Abstract
One way the effects of both ecology and environment on species can be observed in the fossil record is as changes in geographical distribution and range size. The prevalence of competitive interactions and species replacements in the fossil record has long been investigated and many evolutionary perspectives, including those of Darwin, have emphasized the importance of competitive interactions that ultimately lead one species to replace another. However, evidence for such phenomena in the fossil record is not always manifest. Here we use new quantitative analytical techniques based on Geographical Information Systems and PaleoGIS tectonic reconstructions to consider this issue in greater detail. The abundant, well-preserved fossil marine vertebrates of the Late Cretaceous Western Interior Seaway of North America provide the component data for this study. Statistical analysis of distributional and range size changes in taxa confirms earlier ideas that the relative frequency of competitive replacement in the fossil record is limited to non-existent. It appears that typically, environmental gradients played the primary role in determining species distributions, with competitive interactions playing a more minor role.
Keywords: competitive replacement, GIS, Western Interior Seaway, marine vertebrates
1. Introduction
(a). Historical perspective
A central question in biogeography and evolution is what causes species' distributions to wax and wane through time. Traditionally, a dominant role has been ascribed to competitive interactions between species [1–7]. Classic examples include the decline and replacement of brachiopods by bivalves, mammal-like reptiles by archosaurs, cyclostome bryozoans by cheilostome bryozoans, gymnosperms by angiosperms, multituberculates by rodents and South American mammals by North American fauna; however, these cases for the most part have not been tested in detail [8–10]. The theoretical importance of competition in evolution actually predates Darwinian competitively driven natural selection and can be traced back to the notion of plenitude. Plenitude ascribes a fixed number of ecological niches on the Earth, with rapid evolution of life to fill all available niche space. Once filled, evolution occurs in dynamic equilibrium where individual species may arise and go extinct, but patterns of global diversity remain constant [8,11–13]. Darwin [7] supported this view, particularly with his famous wedge analogy, where species are akin to wedges hammered into a surface—once the surface is filled with wedges, a new wedge may only be driven in at the expense of an older wedge being driven out [13–15]. From this perspective, evolution occurs by a series of competitive replacements through time, species' distributions are predominantly controlled by competitive interactions with contemporaries, and interspecific competition is a primary driver of macroevolution.
An alternative perspective is where an existing species or clade is successful until an external perturbation results in its extinction and later replacement by a new taxon. For instance, a re-examination of the diversity patterns of brachiopods and bivalves by Gould and Calloway found these clades to be as ‘ships that pass in the night’ (Longfellow. In [15]); a view in accord with the notion that abiotic environmental change dictates species' origination and extinction patterns [9,11,14–23].
Of course, these (and other) authors acknowledge that both factors probably play some role in evolution. Thus, here we test for evidence of interspecific competition on species' distributions over macroevolutionary time scales by concentrating on identification of competitive replacements in fossil taxa using Geographical Information Systems (GIS). GIS-based techniques are increasingly recognized as powerful tools for investigating evolutionary patterns and processes [24–28]. These methods allow for quantitative measurement of distribution and range size change during specific temporal intervals. Further, GIS analyses lend themselves to statistical analysis of negative range area correlations in species pairs through time, which can be used as a proxy for evidence of competitive replacement. The focus of this analysis is a set of marine vertebrate species from the exceptionally diverse and complete record of the Late Cretaceous Western Interior Seaway (WIS) of North America. This region has been the subject of palaeobiological and geological study for more than a century and has been intensely sampled. Further, palaeobiological samples can be placed in a detailed stratigraphical context.
(b). Geological setting
The Late Cretaceous covers a 35 Myr period between 100–65 Myr ago. The Earth at this time was in a greenhouse climate state with little or no polar ice [29–32]. As a consequence of this, and higher rates of sea floor spreading, sea level was much higher than today. In particular, central North America was covered by a shallow epicontinental sea, the WIS (i.e. ≤600 m water depth) [32–35]. The WIS represents a foreland basin formed by tectonic loading and lithospheric flexure during uplift of the Rocky Mountains to the west. This basin was inundated episodically from both boreal waters extending south from the Arctic Ocean and tropical waters extending north from the proto-Atlantic/Tethys seas [33,34,36,37]. At the end of the Early Cretaceous (late Albian, approx. 100 Ma), a global sea-level low stand separated the northern and southern arms of the WIS for the last time until the late Maastrichtian (approx. 65 Ma). Cyclic sea-level changes are recorded in the WIS as three major transgressive/regressive events: the Greenhorn Cycle (late Cenomanian-Turonian), which included the sea-level high stand for the Late Cretaceous with eustatic sea levels upwards of 250 m higher than today; the Niobrara Cycle (late Coniacian—early Campanian); and the Claggett/Bearpaw Cycle (Campanian—Maastrichtian [33,34,36]).
Our understanding of the Late Cretaceous WIS is based on over 100 years of field and laboratory work by geologists, palaeoclimatologists and palaeobiologists. As a consequence, the tectonic, environmental and geological history of this area is well understood and extensively palaeobiologically sampled, making it an ideal region for this type of palaeobiogeographical investigation (e.g. [32,33,36–49]). However, extensive sampling does not always equate to representative sampling; consequently, we provide various tests to assess the quality of the WIS record and its use in palaeobiogeographical analyses.
2. Materials and methods
(a). Data collection
A temporal and geographical occurrence database was generated for 10 Late Cretaceous WIS vertebrate taxa. Taxa included three genera of shark: three species of Ptychodus (Ptychodus anonymus, Ptychodus mortoni and Ptychodus whipplei), one species of Cretoxyrhina (Cretoxyrhina mantelli), and two species of Squalicorax (Squalicorax falcatus and Squalicorax kaupi); as well as two genera of mosasaur (Platecarpus sp., and Tylosaurus sp.) and one teleost genus (Xiphactinus sp.). The taxa included in this analysis were chosen because they are common and abundant in the WIS fossil record, persist through at least three geological stages of the Late Cretaceous, and have been well-characterized taxonomically and palaeobiologically. Further, the WIS at this time had no prominent physical barriers that might have prevented interactions between taxa.
Data on species' geographical and stratigraphical ranges were collected through examination of museum collections, fieldwork and survey of the literature. The following museum collections were used: Natural History Museum and Biodiversity Institute (University of Kansas); Peabody Museum of Natural History (Yale University); Texas Memorial Museum (University of Texas–Austin); Sternberg Museum of Natural History (Fort Hays State University); University of Colorado Museum (University of Colorado–Boulder); University of Nebraska State Museum; and the Black Hills Institute (South Dakota). These museums contain important and diverse collections of WIS taxa spanning the majority of Late Cretaceous WIS geography, and taxa in these collections are well-documented geographically and stratigraphically. All museum specimens were personally examined and identification confirmed by the authors. In cases where species identifications lacked confidence, analyses were run at the generic level (e.g. Tylosaurus, Platecarpus, Xiphactinus). To augment information from museums, fieldwork was conducted at Late Cretaceous sites in western South Dakota and southeastern Missouri.
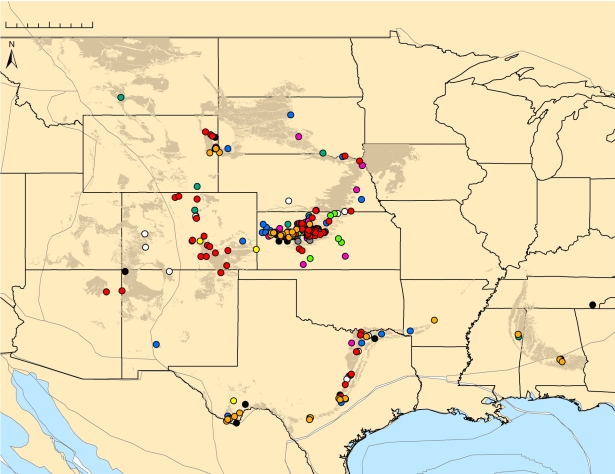
Resolution of geographical locality data was at the county level and better, the standard level of resolution used in other GIS-based palaeobiogeographical analyses (e.g. [24,50,51]). However, most data represent even higher resolution at the 1 mile2 township, range and section. Temporal resolution was at the level of geological stage within the Late Cretaceous and characterized by the formation and member of specimen occurrence. The resulting database consists of 762 total occurrence points; the number of occurrence points per taxon (species and in some cases genus) varies from 31 to 197 (figure 1 and electronic supplementary material, table S1).
Figure 1.
Data points showing occurrence records of Late Cretaceous marine vertebrate specimens analysed in this study. Xiphactinus sp. (pink), Platecarpus sp. (dark green), Tylosaurus sp. (dark blue), Squalicorax kaupi (orange), Squalicorax falcatus (red), Rhinobatos incertus (light green), Ptychodus whipplei (white), Ptychodus mortoni (dark grey), Ptychodus anonymus (light grey) and Cretoxyrhina mantelli (yellow). Present day outcrop of Late Cretaceous sediments is also shown (brown). Scale bar, 0–500 km.
(b). Range reconstructions
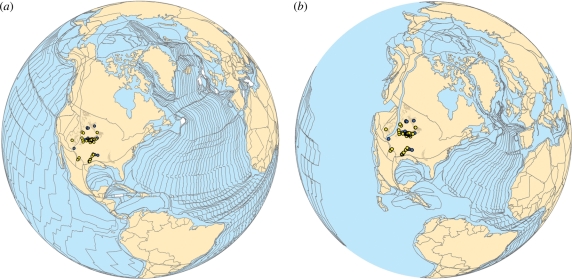
Geographical locality data for each species' occurrence were georeferenced and imported into ArcGIS v. 9.2 for visual representation and spatial analysis [52]. PaleoGIS v. 3.0 [53,54] was then used to reconstruct the palaeogeography of each stage during the Late Cretaceous following the methods of Rode & Lieberman [24] and Stigall & Lieberman [25] (figure 2). This step ensures that distribution and range area reconstructions minimize estimation error owing to tectonic contraction and expansion in the North American plate over the course of the Late Cretaceous.
Figure 2.
Example of PaleoGIS [53] plate tectonic reconstruction. Distribution of Cretoxyrhina mantelli (yellow) and Tylosaurus sp. (blue) are shown. Present day outcrop of Late Cretaceous sediments is also shown (brown). (a) PaleoGIS present day tectonic configuration. (b) PaleoGIS Coniacian reconstruction (approx. 87 Ma).
Once PaleoGIS was used to reconstruct the geography of a particular stage, a 10 km buffer was applied to each specimen occurrence point. Buffering species' locality points helps control for any error in the translation from current geographical location to deep time georeferenced latitude and longitude. Additionally, buffering gives area to point occurrence data, enabling retention of these data in the analysis. ArcGIS was then used to construct least-fit polygons for each taxon at each temporal interval. The spatial analysis software available within this program was used to calculate the area of each reconstructed range. Geographical range data for all taxa are provided in the electronic supplementary material, table S1.
(c). Identifying competition
One way competition can be observed in the fossil record is as changes in species' distribution and range size through time. Benton [9,13] defined ‘candidate competitive replacements’ (CCRs) as species pairs showing negatively correlated abundance and diversity patterns over time. CCRs must involve taxa with overlapping geographical and stratigraphic ranges and should also involve comparisons between taxa with similar habitat, body size and diet. Further, all CCRs must show a distinctly ‘successful’ taxon (the survivor) as well as a distinctly ‘unsuccessful’ taxon, identified by range contraction and extinction within two temporal intervals after the minimum date of origin of the ‘successful’ taxon [9,13]. This pattern can also be identified in the fossil record as negatively correlated geographical range area through time, which can be tested for statistical significance using non-parametric rank correlation in PAST v. 2.01 [55] (Spearmann's ρ and Kendall's τ, p ≤ 0.05); these statistical analyses were corrected for multiple comparisons using the Bonferroni correction.
All taxa under investigation display geographical and stratigraphic overlap. To identify CCRs taxa with similar inferred ecotypes were compared, as taxa within the same ecotype are most likely to have interacted competitively. The taxa in this study can be divided into two general palaeoecologies: species of Cretoxyrhina, Squalicorax, Tylosaurus, Platecarpus and Xiphactinus are inferred to have been pelagic predators (e.g. [32,37,47,56–60]; see [44] for additional discussions of Squalicorax); species of Ptychodus and Rhinobatos are inferred to have had a nekto-benthic, durophagous lifestyle (e.g. [32,37,57,61,62]; see [63,64] for additional discussions of Ptychodus). Comparisons were also conducted by genus, as species within the same genus may be more likely to have the greatest degree of competitive overlap. Finally, an agnostic approach was used, and pairwise comparisons between all taxa were considered.
(d). Analysis of bias
There are many phenomena that can explain why one species range might increase through time while another decreases through time. In addition to competition and other processes discussed below, an incomplete fossil record could artificially produce a pattern mirroring a CCR. Incompleteness of the fossil record is a potential source of bias in any palaeontological study. As previously mentioned, the Late Cretaceous WIS has been exhaustively studied for over a century and is well-characterized both in terms of its geology and palaeontology. Further, it has not undergone significant tectonic modification since the Late Cretaceous. These may all partly serve to obviate the potential problems of an incomplete fossil record. Moreover, some areas within the WIS show exceptional preservation in the form of Konservat Lagerstätte; one of these, the Smoky Hill Chalk member of the Niobrara Formation spans three temporal intervals (Coniacian, Santonian, and Campanian stages) of this study [44,65,66].
However, this does not mean that there might not be certain taphonomic factors conspiring to cloud our understanding of biogeographic patterns in these taxa over time. Because of this, three tests were used to determine if incompleteness or bias in the WIS fossil record is artefactually influencing palaeobiogeographic patterns, including those pertaining to CCRs. First, the robustness of range area reconstructions to potential outliers was tested by resampling occurrence points for each taxon. An ‘n-1’ jack-knifing procedure was used to estimate the resampled mean range size and associated confidence bands for each taxon during each time interval (resampled data available in the electronic supplementary material, table S1). This mean range area was then subjected to non-parametric rank correlation tests and the results were compared with those obtained using original range area calculations (tests on resampled data available in the electronic supplementary material, tables S3 and S4, and discussed more fully below).
The second test compared geographical range size in each taxon to the area of available Late Cretaceous sedimentary outcrop. A high percentage of overlap between the distribution of taxa and available outcrop would suggest that presence/absence of Late Cretaceous geological records may be influencing our results. The third test aimed to identify a correlation between number of data points and geographical range size for each temporal interval. In this case, if sampling bias had an effect on our range size reconstructions, a strong positive correlation between the number of data points and range size would be expected.
3. Results
(a). Competition in the WIS
Tables 1 and 2 show the results of intrageneric range area correlations and correlations by palaeoecotypes, respectively; pairwise comparisons between all taxa are included in the electronic supplementary material, table S2. All species did show changes in distribution and range size through time. The majority of the species comparisons showed no evidence of interspecific competition (e.g. figure 3). A complete set of geographical comparisons for all taxa considered is provided in the electronic supplementary material, figures S1–S43. Some taxa did generally show the basic biogeographic pattern predicted for a CCR (figure 4), however, when analysed, the pattern was not found to be statistically significant. Indeed, no statistically significant negative range area correlations were identified from intrageneric comparisons, within ecotype comparisons, or when all taxa were compared, after the Bonferroni correction was applied. For instance, consider that among the four possible intrageneric comparisons, only S. falcatus and S. kaupi is near significance using Kendall's τ (τ = −0.690, p = 0.052), but the correlation is not significant after a Bonferroni correction for multiple comparisons was applied (new critical p-value of p ≤ 0.013) (table 1). Thus, it appears that for these vertebrate taxa, evidence for CCRs in the Cretaceous WIS is negligible to non-existent.
Table 1.
Intrageneric range area correlations. (A Bonferroni correction [71] for multiple comparisons indicates a critical p-value of p ≤ 0.013 for statistical significance.)
| taxon A | taxon B | Spearman's ρ | p-value | Kendall's τ | p-value |
|---|---|---|---|---|---|
| Squalicorax falcatus | Squalicorax kaupi | −0.812 | 0.072 | −0.690 | 0.052 |
| Ptychodus anonymus | Ptychodus mortoni | −0.185 | 0.742 | −0.077 | 0.828 |
| Ptychodus anonymus | Ptychodus whipplei | 0.936 | 0.025 | 0.833 | 0.019 |
| Ptychodus mortoni | Ptychodus whipplei | 0.092 | 0.883 | 0.077 | 0.828 |
Table 2.
Range area correlations among species with similar palaeoecology. (a) Inferred large, pelagic (circular vertebral centra suggesting fusiform-body) predators; (b) inferred large, nekto-benthic durophagous lifestyle. (A Bonferroni correction [71] for multiple comparisons indicates a critical p-value of p ≤ 0.002 for statistical significance.)
| taxon A | taxon B | Spearman's ρ | p-value | Kendall's τ | p-value |
|---|---|---|---|---|---|
| (a) Inferred pelagic, predatory taxa | |||||
| Cretoxyrhina mantelli | Squalicorax falcatus | 0.928 | 0.022 | 0.828 | 0.020 |
| Cretoxyrhina mantelli | Squalicorax kaupi | −0.882 | 0.036 | −0.786 | 0.027 |
| Cretoxyrhina mantelli | Tylosaurus sp. | −0.765 | 0.097 | −0.643 | 0.070 |
| Cretoxyrhina mantelli | Platecarpus sp. | −0.431 | 0.392 | −0.386 | 0.277 |
| Cretoxyrhina mantelli | Xiphactinus sp. | 0.174 | 0.733 | 0.138 | 0.697 |
| Squalicorax falcatus | Squalicorax kaupi | −0.812 | 0.072 | −0.690 | 0.052 |
| Squalicorax falcatus | Tylosaurus sp. | −0.696 | 0.144 | −0.552 | 0.120 |
| Squalicorax falcatus | Platecarpus sp. | −0.334 | 0.533 | −0.298 | 0.401 |
| Squalicorax falcatus | Xiphactinus sp. | 0.371 | 0.419 | 0.333 | 0.348 |
| Squalicorax kaupi | Tylosaurus sp. | 0.765 | 0.097 | 0.571 | 0.107 |
| Squalicorax kaupi | Platecarpus sp. | 0.770 | 0.108 | 0.617 | 0.082 |
| Squalicorax kaupi | Xiphactinus sp. | 0.058 | 0.933 | 0.000 | 1.000 |
| Platecarpus sp. | Tylosaurus sp. | 0.524 | 0.283 | 0.463 | 0.192 |
| Platecarpus sp. | Xiphactinus sp. | 0.152 | 0.833 | 0.149 | 0.674 |
| Tylosaurus sp. | Xiphactinus sp. | −0.058 | 0.933 | −0.138 | 0.697 |
| (b) Inferred nekto-benthic, durophagous taxa | |||||
| Ptychodus anonymus | Ptychodus mortoni | −0.185 | 0.742 | −0.077 | 0.828 |
| Ptychodus anonymus | Ptychodus whipplei | 0.936 | 0.025 | 0.833 | 0.019 |
| Ptychodus anonymus | Rhinobatos incertus | 0.880 | 0.050 | 0.745 | 0.036 |
| Ptychodus mortoni | Ptychodus whipplei | 0.092 | 0.883 | 0.077 | 0.828 |
| Ptychodus mortoni | Rhinobatos incertus | −0.058 | 0.933 | 0.000 | 1.000 |
| Ptychodus whipplei | Rhinobatos incertus | 0.786 | 0.117 | 0.596 | 0.093 |
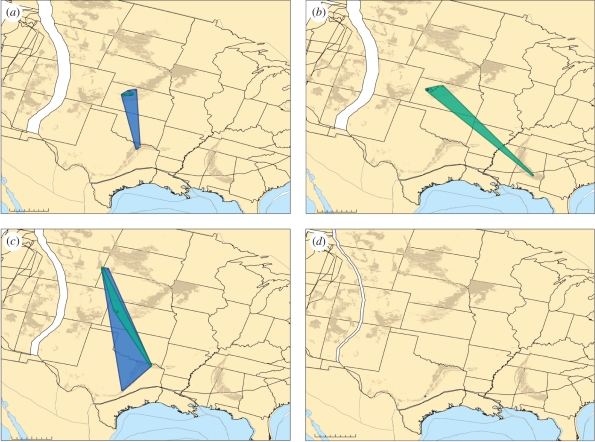
Figure 3.
PaleoGIS [53] range reconstructions illustrating the palaeobiogeographic patterns uncovered for the majority of two-taxon comparisons in this study. Tylosaurus sp. (blue) and Platecarpus sp. (dark green) distributions are shown during the Late Cretaceous. Late Cretaceous stages: (a) Coniacian, (b) Santonian, (c) Campanian, (d) Maastrichtian. These taxa do not show a statistically significant negative range area correlation through time and thus are not identified as CCRs. Present day outcrop of Late Cretaceous sediments is also shown (brown). Scale bars, (a–d) = 0–500 km.
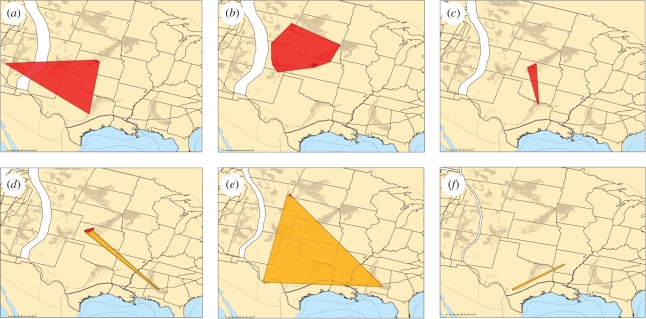
Figure 4.
PaleoGIS [53] range reconstructions illustrating the general predicted geographical pattern of a CCR, although the negative relationship in range size is not statistically significant. Squalicorax falcatus (red), and S. kaupi (orange) distributions are shown during the Late Cretaceous. Late Cretaceous stages: (a) Cenomanian, (b) Turonian, (c) Coniacian, (d) Santonian, (e) Campanian and (f) Maastrichtian. Squalicorax falcatus shows stable, though dynamic, range size until the origination of S. kaupi in the Coniacian (c). After this time, S. falcatus experiences sequential decrease in range size resulting in extinction at the end Campanian (e). This example illustrates a negative relationship between the range area of two ecologically similar species within the same genus, and thus could represent a competitive replacement of S. falcatus by S. kaupi. Present day outcrop of Late Cretaceous sediments is also shown (brown). Scale bars, (a–f) = 0–500 km.
(b). Analysis of bias
Geographical range estimations using this palaeobiogeographic method may be susceptible to artificial inflation by widely flung single occurrence points. In order to assess the influence of these potential outliers on our range reconstructions, and thus pertaining to the identification of statistically significant CCRs, we re-ran all the pairwise comparisons using the estimated mean geographic range calculated by jack-knifing (electronic supplementary material, table S3). The results are identical: before or after correcting for multiple comparisons, no statistically significant intrageneric or within-ecotype CCRs were identified; when all taxa were compared, only two CCRs appeared statistically significant before the Bonferroni correction was applied: they were no longer significant after correction for multiple comparisons. Thus, the results from the analysis of the original data and the resampled data are equivalent and the data appear robust to resampling. Consequently, outliers are not likely to be playing a significant role in influencing the results.
To test for the effect of available outcrop area on species distributions during the Late Cretaceous, we compared species' geographical range size with the area of Late Cretaceous sedimentary record; the approximate margins of the WIS for early, middle and late stages of the Late Cretaceous, along with the occurrence records parsed by stage, are shown in the electronic supplementary material, figure S44. Taxa were shown to occupy only 4–37% of potential habitat. Because taxa are not present in all or even the majority of available outcrop area during this time period, it is unlikely that the simple availability of Late Cretaceous sedimentary records is controlling the patterns of distribution and range size change observed in this analysis.
A correlation of number of unique geographical localities sampled with the size of geographical range reconstruction for each temporal interval in this analysis is shown in table 3 (for correlation statistics using resampled means, see the electronic supplementary material, table S4). The number of unique localities was used to test for sampling bias (instead of all sampled occurrences) because this reduces artificial inflation of points sampled and maximizes the potential for finding a significant correlation (thus the test is most sensitive to identifying sampling bias). None of the stages during the Late Cretaceous show significant correlations between number of data points and size of geographical range (p ≫ 0.007 using Bonferroni correction for multiple comparisons) except the Coniacian stage (p = 0.001; table 3); the same is true of the resampled data (electronic supplementary material, table S4).
Table 3.
Correlation results between the number of unique geographical localities sampled and reconstructed geographical range size for each stage during the Late Cretaceous. (A Bonferroni correction [71] for multiple comparisons indicates a critical p-value of p ≤ 0.007 for statistical significance.)
| stage | Spearman's ρ | p-value | Kendall's τ | p-value |
|---|---|---|---|---|
| Cenomanian | 0.886 | 0.016 | 0.733 | 0.039 |
| Turonian | 0.775 | 0.049 | 0.683 | 0.031 |
| Coniacian | 0.905 | 0.001 | 0.805 | 0.001 |
| *Coniaciana | 0.700 | 0.233 | 0.600 | 0.142 |
| Santonian | 0.551 | 0.163 | 0.743 | 0.101 |
| Campanian | 0.764 | 0.056 | 0.651 | 0.040 |
| Maastrichtian | 0.886 | 0.667 | 0.817 | 0.201 |
| total (combined) | 0.733 | 0.020 | 0.556 | 0.025 |
a*Coniacian represents correlation between number of unique geographical localities and reconstructed range size after removing taxa that either originate or go extinct during this stage.
Many (though not all) taxa have small geographical range size during the Coniacian; it is possible that this represents a bias in collection or preservation. On the other hand, this stage is the point of origin or extinction for a number of taxa studied (e.g. Tylosaurus sp., Platecarpus sp., S. kaupi originate; Ptychodus anonymus and Ptychodus whipplei go extinct). Species commonly have small geographical range size at the point of origination and extinction (particularly if speciation occurs allopatrically in small isolated populations and extinction first involves reduction to a single population). To assess the influence of this phenomenon, these taxa were removed and the correlation statistics re-run (table 3 and the electronic supplementary material, table S4). Excluding taxa originating or going extinct, the number of sampled localities during the Coniacian is no longer significantly correlated with the reconstructed range size in both the original and the resampled data (see table 3 and the electronic supplementary material, table S4). Thus, the uniquely small range size of these taxa was probably causing the suggested sampling bias during this interval.
4. Discussion
This study uses new techniques in quantitative biogeographic analysis to test for the role of competitive replacement in the fossil record. We focused on species' distributions in the abundant representatives of the vertebrate fauna from the Late Cretaceous WIS, specifically looking for two-taxon comparisons suggesting competitive replacement. No two-taxon comparisons showed any statistical evidence of significant, negative geographical range correlations. These results reiterate previous analyses indicating little evidence for competitive replacement [9,13]. Further, this suggests that something other than interspecific competition plays the predominant role in influencing species distributions over macroevolutionary time scales. Such processes were most probably abiotic environmental changes, both climatic and tectonic, as these have been shown to have had a significant impact on species distributions and macroevolution at other times in the history of life [20–22,24,25,50,67–69]. There could, however, also be a substantial contribution from ecological factors such as food-source tracking, intraspecific interactions, etc.
It is worth noting that competitive replacement may be more prevalent among species that are rare and/or geographically restricted. Such cases are difficult to identify in the fossil record, and thus by necessity our study focused on more abundant and potentially more ‘successful’ taxa from the outset. As a consequence, even though we attempted to maximize recovery of CCRs by using broad definitions of palaeoecological similarity, our estimate of the frequency of CCRs is most surely an underestimate. Nonetheless, it is based on quantitative and detailed investigation of these groups, and thus the best estimate possible at present.
Moreover, while we believe that our analysis includes real species using a phylogenetic species concept, it is impossible to exclude the possibility that some of these species actually represent ecomorphs within a single lineage. If this were the case, then instead of identifying cases of competitive replacement between species, our analysis would be testing for intraspecific interactions occurring between co-occurring ecomorphs. The apparent non-prevalence of competitive replacement within potentially adaptive lineages then might suggest that ecomorph evolution also may not be strongly influenced by these types of competitive interactions.
Ultimately, this study provides little evidence that CCRs play a defining role in shaping species' distributions at the macroevolutionary scale. The driving force is instead likely to be abiotic environmental factors, such as climate and sea-level changes, that determine species distribution and range size. Other ecological factors may have been important as well, but interspecific competition does not appear to have had a major effect on macroevolutionary patterns of species in the fossil record [17,20–22,39,68,70].
Acknowledgements
We thank Erin Saupe, Paulyn Cartwright, Greg Edgecombe and two anonymous reviewers for their helpful discussions and comments on previous drafts. We are also indebted to Daniel Brinkman, George Corner, Tonia Culver, Michael Everhart, Neal Larson, Desui Miao, Lyndon Murray, Daniel Williams and Laura Wilson for their assistance with museum collections and pinpointing the most accurate geographical/stratigraphic specimen data and to Peg Yacobucci and Richard Mackenzie for providing generous assistance with figures. This research was supported by the Madison and Lila Self Graduate Fellowship, Panorama Society Small Grant Competition, Yale Peabody Museum Schuchert and Dunbar Grants in Aid Programme, University of Kansas Department of Geology, NSF DEB-0716162 and NSF DBI-0346452.
References
- 1.MacArthur R. H., Wilson E. O. 1972. The theory of island biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Van Valen L. M. 1973. A new evolutionary law. Evol. Theory 1, 1–30 [Google Scholar]
- 3.Vermeij G. J. 1987. Evolution and escalation: an ecological history of life. Princeton, NJ: Princeton University Press [Google Scholar]
- 4.Jackson J. B. C., McKinney F. K. 1990. Ecological processes and progressive macroevolution of marine clonal benthos. In Causes of evolution (eds Ross R. M., Allmon W. D.), pp. 173–209 Chicago, IL: University of Chicago Press [Google Scholar]
- 5.Rosenzweig M. L., McCord R. D. 1991. Incumbent replacement: evidence for long-term evolutionary progress. Paleobiology 17, 202–213 [Google Scholar]
- 6.Sepkoski J. J., Jr, McKinney F. K., Lidgard S. 2000. Competitive displacement among post-Paleozoic cyclostome and cheilostome bryozoans. Paleobiology 26, 7–18 (doi:10.1666/0094-8373(2000)026<0007:CDAPPC>2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 7.Darwin C. 1859. On the origin of species by means of natural selection; or the preservation of favoured races in the struggle for life (reprinted 1st edn). Cambridge, MA: Harvard University Press; [PMC free article] [PubMed] [Google Scholar]
- 8.Benton M. J. 1987. Progress and competition in macroevolution. Biol. Rev. 62, 305–338 10.1111/j.1469-185X.1987.tb00666.x (doi:10.1111/j.1469-185X.1987.tb00666.x) [DOI] [Google Scholar]
- 9.Benton M. J. 1996. Testing the roles of competition and expansion in tetrapod evolution. Proc. R. Soc. Lond. B 263, 641–646 10.1098/rspb.1996.0096 (doi:10.1098/rspb.1996.0096) [DOI] [Google Scholar]
- 10.Rayner R. J., Masters J. C. 1995. A good loser is still a loser: competition and the South African fossil record. S. Afr. J. Sci. 91, 184–189 [Google Scholar]
- 11.Cifelli R. L. 1981. Patterns of evolution among the Artiodactyla and Perissodactya (Mammalia). Evolution 35, 433–440 10.2307/2408192 (doi:10.2307/2408192) [DOI] [PubMed] [Google Scholar]
- 12.Walker T. D., Valentine J. W. 1984. Equilibrium models of evolutionary species diversity and the number of empty niches. Am. Nat. 124, 887–899 10.1086/284322 (doi:10.1086/284322) [DOI] [Google Scholar]
- 13.Benton M. J. 1996. On the nonprevalence of competitive replacement in the evolution of tetrapods. In Evolutionary paleobiology (eds Jablonski D., Erwin D. H., Lipps J.), pp. 185–210 Chicago, IL: Chicago University Press [Google Scholar]
- 14.Gould S. J. 1985. The paradox of the first tier: an agenda for paleobiology. Paleobiology 11, 2–12 [Google Scholar]
- 15.Gould S. J., Calloway C. B. 1980. Clams and brachiopods: ships that pass in the night. Paleobiology 6, 383–396 [Google Scholar]
- 16.Eldredge N., Cracraft J. 1980. Phylogenetic patterns and the evolutionary process. New York, NY: Columbia University Press [Google Scholar]
- 17.Vrba E. S. 1980. Evolution, species and fossils: how does life evolve? S. Afr. J. Sci. 76, 61–84 [Google Scholar]
- 18.Vrba E. S. 1985. Environment and evolution: alternative causes of the temporal distribution of evolutionary events. S. Afr. J. Sci. 81, 229–236 [Google Scholar]
- 19.Masters J. C., Rayner R. J. 1993. Competition and macroevolution: the ghost of competition yet to come? Biol. J. Linn. Soc. Lond. 49, 87–98 10.1111/j.1095-8312.1993.tb00686.x (doi:10.1111/j.1095-8312.1993.tb00686.x) [DOI] [Google Scholar]
- 20.Benton M. J. 2009. The Red Queen and the Court Jester: species diversity and the role of biotic and abiotic factors through time. Science 323, 728–732 10.1126/science.1157719 (doi:10.1126/science.1157719) [DOI] [PubMed] [Google Scholar]
- 21.Barnosky A. D. 2001. Distinguishing the effects of the Red Queen and Court Jester on Miocene mammal evolution in the northern Rocky Mountains. J. Vertebr. Paleontol. 21, 172–185 10.1671/0272-4634(2001)021[0172:DTEOTR]2.0.CO;2 (doi:10.1671/0272-4634(2001)021[0172:DTEOTR]2.0.CO;2) [DOI] [Google Scholar]
- 22.Flagstad O., Syversten P. O., Stenseth N. C., Jakobsen K. S. 2001. Environmental change and rates of evolution: the phylogeographic pattern within the hartebeest complex as related to climatic variation. Proc. R. Soc. Lond. B 268, 667–677 10.1098/rspb.2000.1416 (doi:10.1098/rspb.2000.1416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieberman B. S., Miller W., III, Eldredge N. 2007. Paleontological patterns, macroecological dynamics, and the evolutionary process. Evol. Biol. 34, 28–48 10.1007/s11692-007-9005-4 (doi:10.1007/s11692-007-9005-4) [DOI] [Google Scholar]
- 24.Rode A. L., Lieberman B. S. 2004. Using GIS to unlock the interactions between biogeography, environment, and evolution in Middle and Late Devonian brachiopods and bivalves. Palaeogeogr. Palaeoclimatol. Palaeoecol. 211, 345–359 10.1016/j.palaeo.2004.05.013 (doi:10.1016/j.palaeo.2004.05.013) [DOI] [Google Scholar]
- 25.Stigall A. L., Lieberman B. S. 2006. Quantitative palaeobiogeography: GIS, phylogenetic biogeographical analysis, and conservation insights. J. Biogeogr. 33, 2051–2060 10.1111/j.1365-2699.2006.01585.x (doi:10.1111/j.1365-2699.2006.01585.x) [DOI] [Google Scholar]
- 26.Costa G. C., Wolfe C., Shepard D. B., Caldwell J. P., Vitt L. J. 2008. Detecting the influence of climatic variables on species distributions: a test using GIS niche-based models along a steep longitudinal environmental gradient. J. Biogeogr. 35, 637–646 10.1111/j.1365-2699.2007.01809.x (doi:10.1111/j.1365-2699.2007.01809.x) [DOI] [Google Scholar]
- 27.Kozak K. H., Graham C. H., Wiens J. J. 2008. Integrating GIS-based environmental data into evolutionary biology. Trends Ecol. Evol. 23, 141–148 [DOI] [PubMed] [Google Scholar]
- 28.Butler R. J., Barrett P. M., Penn M. G., Kenrick P. 2010. Testing coevolutionary hypotheses over geological timescales: interactions between Cretaceous dinosaurs and plants. Biol. J. Linn. Soc. 100, 1–15 10.1111/j.1095-8312.2010.01401.x (doi:10.1111/j.1095-8312.2010.01401.x) [DOI] [Google Scholar]
- 29.Barron E. J. 1983. A warm, equable Cretaceous: the nature of the problem. Earth Sci. Rev. 19, 305–338 10.1016/0012-8252(83)90001-6 (doi:10.1016/0012-8252(83)90001-6) [DOI] [Google Scholar]
- 30.Huber B. T., Norris R. D., MacLeod K. G. 2002. Deep-sea paleotemperature record of extreme warmth during the Cretaceous. Geology 30, 123–126 (doi:10.1130/0091-7613(2002)030<0123:DSPROE>2.0.CO;2) [DOI] [Google Scholar]
- 31.Spicer B. 2002. Changing climate and biota. In The Cretaceous world (ed. Skelton P.), pp. 85–162 Cambridge, UK: Cambridge University Press [Google Scholar]
- 32.Everhart M. J. 2005. Oceans of Kansas: a natural history of the Western Interior Sea. Bloomington, IN: Indiana University Press [Google Scholar]
- 33.Hattin D. E. 1982. Stratigraphy and depositional environment of the Smoky Hill Chalk Member, Niobrara Chalk (upper Cretaceous) of the type area, western Kansas. Kans. Geol. Survey Bull. 225, 1–108 [Google Scholar]
- 34.Kauffman E. G., Caldwell W. G. E. 1993. The Western Interior Basin in space and time. In Evolution of the Western Interior Basin. Geol. Assoc. Can. Spec. Paper 39 (eds Caldwell W. G. E., Kauffman E. G.), pp. 1–30 St. John's, Canada: Geological Association of Canada [Google Scholar]
- 35.Poulsen C. J., Barron E. J., Arthur M. A., Peterson W. H. 2001. Response of the mid-Cretaceous global oceanic circulation to tectonic and CO2 forcings. Paleoceanography 16, 576–592 10.1029/2000PA000579 (doi:10.1029/2000PA000579) [DOI] [Google Scholar]
- 36.Kauffman E. G. 1984. Paleobiogeography and evolutionary response dynamic in the Cretaceous Western Interior Seaway of North America. In Jurassic-Cretaceous biochronology and paleogeography of North America. Geol. Assoc. Can. Spec. Paper 27 (ed. Westermann G. E. G.), pp. 273–306 St. John's, Canada: Geological Association of Canada [Google Scholar]
- 37.Shimada K., Schumacher B. A., Parkin J. A., Palermo J. M. 2006. Fossil marine vertebrates from the lowermost Greenhorn Limestone (upper Cretaceous: Middle Cenomanian) in southeastern Colorado. J. Paleontol. 80, 1–45 10.1666/0022-3360(2006)80[1:FMVFTL]2.0.CO;2 (doi:10.1666/0022-3360(2006)80[1:FMVFTL]2.0.CO;2) [DOI] [Google Scholar]
- 38.Hancock J. M., Kauffman E. G. 1979. The great transgressions of the Late Cretaceous. J. Geol. Soc. Lond. 136, 175–186 10.1144/gsjgs.136.2.0175 (doi:10.1144/gsjgs.136.2.0175) [DOI] [Google Scholar]
- 39.Nicholls E. L., Russell A. P. 1990. Paleobiogeography of the Cretaceous Western Interior Seaway of North America: the vertebrate evidence. Palaeogeogr. Palaeoclimatol. Palaeoecol. 79, 149–169 10.1016/0031-0182(90)90110-S (doi:10.1016/0031-0182(90)90110-S) [DOI] [Google Scholar]
- 40.Glancy T. J., Jr, Arthur M. A., Barron E. J., Kauffman E. G. 1993. A paleoclimate model for the North American Cretaceous (Cenomanian-Turonian) epicontinental sea. In Evolution of the Western Interior Basin. Geol. Assoc. Can. Spec. Paper 39 (eds Caldwell W. G. E., Kauffman E. G.), pp. 219–242 St. John's, Canada: Geological Association of Canada [Google Scholar]
- 41.Russell D. A. 1993. Vertebrates in the Cretaceous Western Interior Sea. In Evolution of the Western Interior Basin. Geol. Assoc. Can. Spec. Paper 39 (eds Caldwell W. G. E., Kauffman E. G.), pp. 665–680 St. John's, Canada: Geological Association of Canada [Google Scholar]
- 42.Schroder-Adams C. J., Leckie D. A., Bloch J., Craig J., McIntyre D. J., Adams P. J. 1996. Paleoenvironmental changes in the Cretaceous (Albian to Turonian) Colorado Group of western Canada: microfossil, sedimentological, and geochemical evidence. Cret. Res. 17, 311–365 [Google Scholar]
- 43.Sageman B. B., Rich J., Arthur M. A., Birchfield G. E., Dean W. E. 1997. Evidence for Milankovich periodicities in Cenomanian-Turonian lithologic and geochemical cycles, Western Interior U.S.A. J. Sediment. Res. 67, 286–302 [Google Scholar]
- 44.Schwimmer D. R., Stewart J. D., Williams G. D. 1997. Scavenging by sharks of the genus Squalicorax in the Late Cretaceous of North America. Palaios 12, 71–83 10.2307/3515295 (doi:10.2307/3515295) [DOI] [Google Scholar]
- 45.Keller G., Berner Z., Adatte T., Stueben D. 2004. Cenomanian-Turonian and δ13C, and δ18O sea level and salinity variations at Pueblo, Colorado. Palaeogeogr. Palaeoclimatol. Palaeoecol. 211, 19–43 10.1016/j.palaeo.2004.04.003 (doi:10.1016/j.palaeo.2004.04.003) [DOI] [Google Scholar]
- 46.Everhart M. J. 2001. Revisions to the biostratigraphy of the Mosasauridae (Squamata) in the Smoky Hill Chalk Member of the Niobrara Chalk (Late Cretaceous) of Kansas. Trans. Kans. Acad. Sci. 104, 59–78 10.1660/0022-8443(2001)104[0059:RTTBOT]2.0.CO;2 (doi:10.1660/0022-8443(2001)104[0059:RTTBOT]2.0.CO;2) [DOI] [Google Scholar]
- 47.Becker M. A., Chamberlain J. A., Jr, Wolf G. E. 2006. Chondrichthyes from the Arkadelphia Formation (upper Cretaceous: upper Maastrichtian) of Hot Spring County, Arkansas. J. Paleontol. 80, 700–716 10.1666/0022-3360(2006)80[700:CFTAFU]2.0.CO;2 (doi:10.1666/0022-3360(2006)80[700:CFTAFU]2.0.CO;2) [DOI] [Google Scholar]
- 48.Cobban W. A., Walaszczyk I., Obradovich J. D., McKinney K. C. 2006. A USGS Zonal table for the Upper Cretaceous middle Cenomanian-Maastrichtian of the Western Interior of the United States based on ammonites, inoceramids, and radiometric ages. U.S.G.S., Open-file Report 2006-1250, pp. 46 Denver, CO: United States Geological Survey [Google Scholar]
- 49.Ufnar D. F., Ludvigson G. A., Gonzalez L., Grocke D. R. 2008. Precipitation rates and atmospheric heat transport during the Cenomanian greenhouse warming in North America: estimates from a stable isotope mass-balance model. Palaeogeogr. Palaeoclimatol. Palaeoecol. 266, 28–38 10.1016/j.palaeo.2008.03.033 (doi:10.1016/j.palaeo.2008.03.033) [DOI] [Google Scholar]
- 50.Henricks J. R., Lieberman B. S., Stigall A. L. 2008. Using GIS to study palaeobiogeographic and macroevolutionary patterns in soft-bodied Cambrian arthropods. Palaeogeogr. Palaeoclimatol. Palaeoecol. 264, 163–175 [Google Scholar]
- 51.Maguire K. C., Stigall A. L. 2009. Using ecological niche modeling for quantitative biogeographic analysis: a case study of Miocene and Pliocene Equinae in the Great Plains. Paleobiology 35, 587–611 10.1666/0094-8373-35.4.587 (doi:10.1666/0094-8373-35.4.587) [DOI] [Google Scholar]
- 52.Environmental Systems Research Institute 2006. ArcGIS version 9.2. Redlands, CA: ESRI [Google Scholar]
- 53.Rothwell Group, LP 2007. PaleoGIS for ArcGIS 9.x. Houston, TX [Google Scholar]
- 54.Ross M. I., Scotese C. R. 2000. PaleoGIS/Arcview 3.5, PALEOMAP Project. Arlington, TX: University of Texas at Arlington [Google Scholar]
- 55.Hammer Ø., Harper D. A. T., Ryan P. D. 2001. PAST: Paleontological Statistics Software Package for education and data analysis. Palaeontol. Electron. 4, 1–9 [Google Scholar]
- 56.Russell D. A. 1967. Systematics and morphology of American mosasaurs (Reptilia, Sauria). Bull. Peabody Mus. Nat. Hist. 23, 1–241 [Google Scholar]
- 57.Williamson T. E., Kirkland J. I., Lucas S. G. 1993. Selachians from the Greenhorn Cyclothem (‘Middle’ Cretaceous: Cenomanian-Turonian), Black Mesa, Arizona, and the paleogeographic distribution of Late Cretaceous Selachians. J. Paleontol. 67, 447–474 [Google Scholar]
- 58.Rothschild B. M., Martin L. D., Schulp A. S. 2005. Sharks eating mosasaurs, dead or alive? In Proc. of the First Mosasaur Meeting. Netherl. J. Geosci. vol. 84 (eds Schulp A. S., Jagt J. W. M.), pp. 335–340 [Google Scholar]
- 59.Shimada K., Cicimurri D. J. 2005. Skeletal anatomy of the Late Cretaceous shark, Squalicorax (Neoselachii: Anacoracidae). Paläontol. Zeit. 79, 241–261 [Google Scholar]
- 60.Shimada K. 1997. Paleoecological relationships of the Late Cretaceous Lamniform shark, Cretoxyrhina mantelli (Agassiz). J. Paleontol. 71, 926–933 [Google Scholar]
- 61.Stewart J. D. 1988. Paleoecology and the first North American west coast record of the shark genus Ptychodus. J. Vertebr. Paleontol. 8, 27A [Google Scholar]
- 62.Everhart M. J. 2007. New stratigraphic records (Albian-Campanian) of Rhinobatos sp. (Chondrichthyes; Rajiformes) from the Cretaceous of Kansas. Trans. Kans. Acad. Sci. 110, 225–235 10.1660/0022-8443(2007)110[225:NSRAOR]2.0.CO;2 (doi:10.1660/0022-8443(2007)110[225:NSRAOR]2.0.CO;2) [DOI] [Google Scholar]
- 63.Hamm S. A. 2008. Systematic, stratigraphic, geographic, and palaeoecological distribution of the Late Cretaceous shark genus Ptychodus within the Western Interior Seaway. Unpublished Master's thesis, University of Texas at Dallas [Google Scholar]
- 64.Hamm S. A. 2010. The Late Cretaceous shark, Ptychodus rugosus (Ptychodontidae) in the Western Interior Sea. Trans. Kans. Acad. Sci. 113, 44–55 10.1660/062.113.0203 (doi:10.1660/062.113.0203) [DOI] [Google Scholar]
- 65.Meyer D. L., Milsom C. V. 2001. Microbial sealing in the biostratinomy of Uintacrinus Lagerstätten in the Upper Cretaceous of Kansas and Colorado, USA. Palaios 16, 535–546 [Google Scholar]
- 66.Bottjer D. J. 2002. Smoky Hill Chalk: spectacular Cretaceous marine fauna. In Exceptional fossil preservation: a unique view on the evolution of marine life (eds Bottjer D. J., Etter W., Hagadorn J. W., Tang C. M.), pp. 353–364 New York, NY: Columbia University Press [Google Scholar]
- 67.Lieberman B. S., Eldredge N. 1996. Trilobite biogeography in the Middle Devonian: geological processes and analytical methods. Paleobiology 22, 66–79 [Google Scholar]
- 68.Gates T. A., et al. 2010. Biogeography of terrestrial and freshwater vertebrates from the Late Cretaceous (Campanian) Western Interior of North America. Palaeogeogr. Palaeoclimatol. Palaeoecol. 291, 371–387 10.1016/j.palaeo.2010.03.008 (doi:10.1016/j.palaeo.2010.03.008) [DOI] [Google Scholar]
- 69.Lieberman B. S. 2000. Paleobiogeography. New York, NY: Kluwer Academic Press [Google Scholar]
- 70.Venditti C., Meade A., Pagel M. 2010. Phylogenies reveal new interpretation of speciation and the Red Queen. Nature 463, 349–352 10.1038/nature08630 (doi:10.1038/nature08630) [DOI] [PubMed] [Google Scholar]
- 71.Sokal R. R., Rohlf F. J. 1995. Biometry: the principles and practices of statistics in biological research, 3rd edn. New York, NY: W.H. Freeman & Co [Google Scholar]