Abstract
Thin sections of petrified fossils made during the latter part of the nineteenth and early twentieth centuries to investigate the internal tissue systems of plants now provide an important new source of information on associated micro-organisms. We report a new heterokont eukaryote (Combresomyces williamsonii sp. nov.) based on exquisitely preserved fossil oogonia, antheridia and hyphae from the Carboniferous (Pennsylvanian: Bashkirian stage) of UK. The structure of the oogonia and antheridia and features observed within the hyphae demonstrate a relationship with Oomycetes (Peronosporomycetes). The fossil micro-organism was documented in situ in petrified stem cortex and rootlets of the extinct seed fern Lyginopteris oldhamia (Pteridospermales). The main observed features point towards a pythiaceous Oomycete but links to biotrophic Albuginales or Peronosporaceae cannot be ruled out owing to the observation of a possible haustorium. Our study provides the earliest evidence for parasitism in Oomycetes.
Keywords: Oomycetes (Peronosporomycetes), Lyginopteris, parasite, oogonium, antheridium, fertilization tube
1. Introduction
Oomycetes are microscopic eukaryotes that are common saprophytes and parasites of plants, animals and fungi. Some species are known to cause serious diseases, and several have great economic impact (e.g. Phytophthora infestans Potato Blight, Pythium spp damping-off of seedlings). The group was renamed Peronosporomycetes by Dick [1], but here we follow the classification and nomenclature of Cavalier-Smith & Chao [2]. We retain the name Oomycetes because of its wider currency in the mycological and plant pathological communities. Oomycetes are an ancient group, but their evolutionary history is mostly inferred from molecular phylogenetic studies of living species [3–16]. Bhattacharya et al. [17] suggested a mean age for the split between Oomycetes and Bacillariophyta of 936 Ma (range: 1150–770 Ma). Internal calibration of the Oomycetes molecular phylogeny has not yet been attempted, but Dick [1,18] presented a compelling case for a comparatively recent (Cenozoic Era) radiation of the phylogenetically derived Peronosporales (Downy Mildews). Fossil evidence prior to the Cretaceous Period is rare, and where fossils have been documented their affinities are difficult to establish [19]. Recent work, however, provides compelling evidence for Oomycetes in Palaeozoic and early Mesozoic ecosystems. The oldest fossils come from the 400 Myr old Rhynie Chert [20]. Records from the Carboniferous and Triassic Periods provide clear evidence of Oomycetes associated with plant debris [21,22], and they have also been described within the tissues of the Carboniferous lycopod Lepidodendron [23] and in the reproductive organs of a fern in the extinct Zygopteridales [24]. Here we document the first evidence of Oomycetes in seed ferns (pteridosperms), extending the known diversity of fossil members and their distribution to a third key element of the Carboniferous mire environments.
2. Material and methods
During the late nineteenth and early twentieth centuries the study of fossil plants was revolutionized through the introduction of the thin section technique. This enabled the anatomy of petrified fossils to be studied in detail, and large collections of thin sections were accumulated, especially in France and Great Britain. These collections are now an invaluable new source of data on micro-organism–plant associations. We reinvestigated the Oliver and Williamson Collections housed at the Natural History Museum, London, focusing on the pteridosperm Lyginopteris. Two hundred slides were studied. An additional 35 slides were examined from the Williamson Collection at the Manchester Museum. Specimens in both collections were originally prepared from coal balls using traditional thin sectioning techniques. Coal balls are calcareous concretions that occur locally within coal seams of Late Carboniferous–Lower Permian age. Occurrences in Britain are limited stratigraphically to a small number of seams of the Lower Coal Measures of Yorkshire and Lancashire [25,26]. They are of Westphalian A age (= second part of Bashkirian). These permineralized peats preserve the uncompressed remains of the coal-forming plants in exquisite anatomical detail as well as the micro-organisms that they contain.
Thin sections of coal balls were examined using transmitted light microscopy. Images were taken using Leitz Ortholux II and Leica Wild MC3 light microscopes in the fossil plant Laboratory at the Natural History Museum (London) and using a Leitz Dialux 20 EB microscope at the Laboratoire Mycorhizes (Angers, France). Micro-organism/plant interactions were observed in the two series of slides. The slides under accession numbers NHMUK PB.OC.1068 (Oliver Collection), NHMUK PB.WC.1144.A, NHMUK PB.WC.1144.D, NHMUK PB.WC.1144.E (Williamson collection) are housed in the Natural History Museum, London, UK (NHMUK). Those under accession numbers MANCH R.1077, MANCH R.1081.a, MANCH R.1081.b, MANCH R.1081.c, MANCH R.1081.e, MANCH R.1081.g are housed in the Manchester Museum, UK (MANCH).
3. Results
The stem of the pteridosperm Lyginopteris oldhamia is characterized by a distinctive outer cortex (termed dictyoxylon cortex) composed of radially aligned fibrous bands that anastomose vertically, forming a net-like structure in tangential longitudinal section. Parenchymatous cells separate these bands (figure 1a). We report a micro-organism colonizing structurally preserved rootlets and the outer dictyoxylon cortex of stems of L. oldhamia in young stages of development. The micro-organism has been found within the plant tissues and not in the associated matrix. We observed two populations of the same micro-organism in different slide collections, that we here designate P1 (Natural History Museum, London) and P2 (Manchester Museum). The main differences between the two concerns the size of the structures and some details of the oogonial ornamentation. Other differences are discussed in the following text (see also electronic supplementary material, table S1). The vegetative mycelium is characterized by coenocytic hyphae. These form occasional hyphal knots in the cortex of rootlets and in the dictyoxylon outer cortex of the stems (P1; figure 1b). The diameter of the hyphae typically varies from 12 to 30 µm (P2) but may swell to 30–50 µm (P1; figure 2(iv)). Hyphae appear to extend through the fibres of the cortex, and they develop structures suggestive of intraparietal haustoria on the adjacent parenchyma cells (P2; figure 3c).
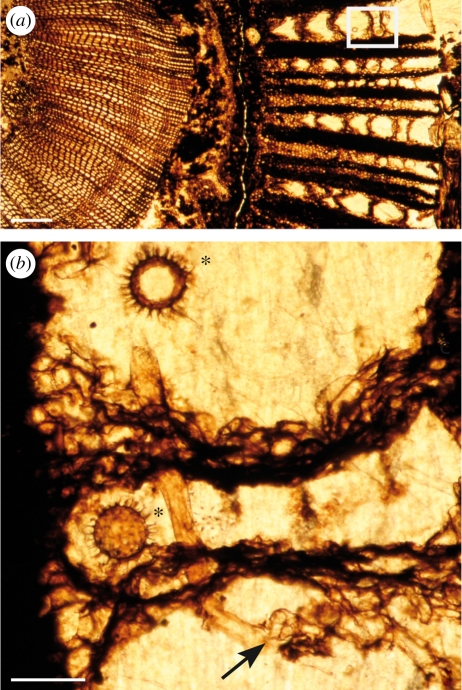
Figure 1.
(a) Overview of a transverse section of Lyginopteris oldhamia stem showing colonization by the Oomycetes in the cortical tissues (frame); the zone in the frame corresponds to (b); scale bar, 400 µm. (b) Hypha (arrow) and oogonia (asterisk) of Combresomyces williamsonii (Holotype) within the parenchyma that separates the fibres of the dictyoxylon outer cortex of the stem. Note the occurrence of a knot of hyphae (arrow); scale bar, 130 µm. All images from slide specimen NHMUK PB.WC.1144.E.
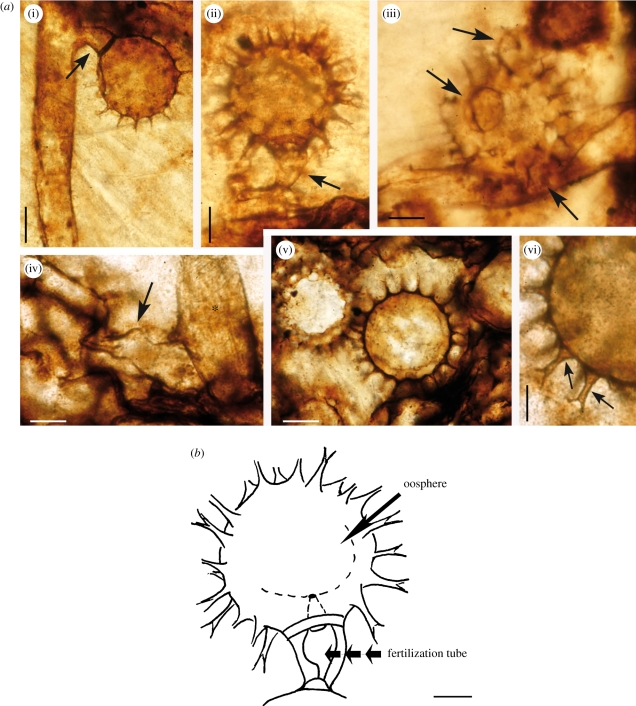
Figure 2.
Combresomyces williamsonii within the outer cortex of the stem of Lyginopteris oldhamia. (a) (i) Oogonium with developing hypogynous antheridium (arrow). A narrowing is observable at the base of the stalk hyphae; scale bar, 35 µm. (ii) Oogonium and hypogynous antheridium (arrow). Note the narrowing at the base of the hyphal stalk). The fertilization tube is discernible (see figure 2b); scale bar, 30 µm. (iii) Oogonium and three paragynous antheridia (arrows; scale bar, 30 µm. (iv) Hyphae with swellings (*) and lobate ornamentation (arrow); scale bar, 30 µm. (v) Oogonia in chains; scale bar, 35 µm. (vi) Oogonium showing projections (arrow); the columnar projections have a wide triangular base (arrow) and dichotomized once (arrow); scale bar, 15 µm. Elements (i,ii,iii,iv,vi): slide specimen NHMUK PB.WC.1144.E; element (v): slide specimen NHMUK PB.WC.1144.D). (b) Interpretive drawing of the fertilization tube corresponding to 2a(ii); scale bar, 25 µm.
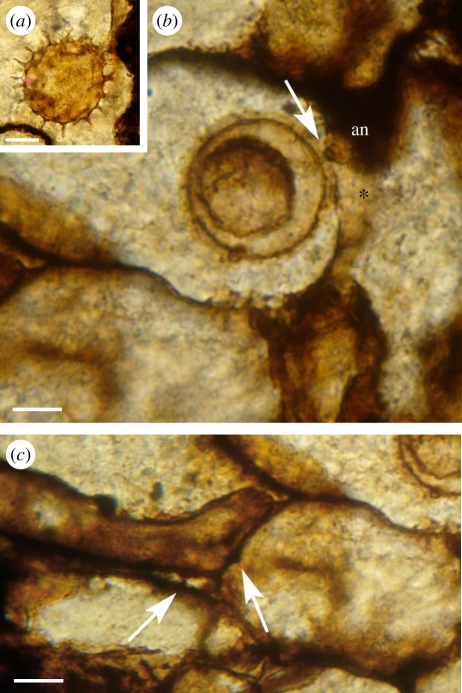
Figure 3.
Combresomyces sp. (a) Oogonium in the parenchymatous intercalation of the stem cortex of Lyginopteris oldhamia; a smaller sphere is discernible on the inside of the oogonium; scale bar, 25 µm. Slide specimen MANCH R.1077. (b,c) Colonization in Lyginopteris stem (in longitudinal section). (b) Note the fertilization tube in the oogonium/antheridium complex (arrow) and the aplerotic oospore; antheridium (an) and antheridium stalk (asterisk); scale bar, 15 µm. (c) Vegetative hyphae developing possible haustoria (arrows) that penetrate the cell walls of the host but not the cytoplasm; scale bar, 15 µm. Slide specimen MANCH R.1081.g.
Ornamented globose structures (90–130 µm (P1) and 50–100 µm (P2) in diameter) occur within the parenchymatous intercalations of the dictyoxylon cortex. We interpret these as oogonia. Some have been observed in connection with vegetative hyphae in the two populations (P1; figures 1b, 2a(i,ii)) and (P2; figure 3a,b), whereas others occur in chains within the parenchyma (P1; figure 2a(v)). Oogonia bear conspicuous projections ranging from 7 to 10 µm in length (P2; figure 3a) and from 12 to 24 µm in length (P1; figure 2a(vi)). The projections are slender and regular in shape and distribution. They are long and columnar with a triangular base, and they dichotomize once, forming extensions (3–7 µm (P1), 0–3 µm (P2)) with occasional tiny dichotomized ends (P1; figure 2a(vi)). Some of the oogonia appear to be empty (P1; figure 1b), whereas others suggest the occurrence of a smaller sphere on the inside ((P1) figure 2a(ii), (P2) figure 3a). We interpret this as the remains of the oospore. This is particularly clear in specimen MANCH R.1077 ((P2), figure 3b) where the plane of section appears to reveal an intracellular oospore, which is 30 µm in diameter. The single oospore is interpreted as aplerotic because of the narrow gap between the oospore and the oogonial wall. The fertilization tube is difficult to illustrate because of its orientation with respect to the oogonia and limitations with imaging depth of field in these fossils. A fertilization tube has been observed in NHMUK PB.WC.1144.E (holotype) and MANCH R.1077. It is discernable in figure 2a(ii) (see also interpretative drawing, figures 2b and 3b). Other structures with club-shaped tips are attached to the sides of the oogonia ((P1) figure 2a(iii), (P2) figure 3b) or form in the oogonial stalk (P1) (figure 2a(i,ii)). Again, because of their depth, some details are difficult to capture in a single image. In a different focal plane, the antheridial stalk (not shown) is visible below the antheridium in the bottom part of figure 2a(iii), but in the illustration we have privileged the main bodies of the antheridia. We have observed both paragynous and hypogynous antheridia. The paragynous forms seem to develop from the oogonial stalk, so we interpret these as probably monoclinous, but the preservation is not sufficient to be conclusive on this point. Three paragynous antheridia attached to the same oogonium can be observed in figure 2a(iii) (P1)—the antheridial hyphae range from 2–15 µm (P2) to 15–20 µm wide (P1).
Taxonomy: after Cavalier-Smith & Chao [2].
Kingdom: Chromista = Straminipila [1].
Super group: Heterokonta.
Phylum: Pseudofungi.
Class: Oomycetes = Peronosporomycetes [1].
Genus: Combresomyces [23] incertae sedis.
Combresomyces williamsonii Strullu-Derrien, Kenrick, Rioult and Strullu. sp. nov.
MycoBank: no. 518661.
Etymology: the specific name honours Prof. William Crawford Williamson (1816–1895), who originally described the fossil plant host.
Diagnosis: ornamented globose oogonia, terminal or in chains, from 90 to 130 µm in diameter (including the projections), thin-walled; conspicuous projections protruding from the surface up to 24 µm. Projections densely and regularly distributed over the entire surface; projections slender and long, columnar, with a triangular base and two extensions, which sometimes dichotomize once at the tips. Oogonia in connection with vegetative hyphae, 30–40 µm wide. Oogonia empty or containing a single spherical aplerotic oospore. Antheridia both paragynous (probably monoclinous) and hypogynous. Antheridial hyphae 15–20 µm wide. Vegetative hyphae coenocytic, sometimes forming knots in the parenchyma of the outer cortex of the stem. Irregular lobate swellings (up to 50 µm wide) sometimes present.
Status: in Lyginopteris stem.
Holotype: oogonia (asterisks) and associated hypha (arrow) in figure 1b (this paper): slide specimen NHMUK PB.WC.1144.E (Williamson Collection, Natural History Museum, London).
Locality: Dulesgate, near Todmorden Moor, West Yorkshire, UK.
Age: Carboniferous: Pennsylvanian: Bashkirian stage (English Lower Coal Measures; ca 315 Ma).
The taxonomic description is based on population P1 (NHMUK). The micro-organism from population P2 (MANCH) probably belongs to the same genus, but to date we are not sure that the species is the same, because of differences in the dimensions of oogonia and ornamentation.
4. Discussion
The exquisitely preserved mico-organisms described here are attributed to the Oomycetes based principally on their coenocytic hyphae and on some highly distinctive features of their oogonia and antheridia. Specifically, the presence of club-shaped antheridia adhering to the outer wall of the globose oogonium is diagnostic (e.g. [1,27–33]). These features also exclude an affinity with Zygomycetes and Chytridiomycetes.
Our new species C. williamsonii resembles most closely Combresomyces cornifer ([23]; see comparisons in the electronic supplementary material, table S1). Both species possess coenocytic hyphae. The oogonia are broadly similar in shape (i.e. mainly globose), they appear to contain a single oospore, and they bear bifurcating projections. Both have paragynous antheridia. However, the fertilization tube produced by the antheridium and shown to be present in C. williamsonii is not yet demonstrated for C. cornifer. The critical differences include: (i) oogonium size, which in C. williamsonii is up to four times that of C. cornifer; (ii) projection shape, which in C. williamsonii shows a clear columnar extension of the triangular projection base. Also, the second bifurcation of the oogonial projections of C. williamsonii is much less distinct than that in C. cornifer; (iii) hyphal diameter, which in C. williamsonii is very much larger than in C. cornifer. Features observed in C. williamsonii but not yet demonstrated in C. cornifer include oogonia in chains, hypogynous antheridium and vegetative features such as lobate hyphal swellings, hyphal knots, a possible haustorium and reproductive structures in organic connection with vegetative hyphae. In our view, these differences warrent recognition at the species level.
The features documented here for C. williamsonii provide a fairly strong link to the plant pathogenic Peronosporales sensu lato (i.e. Pythiales + Peronosporales). The morphology of C. williamsonii is close to some current day Pythium spp. and especially to Pythium ornamentatum, which resembles Pythium acanthicum, Pythium oligandrum and Pythium amasculinum [31]. The observed oogonium morphology and hyphal knots also resemble structures produced by mycoparasites such as P. oligandrum and P. acanthicum [34,35]. As in Pythium spp, the antheridia appear to develop in one of two ways: either as branches derived from the oogonial stalk (monoclinous) or within the oogonial stalk itself (hypogynous). Ornamented oogonia, single oospore and irregular lobate hyphal swellings are also shared features. In common with living P. ornementatum, it has been difficult to observe the number, kind and details of antheridia in the fossil C. williamsonii. The fertilization tube and the single, aplerotic, slightly off centre, oospore are similar to that of modern Pythium, Phytophthora or Albugo spp. Unfortunately, the oospore is not preserved well enough to provide detailed information on wall morphology, which is a key feature used to separate primitive members of the Albuginales from the Peronosporales. Another feature is the conspicuous projections of the oogonia. No modern species has projections of quite the same form, however many species of Pythium are known to possess ornamented oogonia, and this feature has great taxonomic value (e.g. [28,31–33]). The projections are slender and long, columnar, with a triangular base and two extensions, which sometimes dichotomize once at the tips. Straight projections with a large base also characterize P. ornamentatum oogonia, however they are rarely bifurcated. The morphology of the ornamented oogonia suggests that C. williamsonii may be a member of the Pythiales (aceae).
One of the key points of difference between C. williamsonii and previously described Palaeozoic Era Oomycetes relates to our interpretation of its mode of nutrition. We consider various features observed as indicative of parasitism possibly even biotrophic parasitism, whereas the authors of previously described fossil species (e.g. [20,21,23]) are either non-committal or favour a saprophytic interpretation. New features documented here include the clear presence of hyphal knots and evidence that is suggestive of an haustorium. In Lyginopteris, these are associated with intact parenchyma of the outer cortex, and in the part of the cortex where the parenchyma cells became strained and distorted as secondary tissues developed [36]. Hyphal knots form when hyphae develop within the cortex of the stem and haustoria represent outgrowths from hyphae that penetrate the cell walls of the host but not the cytoplasm in order to absorb nutrients. In these respects our fossil resembles the vegetative growth pattern and haustorial development in Phytophthora and other parasitic Peronosporaceae and Albuginaceae (e.g. [1,37,38]). Hyphal knots point to a possible affinity with some mycoparasitic Pythium species [34]. However the Pythiaceae, which are not biotrophic, do not possess haustoria. So, links to biotrophic Albuginales or Peronosporaceae cannot be ruled out.
Notwithstanding the above similarities to living members of the Oomycetes, the phylogenetic position of C. williamsonii can only be specified in very general terms. Recent molecular systematic studies of living Oomycetes shed new light on the evolution of the group and provide a useful framework for considering the significance and broader relevance of the early fossils (see the electronic supplementary material, figure S4). It seems probable that Oomycetes are extremely ancient, diverging from Bacillariophyta during the Neoproterozoic [17] before making the transition to the land. Because of its terrestrial habitat and oogamous form of reproduction, C. williamsonii may therefore be a comparatively derived form. A close relationship with the Pythiales (Aceae) seems likely based on the localization of the organism in the rootlets and the cortex of the stem and on the features documented above. However this micro-organism could conceivably be in the biotrophic Peronosporaceae or Albuginales. In either case, the features observed indicate that parasitism and possibly even biotrophy was present in the Oomycetes during the latter part of the Carboniferous Period (ca 315 Ma).
5. Conclusion
Recent research provides compelling evidence that oogamous Oomycetes were an important component of early terrestrial ecosystems. Oomycetes have been documented in the tissue systems of three of the dominant plant groups of Carboniferous mire ecosystems: lycopods, ferns, and here in the extinct pteridosperms (seed ferns). Much of the new fossil data comes from historical slide collections dating from the late nineteenth and early twentieth centuries. Initially made to study the anatomy of petrified fossil plants, these are now recognized as an invaluable source of data on plant–micro-organism associations. The new fossil micro-organism is probably most closely related to the Pythiales (aceae) and it provides the earliest evidence for parasitism in Oomycetes.
Acknowledgements
The authors thank Prof. Dick, Prof. Tirilly, Dr A. Lévesque and his collaborators for extensive and useful discussions. They also thank the two anonymous reviewers for their comments. They gratefully acknowledge Dr D. Gelsthrope for making the Williamson slides from the Manchester Museum available. C.S.D. thanks Dr P. Hayes for her welcome in the Department of Palaentology, NHM London under the framework of the SYNTHESYS Programme. This work was partly funded by SYNTHESYS support made available through the European Community-Research Infrastructure Action under the FP6 ‘Structuring the European Research Area Programme’ GB-TAF 4619. ‘The Manchester Museum, The University of Manchester’ is acknowledged for the loan of the slides.
References
- 1.Dick M. W. 2001. Straminipilous Fungi: systematics of the Peronosporomycetes including accounts of the marine straminipilous protists, the plasmodiophorids and similar organisms. London, UK: Kluwer Academic Publishers [Google Scholar]
- 2.Cavalier-Smith T., Chao E. E. Y. 2006. Phylogeny and megasystematics of phagotrophic heterokonts (kingdom Chromista). J. Mol. Evol. 62, 388–420 10.1007/s00239-004-0353-8 (doi:10.1007/s00239-004-0353-8) [DOI] [PubMed] [Google Scholar]
- 3.Dick M. W., Vick M. C., Gibbings J. G., Hedderson T. A., Lopez Lastra C. C. 1999. 18S rDNA for species of Leptolegnia and other Peronosporomycetes: justification of the subclass taxa Saprolegniomycetidae and Peronosporomycetidae and division of the Saprolegniaceae sensu lato into the Leptolegniaceae and Saprolegniaceae. Mycol. Res. 103, 1119–1125 10.1017/S0953756299008643 (doi:10.1017/S0953756299008643) [DOI] [Google Scholar]
- 4.Riethmüller A., Weiss M., Oberwinkler F. 1999. Phylogenetic studies of Saprolegniomycetidae and related groups based on nuclear large subunit ribosomal DNA sequences. Can. J. Bot. 77, 1790–1800 10.1139/cjb-77-12-1790 (doi:10.1139/cjb-77-12-1790) [DOI] [Google Scholar]
- 5.Petersen A. B., Rosendahl S. 2000. Phylogeny of the Peronosporomycetes (Oomycota) based on partial sequences of the large ribosomal subunit (LSU rDNA). Mycol. Res. 104, 1295–1303 10.1017/S0953756200003075 (doi:10.1017/S0953756200003075) [DOI] [Google Scholar]
- 6.Cooke D. E. L., Drenth A., Duncan J. M., Wagels G., Brasier C. M. 2000. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet. Microbiol. 30, 17–32 10.1006/fgbi.2000.1202 (doi:10.1006/fgbi.2000.1202) [DOI] [PubMed] [Google Scholar]
- 7.Hudspeth D. S. S., Nadler S. A., Hudspeth M. E. S. 2000. A COX2 molecular phylogeny of the Peronosporomycetes. Mycologia 92, 674–684 10.2307/3761425 (doi:10.2307/3761425) [DOI] [Google Scholar]
- 8.Riethmüller A., Voglmayr H., Göker M., Weiß M., Oberwinkler F. 2002. Phylogenetic relationships of the downly mildews (Peronosporales) and related groups based on nuclear large subunit ribosomal DNA sequences. Mycologia 94, 834–849 10.2307/3761698 (doi:10.2307/3761698) [DOI] [PubMed] [Google Scholar]
- 9.Spencer M. A., Vick M. C., Dick M. W. 2002. Revision of Aplanopsis, Pythiopsis, and ‘subcentric’ Achlya species (Saprolegniaceae) using 18S rDNA and morphological data. Mycol. Res. 106, 549–560 10.1017/S0953756202005889 (doi:10.1017/S0953756202005889) [DOI] [Google Scholar]
- 10.Hudspeth D. S. S., Strenger D., Hudspeth M. E. S. 2003. A COX2 phylogenetic hypothesis for the downly mildews and white rusts. Fungal Divers. 13, 47–57 [Google Scholar]
- 11.Lévesque C. A., De Cock W. A. M. 2004. Molecular phylogeny and taxonomy of the genus Pythium. Mycol. Res. 108, 1363–1383 [DOI] [PubMed] [Google Scholar]
- 12.Thines M., Spring O. 2005. A revision of Albugo (Chromista, Peronosporomycetes). Mycotaxon 92, 443–458 [Google Scholar]
- 13.Göker M., Voglmayr H., Reithmüller A., Oberwinkler F. 2007. How do obligate parasites evolve? A multi-gene phylogenetic analysis of downy mildews. Fungal Genet. Biol. 44, 105–122 10.1016/j.fgb.2006.07.005 (doi:10.1016/j.fgb.2006.07.005) [DOI] [PubMed] [Google Scholar]
- 14.Blair J. E., Coffey M. D., Park S. Y., Geiser D. M., Kang S. 2008. A multi-locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genet. Biol. 45, 266–277 10.1016/j.fgb.2007.10.010 (doi:10.1016/j.fgb.2007.10.010) [DOI] [PubMed] [Google Scholar]
- 15.Thines M., Voglmayr H., Göker M. 2009. Taxonomy and phylogeny of the downy mildews (Peronosporaceae). In Omycete genetics and genomics: diversity, interactions, and research tools (eds Lamour K., Kamoun S.), pp. 47–75 London, UK: Wiley-Blackwell [Google Scholar]
- 16.Beakes G. W., Sekimoto S. 2009. The evolutionnary phylogeny of Oomycetes—insights gained from studies of holocarpic parasites of algae and invertebrates. In Omycete genetics and genomics: diversity, interactions, and research tools (eds Lamour K., Kamoun S.), pp. 1–24 London, UK: Wiley-Blackwell [Google Scholar]
- 17.Bhattacharya D., Yoon H. S., Hedges S. B., Hackett D. 2009. Eukaryotes. In The timetree of life (eds Hedges S. B., Kumar S.), pp. 116–120 New York, NY: Oxford University Press [Google Scholar]
- 18.Dick M. W. 2002. Towards an understanding of the evolution of the downy mildews. In Advances in downy mildew research (eds Spencer-Phillips P. T. N., Gisi U., Lebeda A.), pp. 1–57 London, UK: Kluwer Academic Publishers [Google Scholar]
- 19.Taylor T. N., Taylor E. L., Krings M. 2009. Paleobotany. The biology and evolution of fossil plants, 2nd edn. New York, NY: Elsevier/Academic Press Inc [Google Scholar]
- 20.Taylor T. N., Krings M., Kerp H. 2006. Hassiella monospora gen. and sp. nov., a microfungus from the 400 million year old Rhynie chert. Mycol. Res 110, 628–632 10.1016/j.mycres.2006.02.009 (doi:10.1016/j.mycres.2006.02.009) [DOI] [PubMed] [Google Scholar]
- 21.Schwendemann A. B., Taylor T. N., Taylor E. L., Krings M., Dotzler N. 2008. Combresomyces cornifer from the Triassic of Antarctica: evolutionary stasis in the Peronosporomycetes. Rev. Pal. Pal. 154, 1–5 [Google Scholar]
- 22.Krings M., Taylor T. N., Galtier J., Dotzler N. 2010. A fossil peronosporomycete oogonium with an unusual surface ornament from the Carboniferous of France. Fungal Biol. 114, 446–450 10.1016/j.funbio.2010.03.006 (doi:10.1016/j.funbio.2010.03.006) [DOI] [PubMed] [Google Scholar]
- 23.Dotzler N., Krings M., Agerer R., Galtier J., Taylor T. N. 2008. Combresomyces cornifer gen. sp. nov., an endophytic Peronosporomycete in Lepidodendron from the Carboniferous of central France. Mycol. Res. 112, 1107–1114 10.1016/j.mycres.2008.03.003 (doi:10.1016/j.mycres.2008.03.003) [DOI] [PubMed] [Google Scholar]
- 24.Krings M., Taylor T. N., Dotzler N., Decombeix A. L. 2010. Galtierella biscalithecae nov. gen. et sp., a Late Pennsylvanian endophytic water mold (Peronosporomycetes) from France. C. R. Palevol 9, 5–11 10.1016/j.crpv.2009.10.002 (doi:10.1016/j.crpv.2009.10.002) [DOI] [Google Scholar]
- 25.Stewart W. N., Rothwell G. W. 1993. Paleobotany and the evolution of plants, 2nd edn. Cambridge, UK: University Press [Google Scholar]
- 26.Galtier J. 1997. Coal-ball floras of the Namurian-Westphalian of Europe. Rev. Pal. Pal. 95, 51–72 [Google Scholar]
- 27.Seymour R. L. 1970. The genus Saprolegnia. Nova Hedwigia 19, 1–124 [Google Scholar]
- 28.Van der Plaats-Niterink A. J. 1981. Monograph of the genus Pythium. Stud. Mycol. 21, 1–242 [Google Scholar]
- 29.Dick M. W. 1969. Morphology and taxonomy of the Oomycetes, with special reference to Saprolegniaceae, Leptomitaceae and Pithiaceae. New Phytol. 68, 751–775 10.1111/j.1469-8137.1969.tb06478.x (doi:10.1111/j.1469-8137.1969.tb06478.x) [DOI] [Google Scholar]
- 30.Dick M. W. 1995. Sexual reproduction in the Peronosporomycetes (chromistan fungi). Can. J. Bot. 73, S712–S724 [Google Scholar]
- 31.Paul B. 1987. A new species of Pythium with ornamented oogonia from Algeria. Mycologia 79, 797–802 10.2307/3807835 (doi:10.2307/3807835) [DOI] [Google Scholar]
- 32.Paul B. 1999. Pythium ornacarpum: a new species with ornamented oogonia isolated from soil in France. FEMS Microbiol. Lett. 180, 337–344 10.1111/j.1574-6968.1999.tb08815.x (doi:10.1111/j.1574-6968.1999.tb08815.x) [DOI] [PubMed] [Google Scholar]
- 33.Paul B. 2006. A new species of Pythium isolated from a vineyard in France. FEMS Microbiol. Lett. 263, 194–199 10.1111/j.1574-6968.2006.00422.x (doi:10.1111/j.1574-6968.2006.00422.x) [DOI] [PubMed] [Google Scholar]
- 34.Deacon J. W. 1976. Studies on Pythium oligandrum, an aggressive parasite of other fungi. Trans. Br. Mycol. Soc. 66, 383–391 10.1016/S0007-1536(76)80206-9 (doi:10.1016/S0007-1536(76)80206-9) [DOI] [Google Scholar]
- 35.Hoch H. C., Fuller M. S. 1977. Mycoparasitic relationships. I. Morphological features of interactions between Pythium acanthicum an several fungal hosts. Arch. Microbiol. 111, 207–224 10.1007/BF00549358 (doi:10.1007/BF00549358) [DOI] [Google Scholar]
- 36.Masselter T., Rowe N. P., Speck T. 2007. Biomechanical reconstruction of the carboniferous seed fern Lyginopteris oldhamia: implications for growth from reconstruction and habit. Int. J. Plant Sci. 168, 1177–1189 10.1086/520720 (doi:10.1086/520720) [DOI] [Google Scholar]
- 37.Zimmer R. C., McKeen W. E., Campbell C. G. 1990. Development of Peronospora ducometi in buck wheat. J. Plant Physiol. 12, 247–254 [Google Scholar]
- 38.Spencer-Phillips P. T. N. 1997. Function of fungal haustoria in epiphytic and endophytic infections. Adv. Bot. Res. 24, 309–333 [Google Scholar]