Abstract
In some of the most complex animal societies, individuals exhibit a cooperative division of labour to form castes. The most pronounced types of caste formation involve reproductive and non-reproductive forms that are morphologically distinct. In colonies comprising separate or mobile individuals, this type of caste formation has been recognized only among the arthropods, sea anemones and mole-rats. Here, we document physical and behavioural caste formation in a flatworm. Trematode flatworm parasites undergo repeated clonal reproduction of ‘parthenitae’ within their molluscan hosts forming colonies. We present experimental and observational data demonstrating specialization among trematode parthenitae to form distinct soldier and reproductive castes. Soldiers do not reproduce, have relatively large mouthparts, and are much smaller and thinner than reproductives. Soldiers are also more active, and are disproportionally common in areas of the host where invasions occur. Further, only soldiers readily and consistently attack heterospecifics and conspecifics from other colonies. The division of labour described here for trematodes is strongly analogous to that characterizing other social systems with a soldier caste. The parallel caste formation in these systems, despite varying reproductive mode and taxonomic affiliation, indicates the general importance of ecological factors in influencing the evolution of social behaviour. Further, the ‘recognition of self’ and the defence of the infected host body from invading parasites are comparable to aspects of immune defence. A division of labour is probably widespread among trematodes and trematode species encompass considerable taxonomic, life history and environmental diversity. Trematodes should therefore provide new, fruitful systems to investigate the ecology and evolution of sociality.
Keywords: sociality, eusociality, colony, defence, Cerithidea californica
1. Introduction
When considering flatworms, one does not generally imagine colonies, much less a division of labour to form standing armies and pools of reproductive morphs. The phenomena of behavioural specialization, polymorphism and caste formation have long engaged students of other animal groups, primarily the social insects [1–7]. The most extreme types of sociality involve the formation of a non-reproducing caste that differs not only behaviourally but also morphologically from the reproductive caste [2]. In groups comprised of separate or mobile individuals, such caste formation has been recognized only for insects (e.g. [1,8–10]), snapping shrimp [11], a sea anemone [12,13] and mole-rats [14,15]. Here, we present experimental and observational evidence for behavioural and morphological caste formation within colonies of a trematode flatworm.
Both ecological factors and genetic relatedness can influence caste evolution [5,7,16]. A degree of relatedness is important for indirect fitness advantages to favour individuals sacrificing personal reproduction to help relatives [17]. However, high relatedness by itself is clearly insufficient for selection of castes [5,7]. In addition to relatedness, particular ecological conditions (such as resource distribution, dispersal mortality and competition) appear necessary to drive the evolution of non-reproducing castes. Groups composed of clonal individuals should be particularly useful to examine the determinants of caste formation [13,18]. The maximal and constant relatedness among clone-mates removes the genetic conflict potentially characterizing other social systems. This can clarify how ecological factors influence the costs and benefits involved in selection for non-reproducing castes.
Trematode flatworms exhibit repeated clonal reproduction of parthenitae in molluscan hosts, following infection by a single, diploid larva that was sexually produced (typically by a hermaphroditic parent). Parthenitae clonally produce more of themselves and dispersive offspring (cercariae), each developing from a single, unfertilized cell [19]. The trematode parthenitae block host reproduction and can reach an aggregate mass of 16–41% of the infected host soft tissue mass (e.g. [20]). The parthenitae cooperatively live together to reproduce and operate the stolen host body—often for years [21]—thus forming a colony. Parthenitae of some trematode species are well known to not only reproduce, but also to kill heterospecific parthenitae when two species contemporaneously infect the same snail [22–26]. This interspecific antagonism generally follows a dominance hierarchy. Most commonly, species whose parthenitae have mouthparts (and are called ‘rediae’) dominate and actively prey upon parthenitae of subordinate species, which often have smaller mouths or no mouths at all. Previous research has not recognized a division of labour among parthenitae where some specialize on antagonism and others on reproduction.
During our work on trematodes in marine snails, we observed the occurrence of two very distinct redia morphologies within colonies of several trematode species in numerous locations throughout the world. There were ‘primary morphs’, which appeared to be the reproducing rediae typically considered and described in the literature. Additionally, there were ‘secondary morphs’, which possessed many of the traits typically associated with immature reproductives [19]. However, several readily apparent attributes of secondary morphs suggested that they were more than solely immature reproductives. In addition to not actively reproducing and being small and thin, secondary morphs comprised a discrete size class, had relatively huge muscular pharynxes and guts, were very active, and were disproportionately common in regions of the snail where new trematode infections occur. Further, we sometimes noted the secondary morphs aggregating around and attacking invading heterospecific trematodes. Based on these observations, we conducted a series of studies to rigorously examine three general questions. First, are secondary morphs simply immature reproductives, or do secondary and primary morphs represent distinct morphological and behavioural castes? Second, if there are two castes, is one specialized for defence and the other for reproduction? Third, if secondary morphs do form a caste, does the evidence indicate that they comprise a permanent caste or a temporal caste? Temporal castes occur when individuals specialize on different tasks at different ages, as commonly occurs in many social insects [2]. We chose to focus on a locally common, yet undescribed, echinostomatid trematode species, Himasthla sp. B (HIMB) that infects the California horn snail, Cerithidea californica (see §2 and electronic supplementary material for more details about the species).
2. Material and methods
(a). Study system
Himasthla sp. B (HIMB) is a member of a guild of over 18 trematode species that infect the California horn snail, C. californica (Haldeman 1840) [27], which lives in Pacific coast estuaries of California and Baja California [28]. Researchers have included HIMB in studies of this trematode guild for over 65 years (see electronic supplementary material).
The physical caste polymorphism characterizing HIMB rediae occurs within the context of the serial polymorphism characterizing HIMB's complex life cycle (electronic supplementary material, figure S1).
(b). Collections
We haphazardly collected all HIMB colonies (that is, HIMB-infected California horn snails) from the intertidal flats of Carpinteria Salt Marsh, Santa Barbara County, CA, USA. We maintained infected snails in the laboratory for up to three weeks before processing.
(c). Morphology
In summer 2006, we measured 14–35 individuals of each redia morph from each colony. We used random grid numbers to collect up a target of 10 of each morph from each of the three snail regions (mantle, basal visceral mass and gonad/digestive gland). We assigned each encountered redia to one of the morph categories. We fixed rediae in 10 per cent hot formalin and transferred them to 70 per cent EtOH. We took length and width measurements on glycerin-mounted specimens using a compound scope ocular micrometer at 40–100X magnification. We calculated volume of bodies and pharynxes by approximating redia shape to a cylinder. We have deposited the voucher specimens from six of the above colonies at the United States National Parasite Collection (USNPC nos 102948–102953).
(d). Activity
Trials took place in spring and summer 2008. For every trial (each with a different colony), we placed 20 secondary morphs and 20 primary morphs into separate 1.5 ml glass wells (10 redia per well) with filtered sea water. We then quantified individual movement for each visible redia at three times (10, 20 and 30 min), by taking two photomicrographs of all rediae in a well, 2 s apart. We superimposed the images over each other to quantify body area movement using the Image Layering Toolbox plug-in for NIH ImageJ software. For each trial and redia morph, we calculated individual movement, first averaging within each time, then among the three times. Ambient room temperature ranged from 22°C to 22.5°C.
In addition to quantifying movement rates of each redia morph, we designed this experiment to detect whether rediae changed activity in response to the presence of a heterospecific (EUHA) compared with the presence of same colony secondary morphs. Thus, for each trial, 10 heterospecific rediae were placed in one of the wells with 10 secondary morphs and one of the wells with 10 primary morphs. As a control, 10 secondary morphs from same colony were placed in the other two wells with the other 10 secondary morphs and primary morphs. Treatments were dilute (individuals usually did not contact one another) and we detected no change in activity, given treatment type (Poisson regression (PReg): p = 0.63 main effect and p = 0.33 for interaction between treatment and redia type). Therefore, to best characterize the activity of each redia type, we then simply averaged among treatments.
(e). Attack rates
We placed HIMB secondary morphs of the focal colony into a different sterile well with filtered sea water. The trials took place in autumn 2009 at ambient room temperature, which ranged from 20.5°C to 24.4°C. In varying order among trials, into each well, we then added parthenitae from the same or different conspecific and heterospecific colonies, after rinsing parthenitae four times with filtered sea water. The 11 trials had four consistent treatments: heterospecific (EUHA), non-kin conspecific 1, non-kin conspecific 2 and same colony. We extended some trials by adding 12 additional heterospecific treatments, involving seven species (electronic supplementary material, figure S4). The first two trials used 20 individuals of each redia type placed in 1.5 ml glass wells. The other nine trials used 15 of each redial type in 0.32 ml plastic wells. There were no differences in the number of attacks observed in the first two trials compared with the subsequent nine (PReg: p = 0.187; χ2 = 1.9, d.f. = 1, n = 44). However, the analysis examined the effect of treatment on observed per capita attack rates while controlling for any effect of trial.
To quantify attack frequency, we observed each well for 30 s using a stereomicroscope. An attack was scored when a redia used its mouth to latch onto another redia. Attack rates for conspecific treatments are conservatively halved because non-kin probably attacked as well and we could not reliably differentiate the focal HIMB from the non-kin conspecifics.
Except as indicated in the main text and figure legend, the experiment comparing attack rates of secondary morphs to primary morphs followed the same procedures described for the attack trials above, and used 1.5 ml glass wells.
(f). Redia censuses and distribution within host
We performed total redia counts for HIMB colonies from 51 infected snails collected over the course of a year (February 2006–January 2007). We carefully cracked snail shells and removed the infected snail body. To perform counts by snail body region, we used a razor blade to separate the three body regions: the mantle, middle (basal visceral mass) and gonad/digestive gland. We counted rediae using a stereomicroscope by squashing tissue between two glass plates. Approximately one out of 1000 (129/91229) rediae were loose in the dissection dish. We included them in total counts but did not assign them to a particular body region.
(g). Statistical analyses
Analyses controlled for the effect of the specific colony or trial, as appropriate. To model the power relationship between the width and the length, we used a mixed-effects general linear model [29] on log10-transformed width and length data. We used Poisson regression (PReg) [30] to adequately capture the error distribution and heterogeneity of variance for some morphometric and caste numbers analyses. PReg used a ln-link and an overdispersion parameter as necessary. We multiplied the fractional pharynx to body volume data by 104 to acquire a lower bound of 1. We simplified models by removing non-significant interactions (p > 0.05). For activity and attack analyses, we used paired t-tests to test hypotheses concerning caste differences or secondary morph responses to self and non-self. We ensured assumptions were met concerning data distributions by examining standardized deviance or residual by predicted plots, and normal quantile plots with Lilliefors curves [30]. We performed all statistical tests using JMP v. 8 software.
3. Results and discussion
(a). Secondary and primary morphs differ in body size, body shape and relative mouthpart size
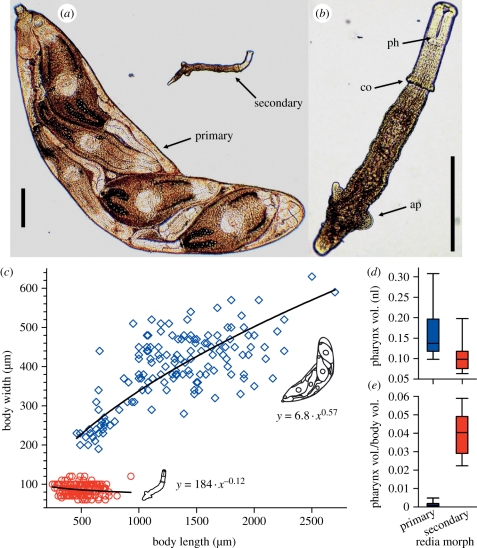
We randomly selected and measured 173 secondary morphs and 143 primary morphs from seven HIMB colonies. Secondary morphs were much smaller than primary morphs, never overlapping in volume or width (figure 1a–c and electronic supplementary material, figure S2). On average, secondary morphs were 0.52–3.8% the volume of primary morphs from the same colony (Poisson regression (PReg): p < 0.0001, χ2 = 1294, d.f. = 1, n = 316), and were 26–54% the length (PReg: p < 0.0001, χ2 = 951, d.f. = 1) and 18–29% the width (PReg: p < 0.0001, χ2 = 3512, d.f. = 1).
Figure 1.
Secondary morph and primary morph redia dimorphism of the trematode, Himasthla sp. B (HIMB). Secondary morphs are putative soldiers and primary morphs are putative reproductives. (a) A secondary morph next to a primary morph that contains five developed dispersive offspring (cercariae). (b) Close-up of a secondary morph indicating the large pharynx (ph), collar (co) and posterior appendages (ap). (c) Body width versus length for 173 secondary morphs (red circles) and 143 primary morphs (blue diamonds) from seven colonies. Secondary morphs and primary morphs have very different width to length relationships (general linear model using log10-transformed variables: full model R2 = 0.97, p < 0.0001; interaction p < 0.0001, F1,17 = 118; 95% CI for exponents: secondary morphs, −0.21, −0.031; primary morphs, 0.48, 0.65). (d) Secondary morphs overlap with primary morphs in absolute pharynx size, and the 68% average size decrease is not significant (Poisson regression: p = 0.22, χ2 = 1.5, d.f. = 1, n = 316). (e) Secondary morph pharynx size relative to body size is 22 times larger than for primary morphs (PReg on × 104 data: p = 0.011, χ2 = 6.4, d.f. = 1). These data represent morphs from all colonies, but analyses controlled for any effects of individual colony. Electronic supplementary material, figures S2 and S3 present individual data for each colony. Scale bars (a,b) 0.2 mm.
Secondary morphs also had different body shapes than primary morphs. Secondary morphs possessed collars and pronounced posterior appendages (figure 1a,b). These structures were missing in primary morphs (figure 1a). Additionally, secondary morphs exhibited a very different width to length relationship than did primary morphs (figure 1c). Secondary morphs slightly decreased in width with increasing length, whereas primary morphs strongly increased. Further, secondary morphs were relatively much thinner than primary morphs (figure 1a,c) with mean length to width ratios within individual colonies ranging from about 3 : 1 to 12 : 1 versus 11/2 : 1 to 6 : 1 for primary morphs (PReg: p < 0.0001, χ2 = 93.3, d.f. = 1, n = 316). The occupancy of discrete areas of morphospace is consistent with the hypothesis that secondary and primary morphs comprise two functionally specialized castes, whether temporal or permanent.
Evidence from study of interspecific antagonism indicates that the size of muscular pharynxes is important for the ability of rediae to kill heterospecifics [22]. Despite secondary morphs being only 1.6 per cent the body size of primary morphs, secondary morph pharynxes strongly overlapped in size with the pharynxes of primary morphs, being statistically indistinguishable from those of primary morphs in the same colony (figure 1d and electronic supplementary material, figure S3). Secondary morph pharynxes were 4 per cent their body volume versus only 0.2 per cent for primary morphs (figure 1e and electronic supplementary material, figure S3). Their relatively large muscular pharynxes may enhance the ability of secondary morphs to perform an active defensive role.
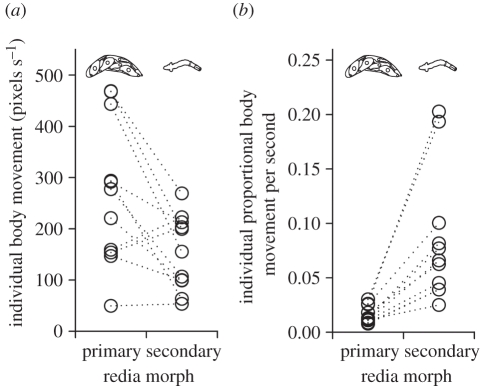
(b). Secondary morphs are more active than primary morphs
We quantified the body movement of 195 secondary morphs and 198 primary morphs from 11 HIMB colonies, using a series of photomicrographs to make 1139 observations of individual body movement, in vitro. Secondary morphs regularly bent, extended and contracted their bodies, whereas primary morphs barely moved. Because primary morphs are about 64 times the size of secondary morphs, their slight movement translated into absolute movement that was on average 1.3 times greater than that of secondary morphs (figure 2a). However, secondary morphs proportionally moved five times more than did primary morphs (figure 2b). The fat, slow, ‘slug-like’ morphology and activity of primary morphs compared with secondary morphs are consistent with the hypothesis of an adaptive division of labour, with primary morphs specializing on reproduction and secondary morphs specializing on an active role in defence.
Figure 2.
Himasthla sp. B (HIMB) secondary morph and primary morph redia in vitro activity rates. (a) Mean absolute body movement rates, and (b) mean proportional body movement for secondary morphs and primary morphs from 11 different trials, each with individuals from a different colony. Each datum represents mean individual movement of up to 20 randomly selected individuals of each caste from each colony observed at three times. Statistics are from paired t-tests using trials as replicates. Dotted lines connect data from same trial (colony). (a) t10 = 3.1, p = 0.011. (b) t10 = 4.3, p = 0.016.
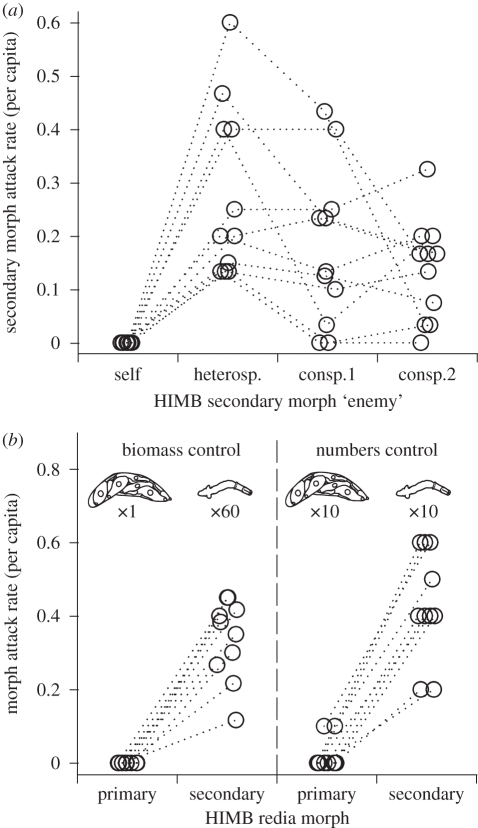
(c). Secondary morphs readily attack heterospecifics and conspecifics from other colonies
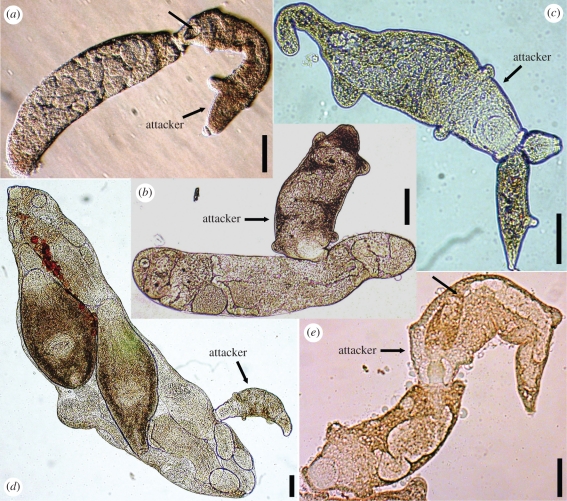
In 11 out of 11 in vitro trials, secondary morphs attacked parthenitae of the common heterospecific, Euhaplorchis californiensis (EUHA) and never attacked HIMB secondary morphs from the same colony (figures 3a and 4a–b). HIMB secondary morphs also attacked, 100 per cent of the time, parthenitae of six different species in 12 additional heterospecific treatments (figure 4c–d and electronic supplementary material, figure S4). Attacked heterospecifics included species subordinate to HIMB in the interspecific dominance hierarchy characterizing the assemblage of trematodes that infect the California horn snail (§2). Attacked heterospecifics also included secondary morphs (putative soldiers) and primary morphs of species dominant to HIMB (figure 4c–d and electronic supplementary material, figure S4). Attacking secondary morphs used their mouths to attach to heterospecifics and usually did not let go. Secondary morphs either ingested the entire heterospecific or lacerated the body wall, removing the contents of the heterospecific parthenitae (figure 4a–d).
Figure 3.
Himasthla sp. B (HIMB) secondary morph rediae readily attack heterospecifics and non-kin conspecifics whereas primary morphs do not. (a) The mean number of attacks by each secondary morph observed 2 h after placing 15 or 20 secondary morphs from each of 11 colonies with the same amount of (i) secondary morph rediae from the same colony, (ii) heterospecific (E. californiensis-EUHA) rediae (t10 = 5.7, p = 0.0002), or (iii) conspecific secondary morphs from two other colonies (t10 = 3.9, p = 0.003; t10 = 4.8, p = 0.0008). Statistics reflect paired t-tests comparing attack rates to those on ‘self’. The two trials where secondary morphs did not attack both conspecifics were also trials with the lowest attack rates in the heterospecific treatment. Electronic supplementary material, figure S4 presents the actual number of attacks, additional detail, and the results for the additional heterospecific treatments. (b) The mean number of attacks by each morph observed 2 h after placing secondary morphsand primary morphs with 60 heterospecific (EUHA) rediae. The left pair pits an approximately equivalent biomass of primary morphs or secondary morphs against the heterospecifics (t10 = 9.7, p = 0.0001), while the right pair pits an equivalent number (t10 = 9.5, p = 0.0001). In (a) and (b), paired t-tests used trials as replicates, and dotted lines connect data from same trial (colony).
Figure 4.
Himasthla sp. B (HIMB) secondary morph rediae attacking heterospecifics and conspecific secondary morphs from other colonies. (a) A secondary morph swallowing a heterospecific (E. californiensis-EUHA), the posterior end of which is visible in the secondary morph's pharynx cavity (unlabelled black arrow). (b) A secondary morph has lacerated the side of a heterospecific (EUHA), and is ingesting the heterospecific's offspring (the black eyes of which are visible in the secondary morph's intestine). (c) A HIMB secondary morph attacking a heterospecific (Parorchis acanthus, PARO) secondary morph. (d) A secondary morph attacking a primary morph of the heterospecific, PARO. (e) A HIMB secondary morph ingesting a HIMB secondary morph from a different colony. The posterior end of the attacked secondary morph (unlabelled black arrow) fills the anterior 40% of the attacker's intestine. All photos taken through a normal bright field compound microscope, sometimes using phase rings for contrast. scale bars, 0.2 mm.
Do secondary morphs also attack conspecifics? In each of the above 11 trials, we also included two treatments with secondary morphs from different conspecific colonies (different infected snails). In every trial (and 20 of the 22 replicates), HIMB secondary morphs also readily attacked conspecific secondary morphs from other colonies (figures 3a and 4e). This result is contrary to the lack of non-kin recognition documented for some similarly social animals with soldier morphs, e.g. some gall thrips [9] and aphids [18,31], but is consistent with the recognition-of-self documented for clonal wasp larvae [32] and sea anemones [12]. Although there has been much study of direct antagonism between heterospecific trematode parthenitae [22–26], there has been very little documentation of direct antagonism between conspecifics (but see [33]).
Secondary morphs readily attack heterospecifics and conspecifics, but do they attack more frequently than do primary morphs? We performed another series of 10 trials, with two different secondary morph and two primary morph treatments, each presented with heterospecific (EUHA) rediae. Secondary morphs again consistently attacked the heterospecific in 20/20 replicates (figure 3b). By contrast, primary morphs attacked in only 2/20 replicates and with much lower per capita attack rates (figure 3b), despite their mouths often being in contact with heterospecific rediae. Thus, although primary morphs are capable of attack (documented here, and in previous work [26]), they appear to attack at much lower rates than do secondary morphs. The differential attack rates of secondary morphs compared with primary morphs are consistent with the hypothesis that secondary morphs are specialized for recognizing and killing both heterospecific and conspecific invaders.
(d). Secondary morphs do not reproduce
None of the 173 randomly sampled secondary morphs that we inspected under high magnification had germinal balls (early-stage embryos lose in the body cavity). By contrast, 96 per cent (137/143) of the randomly sampled primary morphs had embryos (Pearson χ2 = 293, p < 0.0001, d.f. = 1, n = 316). Further, most of these (133/137) had later-stage embryos or fully formed dispersive offspring (cercariae). Additionally, the small size of secondary morphs clearly precludes them from producing the dispersive offspring that primary morphs produce, those offspring being much larger than secondary morphs (figure 1a). It is important to note that, despite not actively reproducing, secondary morphs might possess the ability to transition to reproductive morphs. For instance, in immature rediae of confamilial trematodes, the germinal material initially only resides in the tissue at the posterior body cavity [19,34]. Do secondary morphs typically transition to become primary morphs?
(e). Secondary morphs do not appear to generally transition to become primary morphs
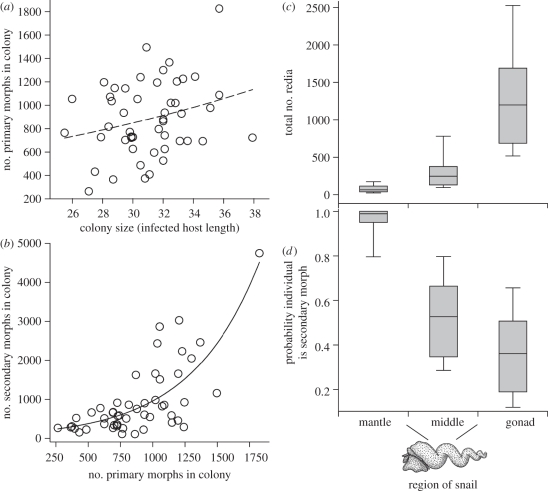
We performed complete redia censuses for 51 mature HIMB colonies (infected snails). Total redia numbers in colonies ranged from 525 to 6564 (1515 median, 1028–2157 i.q.r.). Secondary morphs formed a substantial proportion of the individuals in each of the 51 colonies, averaging 44 per cent (±16% s.d., range 11–73%). We observed no dead or dying primary morphs among the 91 229 rediae encountered in the population censuses. Nor did we encounter any morphs eating other morphs in the same colony. This indicates that there is virtually no turnover of primary morphs that would require a supply of replacements if colony numbers were maintained. Some additional primary morphs must be generated as the host and the colony grows. Indeed, we detected a weak positive association between the number of primary morphs and the host size (figure 5a). However, the average number of primary morphs increased by only approximately 400 from small to large infected hosts. Not only are there far more secondary morphs than this increase in primary morph numbers, but also the number of secondary morphs actually strongly increased with the number of primary morphs (figure 5b). Thus, expansion of these colonies appears to entail continual addition of both secondary and primary morphs.
Figure 5.
Himasthla sp. B (HIMB) secondary morph and primary morph redia caste numbers given colony size, and distribution throughout infected snail bodies. (a) The number of primary morphs weakly increased with infected host body size (Poisson regression (PReg): R2L = 0.064, p = 0.059, χ2 = 3.6, d.f. = 1, n = 49). (b) The number of secondary morphs disproportionately increased with the number of primary morphs (PReg: R2L = 0.54, p < 0.0001, χ2 = 57.9, d.f. = 1, n = 51). (c) The distribution of all individuals in the three regions of the host for 51 HIMB colonies (PReg: overall heterogeneity, p < 0.0001, χ2 = 662, d.f. = 2). (d) Secondary morphs were more dispersed throughout the host body than were primary morphs and comprised almost all of the individuals furthest from the colony locus in the gonad region (GLM linear contrast p < 0.0001, F1,90 = 312). In (c,d), boxes indicate 25th and 75th percentiles, whiskers indicate 10th and 90th percentiles, and the horizontal bars indicate medians.
In our censuses, we detected a small number of intermediate morphs. They were so rare that we did not recover one in our random selection of the 316 rediae for morphometric analyses. We targeted, for close inspection, 42 of these rare intermediates from the seven colonies used for morphometrics. Several lines of evidence indicate that intermediates represent the rare maturing primary morphs involved in the increase in numbers associated with increasing colony size. First, they were intermediate in size, ranging from the size of secondary morphs to that of the smallest primary morphs. Second, they were intermediate in shape, having reduced appendages and collars. Third, intermediates were rare, consistent with them representing a brief, transitional stage. Fourth, although some intermediates (28 of 42) were reproductive, they only contained offspring in early development: 26 had early stage embryos (germinal balls), and two had later stage embryos (procercariae). It remains an open and interesting question whether these maturing primary morphs originated from newly generated clonal offspring, or from rare secondary morphs that have switched roles and are metamorphosing into primary morphs. In any case, the rarity of maturing primary morphs combines with the discrete morphospace occupied by primary and secondary morphs, the lack of primary morph turnover, and the increased number of secondary morphs with increased colony size to indicate that secondary morphs do not represent a temporal caste. That is, secondary morphs do not appear generally to initially specialize on defence and then transition to become reproductives (as documented for some aphid soldiers [18,31]).
(f). Secondary morphs are disproportionally common at invasion fronts
Specialization of castes can involve spatial positioning to optimize conducting caste functional roles (e.g. [35,36]). As for most marine trematodes, the bulk of the HIMB colony resides in the gonadal region of the host [20]. However, new trematode infections initially invade uninfected or infected snails more anteriorly, entering through the mantle or the mid-region of the body [19]. Strikingly, in the 51 censused HIMB colonies, secondary morphs comprised almost 100 per cent of the individuals in the mantle (median 99%, i.q.r. 95–100%), a site for new infections and the region farthest from the primary morph locus in the gonadal region (figure 5c,d). Overall, secondary morphs were more widely dispersed throughout host bodies than were primary morphs. Primary morphs were almost all located in the visceral mass, and, therein, mostly to the gonad region (figure 5c,d). The spatial positioning at invasion fronts is consistent with the hypothesis of a defensive role for secondary morphs, and is similar to that observed for the defensive caste of other social animals, for example, some ants [36], gall aphids [35] and sea anemones [12].
(g). Evidence congruently indicates physical and behavioural specialization to form a non-reproductive soldier caste and a reproductive caste
The discrete morphologies, rarity of intermediate stages, and lack of primary morph turnover signify that secondary and primary morphs form two relatively permanent and physically distinct castes. The greater activity of secondary morphs and their consistent attack rates indicate behavioural differences underlying a specialized functional role, specifically one of defence against invaders. The relatively large mouthparts of secondary morphs may facilitate killing invaders, despite their small body size. The small, thin bodies of secondary morphs may preclude reproduction, but could enhance a defensive role by enabling their increased activity and dispersion throughout host tissues. Their disproportionate presence at invasion fronts is also consistent with a defensive role for secondary morphs, as this probably increases encounter rates with newly established invaders. In short, the data concerning morphology, reproduction, activity, attack rates, censuses and spatial distribution congruently support the hypothesis of a sharply defined division of labour within HIMB colonies. This division of labour consists of primary morphs forming a reproductive caste, and secondary morphs forming a largely permanent, non-reproductive soldier caste that is specialized for encountering, recognizing and killing both heterospecific and conspecific invaders. To our knowledge, such pronounced caste formation has not been recognized for trematodes.
(h). Parallels with other systems indicate the importance of ecology to social evolution
The division of labour recognized and described here for trematodes has strong parallels to other social systems with a soldier caste. First, there are some gall aphids [8,18,31], a sea anemone [12,13] and some parasitoid wasp larvae [10,37]. As for trematode parthenitae, colonies in these systems comprise clonal, separate individuals. However, there are also three sexual systems with a similar soldier caste: some haplodiploid gall thrips [9,38], some diploid wood-nesting termites [1,39] and some diploid sponge-dwelling snapping shrimp [11,40]. All of these systems—whether clonal, haplodiploid sexual or diploid sexual—have traits that fit the ‘fortress-defence’ model of sociality [3,5,6,18,41]. In such societies, the colony resides and feeds in a circumscribed, localized area and monopolizes a defensible, all-important resource (a host, a gall in a plant, a patch on a rock or a piece of wood). The parallel caste formation in these seven systems, despite varying reproductive mode and taxonomic affiliation, emphasizes the probable general importance of ecological factors in influencing the evolution of social behaviour.
(i). Caste formation is probably extensive among trematode species
Several other aspects of the biology and ecology of trematode colonies are consistent with additional factors associated the fortress-defence model for the evolution of sociality [3,5,6,18,41]. First, kin are clustered spatially and temporally to form colonies. This sets the stage for ecological factors to select for cooperative behaviour via indirect fitness advantages [17]. Second, those clustered kin (clonal parthenitae) are genetically identical, further fostering selection for cooperation by removing genetic conflict between individuals. Third, many trematodes have the ability to defend. This effectively translates to the likely existence of genetic variation (among-clones) for defence on which selection could act for specialization of soldiers. Fourth, defence could often be selectively advantageous. Many systems of larval trematodes are interactive, with high levels of invasion by heterospecifics [23,24]. Invasion by conspecifics is also probably important, as indicated by our finding high recognition of and attack rates on conspecifics from other colonies. Fifth, many trematode colonies reside in hosts that can live for years, perhaps decades [21]. This further relates to a potential selective advantage of defence, because, all else being equal, greater longevity of a trematode colony generates a greater cumulative probability of attack. Additionally, greater possible longevity can result in selection for greater defence investment given the greater possible returns from future reproduction, similar to considerations about colony longevity in social gall aphids [18]. Similarly, potential great longevity may also favour minimizing pathogenic effects on the stolen host phenotypes [20,42], which may be a primary feature selecting for small, thin soldiers, rather than simply using large, totipotent rediae for defence. Putting all this together, we predict that trematode soldier castes will more probably evolve in (i) taxa that are typically dominant in interspecific hierarchies, reflecting the ability to defend; (ii) systems where trematodes typically infect a high proportion of hosts, resulting in higher interaction probabilities and a selective advantage to defend; and (iii) among trematodes that infect longer lived hosts, resulting in a selective advantage to defend given higher cumulative interaction probabilities in addition to greater possible returns from future reproduction.
Evidence indicates the widespread existence of trematode castes. First, there are suggestive reports of large and secondary morphs, without the documentation or recognition that the morphs represent castes and not solely immature reproductives (e.g. [43,44]). Second, Lie's [44] observations of ‘immature’ rediae of a trematode species attacking invaders in a freshwater snail indicate that the occurrence of soldier castes may span marine and freshwater systems. Finally, we have noticed probable soldiers in five species other than HIMB (often with more extreme size dimorphism), including species in marine snails from the Persian Gulf, Japan and western North America (electronic supplementary material, table S1).
(j). Future directions
Trematodes should provide remarkable and fruitful systems to study the evolution and ecology of sociality and—as briefly elaborated below—the ecology and evolution of immune defence. There are conservatively an estimated 20 000 trematode species [45,46] infecting over 51 superfamilies of molluscan ‘first intermediate’ hosts [47]. Although not typically recognized as such, each of these trematode species forms colonies or societies (sensu [48]) in their first intermediate hosts. These parasite–host systems encompass a wide range of taxonomic, life history and environmental diversity, providing substantial material for comparative analyses and laboratory investigations. Many interesting issues remain to be explored concerning soldier castes, including caste evolutionary origins, inducibility, plasticity, costs and local adaptation. There are also questions concerning the mechanisms and timing of the documented caste differentiation, including variation in the degree of caste permanency. Additionally, research can examine the details of communication between individuals and the recognition of non-self. Further, defence by trematode soldiers can address immunological principles. The analogy is more direct for trematodes than it is for the ‘social immunity’ [49] considered for social insects. This is because trematodes defend not only a colony, but also a body—namely, the usurped body of the parasitically castrated host within which the trematode colony resides. The soldiers are analogous to giant, specialized macrophages. Finally, it is important to note the likely existence of more subtle forms of division of labour among trematode parthenitae. Providing perhaps the best evidence for this, Sapp et al. [33] documented a single, early-maturing, reproductive morph in an echinostomatid trematode that appeared to serve a specialized defensive role by remaining at the initial infection site. Thus, with Sapp et al. [33], we expect that much unappreciated specialization and division of labour remain to be explored in this widespread and ecologically important group of colonial parasites.
Acknowledgements
We are grateful to Mark Rigby, Kevin Lafferty, Bernie Crespi and the anonymous reviewers for comments; to Mercy Tetteh and Jessica Meyer for laboratory assistance; to California Sea Grant and the NSF/NIH Ecology of Infectious Diseases programme for financial support; and to the University of California Carpinteria Salt Marsh Reserve for access to field sites.
References
- 1.Wilson E. O. 1971. The insect societies. Cambridge, MA: Belknap Press of Harvard University Press [Google Scholar]
- 2.Oster G. F., Wilson E. O. 1978. Caste and ecology in the social insects. Monographs in population biology, 12. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 3.Alexander R. D., Noonan K. M., Crespi B. J. 1991. The evolution of eusociality. In The biology of the naked mole-rat (eds Sherman P. W., Jarvis J. U. M., Alexander R. D.), pp. 3–44 Princeton, NJ: Princeton University Press [Google Scholar]
- 4.Michener C. D. 1969. Comparative social behavior of bees. Annu. Rev. Entomol. 14, 299–342 10.1146/annurev.en.14.010169.001503 (doi:10.1146/annurev.en.14.010169.001503) [DOI] [Google Scholar]
- 5.Korb J., Heinze J. 2008. The ecology of social life: a synthesis. In Ecology of social evolution (eds Heinze J., Korb J.), pp. 245–259 Berlin, Germany: Springer [Google Scholar]
- 6.Queller D. C., Strassmann J. E. 1998. Kin selection and social insects. Bioscience 48, 165–175 [Google Scholar]
- 7.Crespi B. J., Choe J. C. 1997. Explanation and evolution of social systems. In The evolution of social behavior in insects and arachnids (eds Choe J. C., Crespi B. J.), pp. 499–524 Cambridge, NY: Cambridge University Press [Google Scholar]
- 8.Aoki S. 1977. Colophina clematis (Homoptera, Pemphigidae), an aphid species with ‘soldiers’. Kontyu 45, 276–282 [Google Scholar]
- 9.Crespi B. J. 1992. Eusociality in Australian gall thrips. Nature 359, 724–726 10.1038/359724a0 (doi:10.1038/359724a0) [DOI] [Google Scholar]
- 10.Cruz Y. P. 1981. A sterile defender morph in a polyembryonic hymenopterous parasite. Nature 294, 446–447 10.1038/294446a0 (doi:10.1038/294446a0) [DOI] [Google Scholar]
- 11.Duffy J. E. 1996. Eusociality in a coral-reef shrimp. Nature 381, 512–514 10.1038/381512a0 (doi:10.1038/381512a0) [DOI] [Google Scholar]
- 12.Francis L. 1976. Social organization within clones of the sea-anemone Anothopleura elegantissima. Biol. Bull. (Woods Hole) 150, 361–376 10.2307/1540678 (doi:10.2307/1540678) [DOI] [PubMed] [Google Scholar]
- 13.Ayre D. J., Grosberg R. K. 1996. Effects of social organization on inter-clonal dominance relationships in the sea anemone Anthopleura elegantissima. Anim. Behav. 51, 1233–1245 10.1006/anbe.1996.0128 (doi:10.1006/anbe.1996.0128) [DOI] [Google Scholar]
- 14.Jarvis J. U. M. 1981. Eusociality in a mammal—cooperative breeding in naked mole-rat colonies. Science 212, 571–573 10.1126/science.7209555 (doi:10.1126/science.7209555) [DOI] [PubMed] [Google Scholar]
- 15.O'Riain M. J., Jarvis J. U. M., Alexander R., Buffenstein R., Peeters C. 2000. Morphological castes in a vertebrate. Proc. Natl Acad. Sci. USA 97, 13 194–13 197 10.1073/pnas.97.24.13194 (doi:10.1073/pnas.97.24.13194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans H. E. 1977. Commentary: extrinsic versus intrinsic factors in the evolution of insect sociality. Bioscience 27, 613–617 10.2307/1297657 (doi:10.2307/1297657) [DOI] [Google Scholar]
- 17.Hamilton W. D. 1964. Genetical evolution of social behaviour I & II. J. Theor. Biol. 7, 1–52 10.1016/0022-5193(64)90038-4 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 18.Stern D. L., Foster W. A. 1997. The evolution of sociality in aphids: a clone's-eye view. In The evolution of social behavior in insects and arachnids (eds Choe J. C., Crespi B. J.), pp. 151–165 Cambridge, UK: Cambridge University Press [Google Scholar]
- 19.Galaktionov K. V., Dobrovolskij A. A. 2003. The biology and evolution of trematodes: an essay on the biology, morphology, life cycles, transmission, and evolution of digenetic trematodes. Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 20.Hechinger R. F., Lafferty K. D., Mancini F. T., III, Warner R. R., Kuris A. M. 2009. How large is the hand in the puppet? Ecological and evolutionary effects on body mass of 15 trematode parasitic castrators in their snail host. Evol. Ecol. 23, 651–667 10.1007/s10682-008-9262-4 (doi:10.1007/s10682-008-9262-4) [DOI] [Google Scholar]
- 21.Kuris A. M., Lafferty K. D. 2005. Population and community ecology of larval trematodes in molluscan first intermediate hosts. In Marine parasitology (ed. Rohde K.), pp. 321–325, 517–519 Victoria: CSIRO Publications [Google Scholar]
- 22.Lie K. J. 1973. Larval trematode antagonism: principles and possible application as a control method. Exp. Parasitol. 33, 343–349 10.1016/0014-4894(73)90038-6 (doi:10.1016/0014-4894(73)90038-6) [DOI] [PubMed] [Google Scholar]
- 23.Lim H. K., Heyneman D. 1972. Intramolluscan inter-trematode antagonism: a review of factors influencing the host-parasite system and its possible role in biological control. Adv. Parasitol. 10, 191–268 10.1016/S0065-308X(08)60175-X (doi:10.1016/S0065-308X(08)60175-X) [DOI] [PubMed] [Google Scholar]
- 24.Kuris A. M., Lafferty K. D. 1994. Community structure: larval trematodes in snail hosts. Annu. Rev. Ecol. Syst. 25, 189–217 10.1146/annurev.es.25.110194.001201 (doi:10.1146/annurev.es.25.110194.001201) [DOI] [Google Scholar]
- 25.Combes C. 1982. Trematodes: antagonism between species and sterilizing effects on snails in biological control. Parasitology 84, 151–175 10.1017/S0031182000053634 (doi:10.1017/S0031182000053634) [DOI] [Google Scholar]
- 26.Sousa W. P. 1993. Interspecific antagonism and species coexistence in a diverse guild of larval trematode parasites. Ecol. Monogr. 63, 103–128 10.2307/2937176 (doi:10.2307/2937176) [DOI] [Google Scholar]
- 27.Haldeman S. S. 1840. A monograph of the Limniades and other freshwater univalve shells of North America. Philadelphia, PA: J. Dobson [Google Scholar]
- 28.Race M. S. 1981. Field ecology and natural history of Cerithidea californica (Gastropoda: Prosobranchia) in San Francisco Bay. Veliger 24, 18–27 [Google Scholar]
- 29.Quinn G. P., Keough M. J. 2002. Experimental design and data analysis for biologists. Cambridge, UK: Cambridge University Press [Google Scholar]
- 30.Myers R. H., Montgomery D. C., Vining G. G. 2002. Generalized linear models: with applications in engineering and the sciences. Wiley series in probability and statistics. New York, NY: Wiley [Google Scholar]
- 31.Pike N., Foster W. A. 2008. The ecology of altruism in a clonal insect. In Ecology of social evolution (eds Heinze J., Korb J.), pp. 37–56 Berlin, Germany: Springer [Google Scholar]
- 32.Giron D., Strand M. R. 2004. Host resistance and the evolution of kin recognition in polyembryonic wasps. Proc. R. Soc. Lond. B 271, S395–S398 10.1098/rsbl.2004.0205 (doi:10.1098/rsbl.2004.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sapp K. K., Meyer K. A., Loker E. S. 1998. Intramolluscan development of the digenean Echinostoma paraensei: rapid production of a unique mother redia that adversely affects development of conspecific parasites. Invert. Biol. 117, 20–28 10.2307/3226848 (doi:10.2307/3226848) [DOI] [Google Scholar]
- 34.Cort W. W., Ameel D. J., Van Der Woude A. 1954. Germinal development in the sporocysts and rediae of the digenetic trematodes. Exp. Parasitol. 3, 185–216 10.1016/0014-4894(54)90008-9 (doi:10.1016/0014-4894(54)90008-9) [DOI] [PubMed] [Google Scholar]
- 35.Pike N. 2007. Specialised placement of morphs within the gall of the social aphid Pemphigus spyrothecae. BMC Evol. Biol. 7, 18(doi:10.1186/1471-2148-7-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sendova-Franks A. B., Franks N. R. 1995. Spatial relationships within nests of the ant Leptothorax unifasciatus (Latr.) and their implications for the division of labour. Anim. Behav. 50, 121–136 10.1006/anbe.1995.0226 (doi:10.1006/anbe.1995.0226) [DOI] [Google Scholar]
- 37.Strand M. R., Grbic M. 1997. The life history and development of polyembryonic parasitoids. In Parasites and pathogens: effects on host hormones and behavior (ed. Beckage N. E.), pp. 37–56 New York, NY: Chapman & Hall [Google Scholar]
- 38.Chapman T. W., Crespi B. J., Perry S. P. 2008. The evolutionary ecology of eusociality in Australian gall thrips: a ‘model clades’ approach. In Ecology of social evolution (eds Korb J., Heinze J.), pp. 57–83 Berlin, Germany: Springer [Google Scholar]
- 39.Korb J. 2008. The ecology of social evolution in termites. In Ecology of social evolution (eds Heinze J., Korb J.), pp. 151–174 Berlin, Germany: Springer [Google Scholar]
- 40.Duffy E., Morrison C., Macdonald K. 2002. Colony defense and behavioral differentiation in the eusocial shrimp Synalpheus regalis. Behav. Ecol. Sociobiol. 51, 488–495 10.1007/s00265-002-0455-5 (doi:10.1007/s00265-002-0455-5) [DOI] [Google Scholar]
- 41.Crespi B. J. 1994. Three conditions for the evolution of eusociality: are they sufficient? Insectes Sociaux 41, 395–400 10.1007/BF01240642 (doi:10.1007/BF01240642) [DOI] [Google Scholar]
- 42.Taskinen J., Makela T., Valtonen E. T. 1997. Exploitation of Anodonta piscinalis (Bivalvia) by trematodes: parasite tactics and host longevity. Ann. Zool. Fennici 34, 37–46 [Google Scholar]
- 43.Howell M. J., Bearup A. J. 1967. The life histories of two bird trematodes of the family Philophthalmidae. Proc. Linn. Soc. New South Wales 92, 182–194 [Google Scholar]
- 44.Lie K. J. 1969. Role of immature rediae in antagonism of Paryphostomum segregatum to Schistosoma mansoni and larval development in degenerated sporocysts. Z. Parasitenk. 32, 316–323 [DOI] [PubMed] [Google Scholar]
- 45.Poulin R., Morand S. 2004. Parasite biodiversity. Washington, DC: Smithsonian Books [Google Scholar]
- 46.Cribb T. H., Bray R. A., Littlewood D. T. J., Pichelin S. P., Herniou E. A. 2001. The Digenea. In Interrelationships of the Platyhelminthes (eds Littlewood D. T. J., Bray R. A.), pp. 168–185 London, UK: Taylor & Francis [Google Scholar]
- 47.Cribb T. H., Bray R. A., Littlewood D. T. J. 2001. The nature and evolution of the association among digeneans, molluscs and fishes. Int. J. Parasitol. 31, 997–1011 10.1016/S0020-7519(01)00204-1 (doi:10.1016/S0020-7519(01)00204-1) [DOI] [PubMed] [Google Scholar]
- 48.Wilson E. O. 1975. Sociobiology: the new synthesis. Cambridge, MA: The Belknap Press of Harvard University Press [Google Scholar]
- 49.Cremer S., Armitage S. A. O., Schmid-Hempel P. 2007. Social immunity. Curr. Biol. 17, R693–R702 [DOI] [PubMed] [Google Scholar]