Abstract
Animal personality, defined as consistent individual differences across context and time, has attracted much recent research interest in the study of animal behaviour. More recently, this field has begun to examine how such variation arose and is maintained within populations. The habitat-dependent selection hypothesis, which posits that animals with differing personality types may fare better (i.e. have a fitness advantage) in different habitats, suggests one possible mechanism. In the current experiment, we tested whether slow- and fast-exploring black-capped chickadees (Poecile atricapillus), determined by performance in a novel environment exploration task, perform differentially when the demands of an acoustic operant discrimination (cognitive) task were altered following successful task acquisition. We found that slow-exploring birds learn to reverse previously learned natural category rules more quickly than faster exploring conspecifics. In accordance with the habitat-dependent selection hypothesis, and previous work with great tits (Parus major), a close relative of the black-capped chickadee, our results suggest that fast-exploring birds may perform better in stable, predictable environments where forming a routine is advantageous, while slow-exploring birds are favoured in unstable, unpredictable environments, where task demands often change. Our results also support a hypothesis derived from previous work with great tits; slow-exploring birds may be generally more flexible (i.e. able to modify their behaviour in accordance with changes in environmental stimuli) in some learning tasks.
Keywords: animal personality, black-capped chickadee, exploratory behaviour, habitat-dependent selection, instrumental discrimination, learning
1. Introduction
The study of animal personality (also known as temperament [1], behavioural syndromes [2] and coping styles [3]) is concerned with how behavioural characteristics are related across contexts and over time within a species. A survey of personality-related research by Gosling [4] showed that such studies included 66 species spanning a diverse array of taxa. As species are added to this list (e.g. [5–7]; see [8–10] for recent reviews), so too are new behavioural characteristics being linked to existing suites of correlated traits (e.g. boldness correlates with aggressiveness [11]).
The field of animal personality has begun to pose questions about how individual differences in behaviour develop, evolve and how these differences are maintained within a species or a population [8,9,12]. Individuals can differ in their overall personality (or behavioural profile) associated with differing life-history strategies [13–15]. For instance, some individuals may focus on current reproduction and thus behave in a more risk-prone manner, whereas other individuals may focus on future reproduction and behave in a more risk-averse manner [14,16]. Variation in the personality of animals within a species may be maintained when one personality phenotype is rare and has higher fitness compared with alternate personality phenotype(s) (negative frequency-dependent selection [14,16,17]) or is more effective in different local habitats (habitat-dependent selection [1,16]).
While individual variation in exploratory behaviour represents one of the best-studied personality characteristics (e.g. [1,11,18–20]), individual variation in learning ability has received less attention [2,3,21]. Previous work has suggested that individual variation in discrimination learning is related to how black-capped chickadees (Poecile atricapillus) explore a novel environment [6]. Guillette et al. [6] showed that chickadees which are quicker to explore a novel environment are also quicker to learn an acoustic operant discrimination task. The notion that, in general, fast explorers are fast learners is further supported by evidence from a study with wild-caught male starlings (Sturnus vulgaris [22]). Boogert et al. [22] found that starlings which were faster to feed in a novel environment solved a learning task faster than starlings that were slower to feed in a novel environment.
Here, we test the idea that fast explorers may perform better in a stable (predictable) environment where it is adaptive to learn new tasks quickly and commit them to a routine [18,23], while slower exploring animals may benefit in unstable (unpredictable) environments where behavioural flexibility is favoured [24,25]. The notion that personality spans a behavioural continuum (e.g. bold, exploratory → shy, non-exploratory) and that the type of personality that is favoured changes with habitat variation across space or time is supported by studies in several species (e.g. [26]). For example, during range expansions in western blue birds (Sialia mexicana), aggressive individuals are initially favoured when colonizing new areas. The number of aggressive individuals declines rapidly following colonization, presumably because aggressive males tend to provide lower levels of parental care [27]. However, the link between exploration and learning has not yet been thoroughly examined.
Birds that are faster to act when faced with novelty (black-capped chickadees' exploration in a novel environment [6]; starlings' latency to feed in a novel environment [22]) are also faster to learn a cognitive task; however, the cognitive task used in these particular experiments may have inadvertently favoured individuals with bold, exploratory personality types because the task demands were stable, and forming a routine would have been advantageous. Experiments that might favour shyer, less exploratory individuals have yet to be examined. This could be accomplished through testing learning in a dynamic, rather than a stable, environment. If slow-exploring birds perform better in a testing environment where task demands are altered, this suggests that they may have an advantage over fast-exploring birds in certain micro-habitats (in line with the habitat-dependent selection hypothesis).
We trained black-capped chickadees in an instrumental discrimination paradigm that required each bird to respond to rewarded (S+) stimuli, but not respond to non-rewarded (S−) stimuli belonging to different note-type categories (e.g. A notes, B notes) from their namesake chick-a-dee call. Once a bird had learned this task, the reward contingencies associated with each category were reversed. This represents an environmental change that birds need to adapt to in order to maximize their reward (food). Only the reward contingency (i.e. rewarded or non-rewarded) associated with each category changed, so previous learning (discrimination training on categories) will aid birds in the reversal if they are able to exhibit flexibility in their learned behaviour. The number of trials it took each bird to learn the discrimination task served as a baseline for learning with which the number of trials needed to learn the reversal was compared. All birds were first run in a novel environment exploration task, and then run in the operant discrimination task.
2. Methods
(a). Subjects
Thirty birds (second year or after the second year, determined by the shape and colouring of outer tail retrices [28]) were captured between 19 December 2008 and 28 January 2009. Seventeen birds (nine males, eight females) originated from the North Saskatchewan River Valley in Edmonton, Alberta, Canada (53°34′ N, 113°31′ W), and the remaining 13 birds (seven males, six females) originated from Stony Plain Alberta, Canada (53°31′ N, 114°00′ W), 36 km west of Edmonton. Prior to and between testing phases, each bird was housed individually at the University of Alberta in Jupiter Parakeet cages (30 × 40 × 40 cm; Rolf C. Hagen, Inc., Montreal, Canada), which allowed for auditory and visual, but not physical contact among birds. Birds had free access to food (Mazuri Small Bird Maintenance Diet; Mazuri, St Louis, MO, USA), water (vitamin supplemented on alternating days; Prime vitamin supplement, Hagen, Inc., Montreal, Canada), grit and cuttlebone. Birds were given three to five sunflower seeds daily. Birds also received one mealworm (Tenebrio molitor) or superworm (Zophobas morio) three times a week and a mixture of greens and eggs twice a week. Birds were maintained on a light/dark cycle that mimicked the natural light cycle for Edmonton, Alberta, Canada.
(b). Apparatus
(i). Novel environment room
The novel environment room (2.03 × 1.52 × 2.44 m) housed five artificial trees. The artificial trees consisted of a 5 × 5 cm unfinished, wooden ‘trunk’ that was 1.4 m high. There were four, 1 cm diameter unfinished wooden dowel ‘branches’ that extended 20 cm from the trunk. Two upper branches were 5 cm from the top of the trunk, and the other two branches were 20 cm lower and perpendicular to the top branches (following Verbeek et al. [18]). In one end of the room there was a 35 × 24 cm opening behind which the bird was placed in its home cage (30 × 40 × 40 cm). Each session was recorded with a JVC Everio camcorder, fit with a wide angle lens, so behavioural data could be scored at a later date.
(ii). Operant conditioning chamber
A detailed description of the instrumental discrimination apparatus can be found in Sturdy & Weisman [29]. In brief, each bird was placed in a modified budgerigar cage (30 × 40 × 40 cm) that had several perches, a grit cup, cuttlebone, water tube, an opening on one side to allow access to the food hopper and a plastic mesh suspended from the bottom so that birds could not eat spilled food. A request perch with an infra-red beam was situated approximately 5 cm from the opening to the food hopper; another infra-red beam spanned the entrance to the food hopper. A speaker that broadcast stimuli was at perch height next to the food hopper on the outside of the cage. This apparatus was housed in a ventilated sound attenuating chamber.
(iii). Stimulus preparation
The stimuli for the acoustic operant discrimination were exemplars of ‘B’ and ‘C’ notes from the namesake chick-a-dee call of the black-capped chickadee. The chick-a-dee call is composed of four note types; A, B, C and D, produced in a fixed order, but each note type may be omitted or repeated [30]. These note types are perceived as belonging to natural, open-ended categories by black-capped chickadees [31,32]. That is, birds can continue to classify notes as belonging to a natural category when they are tested with novel exemplars. A detailed description of stimulus preparation can be found in Charrier et al. [31]. Briefly, notes were taken from high-quality recordings of black-capped chickadee chick-a-dee calls. Twenty exemplars each of two note types (i.e. 20 B, 20 C, 40 total) were recorded, one note per track, to a recordable compact disc for discriminative stimuli in the instrumental discrimination task.
(iv). Novel environment task
Birds were tested for exploratory behaviour in the novel environment task before initiation of the instrumental discrimination task. Individual birds in their home cages were placed with their cage against the opening to the novel environment room. A clear barrier controlled birds' physical, but not visual, access to the novel environment room. We allowed visual access to the novel environment room because pilot tests for previous experiments (i.e. [6]) revealed that birds rarely left their home cage within 30 min if the barrier to the novel environment was opaque. One hour later the barrier was removed, allowing the bird access to the room. Each bird was recorded via the JVC camcorder suspended from the ceiling for 10 min. All trials were conducted between 11.00 and 15.00 h. Birds were returned to the colony room after testing, and the length of the left tarsus was measured as an index of body size. All birds visited the novel environment between 27 April and 25 May 2009.
(v). Instrumental discrimination task
Preliminary training
Once a bird learned to use the request perch and food hopper to obtain food, preliminary training began. To start a trial, a bird had to wait on the request perch, thus breaking an infra-red beam, for a randomly selected interval of between 900 and 1100 ms. Following this, a note from the pool of 40 notes (20 of each note type) was randomly selected and played (between approx. 70 and 80 dB SPL as measured by a Radio Shack Sound Level Meter (fast setting, A weighting)). If the bird left the request perch before the note had finished playing, the trial terminated and a 30 s inter-trial interval (ITI) with the houselights off ensued (termed a zap). This was to train birds to remain on the perch and attend to each stimulus in its entirety before making a response. If the bird remained on the perch until the note finished playing and then flew to the feeder within 1 s from the termination of the stimulus, it was given 1 s access to food followed by a 30 s ITI, with the houselights on. If the bird listened to the entire note, left the request perch within 1 s of stimulus termination but did not enter the feeder, the trial ended after 1 s. If the bird listened to the entire note and remained on the request perch, the trial ended after 1 s and a 60 s ITI followed, with the houselights on. If the bird left the perch during the 60 s ITI for 1 s, the 60 s ITI was terminated and a new trial initiated. We used preliminary training to train birds to create high, uniform, responses to all training stimuli that would be used in the discrimination phase of the experiment, and to train the birds to listen to each stimulus in its entirety, while also training them to leave the request perch after each stimulus was played (see Charrier et al. [31] for details). This step ensured that birds readily approached all stimuli prior to discrimination training, thus eliminating any inherent bias resulting from individual differences in neophobia that might be present among the birds and that could affect discrimination performance. The criteria to complete preliminary training was six blocks (one block = 500 trials) with over 60 per cent responding to all stimuli, and no more than 3 per cent difference in response to all note types to ensure no initial preferences for note types biased the discrimination. Exemplars were presented equally and in a random order within blocks, and randomized for each block, for each subject.
Discrimination acquisition
Charrier et al. [31] describes the acoustic discrimination and transfer training procedures in great detail. In this phase, breaking the infra-red beam in the food hopper after food-rewarded (S+) notes resulted in 1 s access to food, whereas visits to the feeder following non-rewarded (S−) notes resulted in a 30 s ITI with the house lights extinguished and no food access. All other procedures from preliminary training (e.g., random selection of notes, remaining on the perch until stimulus completion etc.) remained in effect during discrimination training.
Discrimination training was initiated immediately following preliminary training. All 30 birds learned to discriminate the notes (B notes were S+, and C notes were S−). Birds were initially trained to discriminate between 10 S+ and 10 S− stimuli. Next, the birds were presented with the remaining 10 S+ and 10 S− stimuli, referred to as the first set, and second set, respectively, hereafter. Finally, the birds were presented with all 20 S+ and 20 S− stimuli. The criterion to complete each of the phases of the discrimination task was six blocks with a discrimination ratio (DR; calculated by dividing the average percentage of response to S+ notes by the average percentage of response to all (both the S+ and S−) notes, excluding zap trials) of 0.8 or greater with the last two blocks of 0.8 or greater occurring consecutively.
Reversal training
Following completion of discrimination training, all birds were placed on reversal. In reversal, reward contingencies were modified such that C notes were now rewarded (S+) and B notes were now unrewarded (S−); opposite to that of discrimination training. The stages of training and the criterion to complete each of the phases of reversal task were the same as during the discrimination task. All birds were tested in the instrumental discrimination task between 30 April and 18 December 2009.
(vi). Scoring
We scored the number of trees (out of five) visited by each bird in the novel environment room from the recording of each 10 min trial. A score of zero was awarded to a bird that failed to leave the home cage. In the instrumental discrimination task, the number of blocks needed to reach criteria served as the learning scores. The reversal speed score is the learning score for set 1 reversal minus the learning score for set 1 discrimination. The longer (more blocks) it took a bird to learn the reversal task, compared with the initial task, the higher the reversal speed score, reflecting that the bird was slow at reversing relative to a bird with a lower score.
(vii). Statistical analysis
One-sample t-tests were conducted to test whether the DRs were different from chance (chance = 0.5) during various blocks of learning stages. Linear regressions, using residuals from the number of trees perched on (0, 1, 2, 3, 4 or 5) in the novel environment after controlling for sex and tarsus length (as a proxy for estimating body size), were used to predict learning scores. We controlled for sex and body size (both permanent between-individual sources of variation) because males and females may show differences in predictability of some behaviours [9,33,34] and boldness is related to body size in at least some species [1,35]. All analyses were conducted in PSAW Statistics v. 18.
3. Results
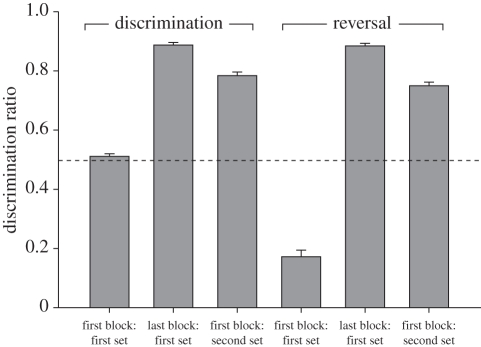
Owing to equipment failure during operant testing, the data for three birds were discarded; leaving 27 individuals (12 from Stony Plain: six male, six female; and 15 from River Valley: seven male, eight female). All numbers reported are mean ± s.e.m. Responding was not different from chance at the start of training (DR first block of first set of discrimination = 0.51 ± 0.01, t26 = 0.86, p = 0.40). When birds were transferred to the second set of stimuli, the DR was significantly higher than chance (DR = 0.78 ± 0.01; t26 = 26.31, p < 0.001; figure 1), demonstrating significant savings as birds moved through the various stages of acquisition (see figure 2 for the number of blocks required to complete each training stage).
Figure 1.
The mean ± s.e.m. for the DR (y-axis) of all birds (n = 27) during various stages of training (x-axis). Each block consisted of 500 trials. Out of 40 total stimuli, the first set contained 10 exemplars of each note, the second set contained the remaining exemplars. During discrimination training, all birds were rewarded for responding to B notes, and not rewarded for C notes, while during reversal training, birds were rewarded for responding to C notes, and not rewarded for B notes. Chance discrimination is indicated by the dashed line.
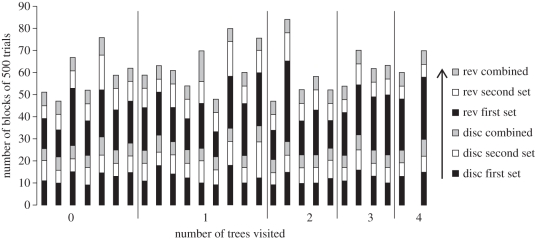
Figure 2.
The number of 500-trial blocks for each individual (y-axis) to complete the different training phases. The first black bar represents the first set for discrimination (disc), the first white bar represents the second set for discrimination, the first grey bar represents the combined sets, the second black bar represents the first set for reversal (rev), the second white bar represents the second set for reversal and the second grey bar represents the combined set for reversal. Individuals are grouped on the x-axis according to how many trees they visited in the novel environment exploration task.
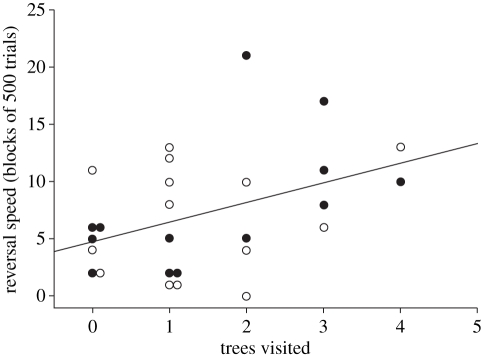
A linear regression predicting the reversal speed score from the number of trees on which a bird perched during the novel environment test showed a significant effect (r2 = 0.20, F1,25 = 6.16, p = 0.02; figure 3). Birds that visited fewer trees learned the reversal task more quickly, compared with baseline scores, than birds that visited more trees. However, there was no relationship between the birds' baseline (discrimination) learning scores and the number of trees on which a bird perched during the novel environment test (r2 = 0.003, F1,25 = 0.006, p = 0.799).
Figure 3.
The number of trees visited in the novel environment (x-axis) and the reversal speed in blocks of 500 trials (y-axis). The reversal speed is the difference between the number of blocks to criterion (six blocks with a DR ≥ 0.80, with the last two blocks occurring consecutively) for the first set of reversal task and the number of blocks to criterion for the first set of the discrimination task. Lower numbers for reversal speed represent birds that learned the reversal faster. Open markers are females and filled markers are males.
4. Discussion
Black-capped chickadees that visited more trees during a 10 min exploration trial took longer to reverse their acoustic discrimination task compared with conspecifics that did not readily enter and explore a novel environment. That is, slower exploring individuals required fewer trials, relative to their baseline, to learn to respond to C notes (C+, from their namesake chick-a-dee call) and withhold responding to B notes (B−) following initial discrimination training on a B+ C− discrimination, compared with faster exploring individuals with the same training. This finding cannot be explained by neophobic tendencies related to exploration because each individual was pre-trained with all discriminative stimuli until they were equally familiar with, and responding to, all stimuli [6].
Our findings support predictions derived from the habitat-dependent hypothesis for maintenance of different personality types within a species [1]. This hypothesis posits that different personality types may fare better in different local habitats. Following this, it has been further hypothesized that fast explorers may fare better in a stable, predictable environment, where they can form stable behavioural routines, while slower exploring birds may fare better in an unpredictable, changing environment because they are more sensitive (and thus adaptable) to changes in the environment. Verbeek et al. [18] found that male great tits that were more likely to explore a novel environment, or approach a novel object, were also more likely to return to a location where food had previously been available. By contrast, birds that were slower to explore a novel environment and approach a novel object altered their foraging habits more rapidly when food locations varied. In another study with great tits, slower exploring birds out-performed faster exploring birds in an avoidance learning task [36]. Once slow birds began to attack aposematic prey, they learned to avoid this prey more rapidly than the fast-exploring birds. The result suggests an underlying difference in learning between slow- and fast-exploring great tits. Exnerová et al. [36] put forth two hypotheses to account for their findings. The first hypothesis was that their results were specific to the avoidance learning task (i.e. the use of aversive stimuli) and that tests which use positive stimuli could yield different results. Our results suggest that, in addition to avoidance learning tasks, tasks using positive reinforcement also yield results in support of the idea that slow-exploring birds are faster to learn some cognitive tasks. Our results also support the second hypothesis put forth by Exnerová et al., that slow birds are generally more flexible. These assertions are in line with characteristics of birds with proactive and reactive personalities that were proposed by Cockrem [37]. Specifically, it was proposed that birds with reactive personalities are likely to be slow explorers, more flexible and sensitive to environmental stimuli.
We expected to find that chickadees which were faster to explore the novel environment would learn the discrimination task (B+ C−) faster than slow-exploring birds, in accordance with Guillette et al. [6]. Interestingly, this finding was not replicated in the current study. A possible explanation may relate to subtle differences in methodology between the two studies. Guillette et al. [6] used three different note-type pairs in the operant discrimination (AB, BC and AC), we only used one note-type pair (BC). Previous work with note-type discriminations and black-capped chickadees has shown that the amount of perceptual similarity between note types mediates the number of trials needed to learn the discrimination [38]. That is, it takes chickadees more trials to discriminate between A and B notes, compared with A and C notes. Perhaps using the B/C note pair was not sufficiently perceptually demanding to uncover differences in learning speed when the task demand was held constant.
We believe that differences in the speed of learning an acoustic discrimination may have fitness consequences for individuals. This is especially true for chickadees because they rely on acoustic communication for survival. Learning to discriminate between these acoustic cues and respond appropriately could potentially have fitness advantages such as being able to discriminate between dominant and subordinate males (e.g. [39]) during mate selection or between territory neighbours, thus avoiding unnecessary aggressive responses (dear enemies sensu [40]), thereby increasing fitness [41].
Although our laboratory task can be considered highly artificial relative to the demands of a natural environment, a recent study by Herborn et al. [42] suggests there may be a strong link between an animal's behaviour on a personality test in the laboratory and behaviour in the wild. Herborn et al. demonstrated that, for blue tits (Cyanistes caeruleus), performance in novel environment exploration tasks in the laboratory and an analogous test in the wild yielded similar results. This result lends support to the validity of examining animal personality in the laboratory. The next step will be to carry out analogous experiments in the wild, to test for the fitness advantages of birds of varying degrees of exploratory tendency, in different micro-habitats.
Acknowledgements
All studies were conducted in accordance with the Canadian Council on Animal Care Guidelines and policies approved by the University of Alberta Biological Sciences Animal Care and Use Committee for Biosciences for the University of Alberta. Chickadees were captured under an Environment Canada Canadian Wildlife Service Scientific permit, Alberta Fish and Wildlife Capture and Research permits and City of Edmonton Parks Permit.
We thank the reviewers and associate editor for their thoughtful comments and suggestions that greatly improved the quality of the manuscript, Marcia Spetch for providing laboratory space, Dawson Clary, Craig Anderson, Shannon Wowk and Jon Gaspar for aid in running instrumental training, and Isaac Lank and Lou Omerzu for outstanding and timely fabrication and technical assistance. This research was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant, an Alberta Ingenuity Fund (AIF) New Faculty Grant, a Canada Foundation for Innovation (CFI) Infrastructure Operating Fund, a CFI New Opportunities Grant along with start-up funding and CFI partner funding from the University of Alberta, Edmonton, Alberta, Canada, to C.B.S. L.M.G. is supported by an Izaak Walton Killam Memorial Scholarship at the University of Alberta. A.R.R. is supported by an NSERC Canada Graduate Scholarship-Doctoral. M.H. is supported by an NSERC Post Graduate Scholarship-Doctoral and an Alberta Ingenuity Graduate Student Scholarship.
References
- 1.Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 [DOI] [PubMed] [Google Scholar]
- 2.Sih A., Bell A. M. 2008. Insights for behavioural ecology from behavioural syndromes. Adv. Study Behav. 38, 227–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koolhaas J. M., Korte S. M., de Boer S. F., Van Der Vegt B. J., Van Reenen C. G., Hopster H., De Jong I. C., Ruis M. A. W., Blokhuis H. J. 1999. Coping styles in animals: current status in behaviour and stress physiology. Neurosci. Biobehav. Rev. 23, 925–935 10.1016/S0149-7634(99)00026-3 (doi:10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- 4.Gosling S. D. 2001. From mice to men: what can we learn about personality from animal research. Psychol. Bull. 127, 45–86 10.1037/0033-2909.127.1.45 (doi:10.1037/0033-2909.127.1.45) [DOI] [PubMed] [Google Scholar]
- 5.Pruitt J. N., Riechert S. E., Jones T. C. 2008. Behavioural syndromes and their fitness consequences in a socially polymorphic spider, Anelosimus studiosus. Anim. Behav. 76, 871–879 10.1016/j.anbehav.2008.05.009 (doi:10.1016/j.anbehav.2008.05.009) [DOI] [Google Scholar]
- 6.Guillette L. M., Reddon A. R., Hurd P. L., Sturdy C. B. 2009. Exploration of a novel space is associated with individual differences in learning speed in black-capped chickadees, Poecile atricapillus. Behav. Process. 82, 265–270 [DOI] [PubMed] [Google Scholar]
- 7.Kurvers R. H. J. M., Eijkelenkamp B., Van Oers K., Van Lith B., Van Wieren S. E., Ydenberg R. C., Prins H. H. T. 2009. Personality differences explain leadership in barnacle geese. Anim. Behav. 78, 447–453 10.1016/j.anbehav.2009.06.002 (doi:10.1016/j.anbehav.2009.06.002) [DOI] [Google Scholar]
- 8.Stamps J., Grothuis T. G. G. 2010. The development of animal personality: relevance, concepts and perspectives. Biol. Rev. 85, 301–325 10.1111/j.1469-185X.2009.00103.x (doi:10.1111/j.1469-185X.2009.00103.x) [DOI] [PubMed] [Google Scholar]
- 9.Schuett W., Tregenza T., Dall S. 2010. Sexual selection and animal personality. Biol. Rev. 84, 217–246 10.111/j.1469-185x2009.00101.x (doi:10.111/j.1469-185x2009.00101.x) [DOI] [PubMed] [Google Scholar]
- 10.Bell A. M., Hankison S. J., Laskowski K. L. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783 10.1016/j.anbehav.2008.12.022 (doi:10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sih A., Bell A. M., Johnson J. C., Ziemba R. E. 2004. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 10.1086/422893 (doi:10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 12.Bell A. M. 2007. Evolutionary biology: animal personalities. Nature 447, 539–540 10.1038/447539a (doi:10.1038/447539a) [DOI] [PubMed] [Google Scholar]
- 13.Stamps J. A. 2007. Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol. Lett. 10, 355–363 10.1111/j.1461-0248.2007.01034.x (doi:10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- 14.Wolf M., Van Doorn G. S., Leimar O., Weissing F. J. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–585 10.1038/nature05835 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 15.Wolf M., Van Doorn G. S., Weissing F. J. 2008. Evolutionary emergence of responsive and unresponsive personalities. Proc. Natl Acad. Sci. USA 105, 15 825–15 830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf M., Van Doorn G. S., Leimar O., Weissing F. J. In press The evolution of animal personalities. In Animal personalities: behaviour, physiology, and evolution (eds Carere C., Maestripieri D.). Chigago, IL: University of Chicago Press [Google Scholar]
- 17.Wilson D. S., Clark A. B., Cloeman K., Dearstyne T. 1994. Shyness and boldness in humans and other animals. Trends Ecol. Evol. 9, 442–445 [DOI] [PubMed] [Google Scholar]
- 18.Verbeek M. E. M., Drent P. J., Wiepkema P. R. 1994. Consistent individual differences in early exploratory behaviour of male great tits. Anim. Behav. 48, 1113–1121 [Google Scholar]
- 19.Verbeek M. E. M., Boon A., Drent P. J. 1996. Explorations, aggressive behaviour and dominance in pair-wise confrontations of juvenile male great tits. Behaviour 133, 945–963 [Google Scholar]
- 20.Sih A., Bell A. M., Johnson J. C. 2004. Behavioural syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 21.Moreira P. S. A., Pulman K. G. T., Pottinger T. G. 2004. Extinction of a conditioned response in rainbow trout selected for high or low responsiveness to stress. Horm. Behav. 46, 450–457 [DOI] [PubMed] [Google Scholar]
- 22.Boogert N. J., Reader S. M., Laland K. N. 2006. The relation between social rank, neophobia, and individual learning in starlings. Anim. Behav. 72, 1229–1239 10.1016/j.anbehav.2006.02.021 (doi:10.1016/j.anbehav.2006.02.021) [DOI] [Google Scholar]
- 23.Dall S. R. X., Houston A. I., McNamara J. M. 2004. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 10.1111/j.1461-0248.2004.00618.x (doi:10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- 24.Nussey D. H., Wilson A. J., Brommer J. E. 2007. The evolutionary ecology of phenotypic plasticity in wild populations. J. Evol. Biol. 20, 831–844 10.1111/j.1420-9101.2007.01300.x (doi:10.1111/j.1420-9101.2007.01300.x) [DOI] [PubMed] [Google Scholar]
- 25.Groothuis T. G. G., Carere C. 2005. Avian personality: characterizations and epigenesis. Neurosci. Biobehav. Rev. 29, 137–150 10.1016/j.neubiorev.2004.06.010 (doi:10.1016/j.neubiorev.2004.06.010) [DOI] [PubMed] [Google Scholar]
- 26.Dingemanse N. J., Both C., Drent P. J., Tinbergen J. M. 2004. Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. Lond. B 217, 847–852 10.1098/rspb.2004.2680 (doi:10.1098/rspb.2004.2680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duckworth R. A., Badyaev A. V. 2007. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA 104, 15 017–15 022 10.1073/pnas.0706174104 (doi:10.1073/pnas.0706174104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyle P. 1997. Identification guide to north American birds. Bolinas, CA: Slate Creek Press [Google Scholar]
- 29.Sturdy C. B., Weisman R. W. 2006. Rationale and methodology for testing auditory cognition in songbirds. Behav. Process. 72, 265–272 10.1016/j.beproc.2006.03.007 (doi:10.1016/j.beproc.2006.03.007) [DOI] [PubMed] [Google Scholar]
- 30.Hailman J. P., Ficken M. S., Ficken R. W. 1985. The ‘chick-a-dee’ calls of Parus atricapillus: a recombinant system of animal communication compared with written English. Semiotica 56, 191–224 10.1515/semi.1985.56.3-4.191 (doi:10.1515/semi.1985.56.3-4.191) [DOI] [Google Scholar]
- 31.Charrier I., Lee T. T. Y., Bloomfield L. L., Sturdy C. B. 2005. Acoustic mechanisms of note type perception in black-capped chickadee calls. J. Comp. Psychol. 119, 371–380 10.1037/0735-7036.119.4.371 (doi:10.1037/0735-7036.119.4.371) [DOI] [PubMed] [Google Scholar]
- 32.Sturdy C. B., Phillmore L. S., Weisman R. G. 2000. Call-note discriminations in black-capped chickadees (Poecile atricapillus). J. Comp. Psychol. 114, 357–364 10.1037/0735-7036.114.4.357 (doi:10.1037/0735-7036.114.4.357) [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa S., Gillespie D. O. S., Hatchwell B. J., Burke T. 2007. Predictable males and unpredictable females: sex difference in repeatability of parental care in a wild bird population. J. Evol. Biol. 20, 1674–1681 10.1111/j.1420-9101.2007.01403.x (doi:10.1111/j.1420-9101.2007.01403.x) [DOI] [PubMed] [Google Scholar]
- 34.Guillette L. M., Bailey A. A., Reddon A. R., Hurd P. L., Sturdy C. B. 2010. Capture order is repeatable in chickadees. Int. J. Comp. Psychol. 23, 216–224 [Google Scholar]
- 35.Brown C., Braithwaite V. A. 2004. Size matters: a test of boldness in eight populations of the poeciliid Brachyraphis episcopi. Anim. Behav. 68, 1325–1329 10.1098/rsbl.2004.0222 (doi:10.1098/rsbl.2004.0222) [DOI] [Google Scholar]
- 36.Exnerová A., Svádová K. H., Fucíková E., Drent P., Stys P. 2010. Personality matters: individual variation in reactions of naive bird predators to aposematic prey. Proc. R. Soc. B 277, 723–728 10.1098/rspb.2009.1673 (doi:10.1098/rspb.2009.1673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cockrem J. F. 2007. Stress, corticosterone responses and avian personalities. J. Orthinol. 148, 169–178 10.1007/s10336-007-0175-8 (doi:10.1007/s10336-007-0175-8) [DOI] [Google Scholar]
- 38.Guillette L. M., Farrell T. M., Hoeschele M., Nickerson C. M., Dawson M. R. W., Sturdy C. B. 2010. Mechanism of call note-type perception in black-capped chickadees (Poecile atricapillus): peak shift in a note-type continuum. J. Comp. Psychol. 124, 109–115 [DOI] [PubMed] [Google Scholar]
- 39.Hoeschele M., Moscicki M. K., Otter K. A., Van Oort H., Fort K. T., Farrell T. M., Lee H., Robson S. J., Sturdy C. B. 2010. Dominance signalled in an acoustic ornament. Anim. Behav. 79, 657–664 10.1016/j.anbehav.2009.12.015 (doi:10.1016/j.anbehav.2009.12.015) [DOI] [Google Scholar]
- 40.Wilson E. O. 1975. Sociobiology: the new synthesis. Cambridge, MA: Belknap Press of Harvard University Press [Google Scholar]
- 41.Stoddard P. K. 1996. Vocal recognition of neighbors by territorial passerines. In Ecology and evolution of acoustic communication in birds (eds Kroodsma D. E., Miller E. H.), pp. 356–374 New York, NY: Cornell University Press [Google Scholar]
- 42.Herborn K. A., Macleod R., Miles W. T. S., Schofield L. A., Arnold K. E. 2010. Personality in captivity reflects personality in the wild. Anim. Behav. 79, 835–894 10.1016/j.anbehav.2009.12.026 (doi:10.1016/j.anbehav.2009.12.026) [DOI] [Google Scholar]