations. 2008. See http://www.fao.org/fileadmin/user_upload/foodclimate/HLCdocs/declaration-E.pdf .