Abstract
Emerging evidence increasingly illustrates the importance of a holistic, rather than taxon-specific, approach to the study of ecological communities. Considerable resources are expended to manage both introduced and native mammalian carnivores to improve conservation outcomes; however, management can result in unforeseen and sometimes catastrophic outcomes. Varanid lizards are likely to be apex- or mesopredators, but being reptiles are rarely considered by managers and researchers when investigating the impacts of mammalian carnivore management. Instances of mesopredator release have been described for Varanus gouldii as a result of fox and cat management in Australia, with cascading effects on faunal community structure. A meta-analysis showing extensive dietary niche overlap between varanids, foxes and cats plus a review of experimental and circumstantial evidence suggests mesopredator release of V. gouldii and about five other medium to large species of varanid lizard is likely in other regions. This highlights the need for managers to adopt a whole-of-community approach when attempting to manage predators for sustained fauna conservation, and that additional research is required to elucidate whether mesopredator release of varanids is a widespread consequence of carnivore management, altering the intended faunal responses.
Keywords: dietary niche overlap, exploitation competition, intraguild predation, introduced predator management, trophic cascade, Varanus
1. Introduction
The predatory impact of introduced carnivores on native prey has been widely documented; however, only recently has much attention been given to the indirect effects that result from competitive interactions between predators [1,2]. Competitive interactions can be complex and shifts in their dynamics have resulted in unpredictable and sometimes profound impacts on lower trophic levels [3,4]. Compelling examples are emerging describing the deleterious impacts that subordinate predators, or mesopredators, inflict on prey species after being released from competition with a dominant predator [5–10]. Some instances of mesopredator release have yielded counterintuitive outcomes; for example, the removal of cats from an island resulted in a decline rather than an increase in the resident seabird colony because rats were released from regulation by cats, resulting in an increase in rat numbers and egg predation rates [4]. A similar result was observed on the North American continent where the nest predation rate suffered by the song sparrow (Melospiza melodia) was lower in years when coyotes (Canis latrans) were present, allegedly owing to the coyotes' suppression of racoons (Procyon lotor), the main nest predator at the site [9].
Mesopredator release involving non-mammalian predators has been reported less frequently than is the case among mammalian predators [8]. This may be because such responses are rarer or because they are investigated less often. Conspicuous examples involving non-mammalian predators include the competitive release of ghost crabs (Ocypode quadrata) after raccoon removal resulting in higher rather than lower sea turtle egg predation [11], mesopredator release of marine fish, elasmobranchs and squid as a consequence of humans over-exploiting top-order predators such as sharks (as reviewed by [12]) and cases of intraguild predation among raptors leading to population level effects when the dominant predator declined in abundance (as reviewed by [13]). Examples of mesopredator release such as these highlight the need for careful consideration of all the potential predators, not only mammalian predators, and their interactions in a system.
Investigations into the impacts of mammalian carnivore management have generally focused on mammalian apex predators and mammalian mesopredators and have largely ignored other mesopredators such as large carnivorous reptiles [14,15]. Varanid lizards are the paramount terrestrial reptilian predator across the Old World and appear to play a role similar to small- to medium-sized carnivorous mammals [16–18]. Changes in varanid lizard populations as a result of mesopredator release may then precipitate changes in lower trophic levels; hence, this group of mesopredators warrants scrutiny.
The Australian continent provides an ideal arena to test the concept of mesopredator release involving carnivorous lizards and eutherian carnivores. Australia has suffered the greatest rate of mammal extinction and range contraction of any continent since European arrival [19] and the introduction and recent establishment of two eutherian carnivores, the red fox (Vulpes vulpes) and feral cat (Felis catus), on the Australian mainland has been implicated in many of these catastrophic declines [2,19–24]. Consequently, both foxes and cats are actively managed to reduce their impacts on agricultural and/or conservation values in many parts of Australia [21,25]. Effective management of these eutherian predators can also extirpate the apex eutherian predator the dingo Canis lupus dingo [26], also widely considered a threat to livestock. As a consequence of effective control of these three predators, varanid lizards could become the apex terrestrial predators across large areas of the Australian mainland.
This review poses the questions of whether varanids can, and do, exhibit a mesopredator release response following removal of foxes or cats, and if so, are some varanid species likely to drive changes in prey community composition? To address these questions we review: (i) the likelihood of competitive and predatory interactions between varanid lizard species and introduced carnivores in Australia based on overlapping ecological niches; (ii) the existing evidence suggesting varanids could impact the faunal community structure; and (iii) the evidence for and potential implications of introduced carnivore management initially on varanid populations and subsequently on faunal composition through trophic cascades. Finally, we propose priorities and approaches for future research.
2. Niche overlap between varanid lizards and eutherian carnivores
Niche overlap between species may be expressed spatially, temporally and by the overlap in resources that they use such as diet or resting sites [27,28]. The varanid lizards with most potential to have competitive interactions with foxes or cats are the larger species whose size as adults permits them to escape the threat of predation by foxes and cats [18] as well as increase the potential for dietary overlap. Wide-ranging terrestrial foraging varanid species may be more likely to encounter foxes, cats and their prey than other varanids such as rock-dwelling, semi-aquatic or ambush predators. The availability of sheltering sites is unlikely to be a source of competition because each species is capable of digging or constructing its own shelter or burrow and to our knowledge neither varanids, nor foxes or cats, have been observed usurping the shelter of the other. Consequently, we explore niche overlap considering dietary intake, foraging habits, body size and activity patterns—both temporal and spatial.
We assessed niche overlap between all 27 recognized species of varanid lizards on the Australian mainland with both foxes and cats, initially by conducting a meta-analysis of dietary intake. All of these predators are considered generalists, taking prey in proportions that largely reflect availability [21,29,30] so the potential exists for considerable overlap. We calculated dietary overlap as defined by the equation:
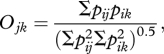 |
where O is the index of overlap, j and k are the species being compared and pi is the frequency of occurrence of the ith food type [31]. The index can range from zero (no overlap) to one (complete overlap). We could not compare the predators in terms of percentage volume of each prey group as this was reported in relatively few studies. The broad food categories common to foxes, feral cats and varanids used in this meta-analysis were mammals, reptiles, reptile eggs, birds, amphibians and invertebrates. Where studies provided frequencies of occurrence only for specific food groups within these broad categories rather than a total frequency, we used the frequency of the most common food group within each broad category. The source literature, sample sizes and ecological characteristics defining each species are given in the electronic supplementary material, table S1.
Overall patterns emerged comparing the diets of each carnivore species. Foxes tend to prey upon medium to small mammals and supplement this with carrion and invertebrates. Feral cats have a similar diet but tend to have a greater proportion of birds and reptiles and less carrion [21,32–34]. Rabbits (Oryctolagus cuniculus) were a key resource for foxes and cats, being commonly eaten in all but two dietary studies reviewed. The diets of larger varanids such as Varanus varius contain a large proportion of mammalian prey, similar to that of foxes, whereas the diets of medium-sized varanids have a higher proportion of reptilian and invertebrate items, akin to that of feral cats. Smaller varanid species mostly consume invertebrates and some small reptiles. Few varanid diet studies were conducted in areas with high densities of rabbits, introducing a potential bias exaggerating dietary differences.
Ontogenetic differences in diet appear to be relatively minor among varanid lizards. Juvenile and adult Varanus gouldii had similar diets in the Great Victoria Desert, Western Australia [35], with around 40 per cent reptilian prey and the remainder mostly invertebrates, and no association was evident between body size variation and dietary intake in V. gouldii or Varanus rosenbergi in mesic southwest Western Australia [36]. Ontogenetic differences in diet have not been reported for foxes or cats. Therefore, the degree of dietary overlap between reptilian and mammalian carnivore species may be reasonably consistent throughout an animal's life.
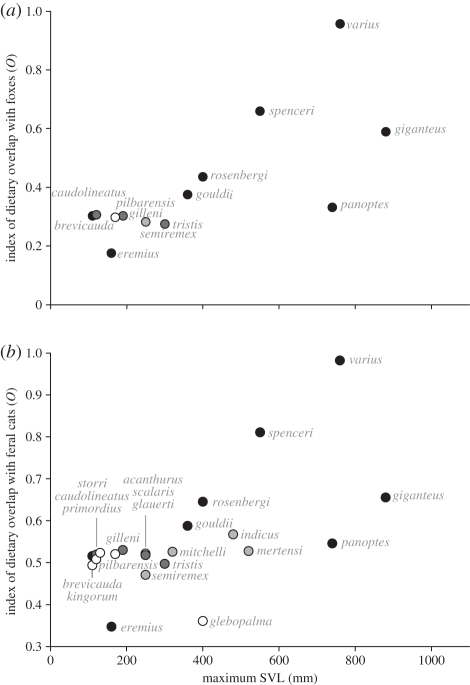
Indices of dietary overlap between introduced foxes and varanid lizards that shared geographical distributions (figure 1a) were high among three species of the largest varanids: V. varius, Varanus giganteus and Varanus spenceri; and moderate for three other large species: V. gouldii, V. rosenbergi and Varanus panoptes (distributions and body sizes from [37,38]). These species are also wide-ranging active foragers suggesting high niche overlap and potential for competitive interactions. Dietary overlap was higher again between these varanid lizards and feral cats (figure 1b). Feral cats also overlap distributions with, and have the potential to compete with, the semi-aquatic V. indicus and V. mertensi which are large species with moderate dietary overlap, but their aquatic foraging habits do differentiate them from cats [37,39,40]. The above varanid lizards have levels of dietary overlap with foxes and cats similar to other carnivore groups where interspecific competition has been demonstrated, such as between conspecific jackals Canis adustus and Canis mesomelas in Zimbabwe [41] or between coyotes C. latrans, gray foxes Urocyon cinereoargenteus and bobcats Lynx rufus in California [42]. Foxes, cats and V. varius have even higher levels of dietary overlap, suggesting the potential for more intense competitive interactions between these species.
Figure 1.
Index of dietary overlap (O) between varanid lizards on the Australian mainland and (a) introduced foxes and (b) feral cats, plotted against the maximum snout-vent length (SVL) of each varanid species. Black symbols represent varanid lizards that are wide-ranging foragers, dark grey are arboreal species, light grey represents semi-aquatic foragers and white indicates those associated with rocky outcrops. Only those varanid species with dietary information and that overlap in geographical distribution with foxes or cats are included. Details of source literature and sample sizes can be found in the electronic supplementary material, table S1.
Limited prey availability may lead to exploitation competition between carnivores with similar diets; however, from the reviewed literature, we could not determine whether shared prey was a limiting resource even though this is probable given the relatively low resource levels over much of the Australian continent. Carrion is seen as an important dietary component of both the mammalian and reptilian carnivores and could well be a key resource leading to competitive interactions [43]. The relative importance of carrion to each species and the intensity of competition for this resource have not been reported.
Aggressive encounters are also recorded more frequently among competitors with greater dietary overlap [44,45], and documented cases exist of direct interactions in the form of intraguild predation. Several cases involve smaller varanid species being preyed upon by cats and foxes [46]. Foxes and cats have also been reported to occasionally include larger varanids in their diet which may represent either predation or scavenging [47–50]. Rarely are cases documented of large adult varanids being preyed upon by cats or foxes; however, on Kangaroo Island, South Australia, feral cats have been reported not only to prey upon sub-adult V. rosenbergi, but opportunistically kill, and in two cases deliberately stalk and kill, adults [50]. Likewise, foxes and cats have been recorded in regurgitated stomach contents of V. varius [51] and in one case four fox cubs were recovered from the stomach of one individual [52].
A general distinction between varanids and the eutherian predators is the temporal separation in activity times. Introduced foxes and feral cats are frequently, though not exclusively, nocturnal foragers, whereas varanids are almost exclusively diurnal. As a consequence direct encounters should be infrequent, but this temporal separation over the day is unlikely to markedly reduce exploitation competition for prey resources. Varanids are highly adept at excavating prey in hiding during the day that introduced predators would otherwise capture when the prey is active at night. A more marked distinction is the seasonal differentiation in foraging activity whereby varanids in the southern latitudes are mostly inactive and undertake limited or no foraging at all in the cooler months [35,53,54] and in the tropics are less active towards the end of the dry season [53,55]. Conversely, mammalian predators must continue to forage and balance greater energetic demands at these times to overcome elevated heat and water loss [56].
3. Varanids and prey community structure
Several examples have been documented describing the impacts varanids can have on native vertebrate populations in Australia. The arrival of toxic cane toads (Bufo marinus) in northern Australia precipitated a marked decline in V. panoptes, which in turn has seen the nest predation rate of pig nosed turtles (Carettochelys insculpta) decline from around 20 per cent to almost nothing [57]. Varanus panoptes has been suggested to have a similar impact on flatback (Natator depressus) and Olive Ridley (Lepidochelys olivacea) sea turtle populations owing to both their extensive use of turtle breeding habitat along the northern Australian coastline and the dominance of turtle eggs in their diet [58]. In an experiment where exclosures were erected to exclude predators including V. gouldii from a natural habitat, the density of small reptiles significantly increased [59]. In another example, recovery of a ringtail possum (Pseudocheirus peregrinus) population after fire was limited by a proportional increase in predation rates by lace monitors and diamond pythons (Morelia spilota spilota) over and above predation by foxes and cats [60]. In the Great Victoria Desert, Pianka [35] described V. gouldii as a keystone predator as it consumes the largest array of reptile species including three other species of Varanus, as well as its own kind.
Instances of varanids being used for pest control have been documented, several of which attest to their impact on prey populations. Varanus rosenbergi were reportedly introduced to Revesby Island, South Australia, to reduce the number of snakes [61,62]. This introduction is thought to have had an entirely different consequence to that intended as it was considered a contributing factor to the extinction of the vulnerable greater stick nest rat (Leporillus conditor) from the island [61]. In India, varanids are protected because they eat crabs that plague rice paddy dykes [63]. Varanus indicus on the Marshall and Palau islands is considered a nuisance because they raid chicken coops and compete with humans for food resources such as crabs [64–66]. To rid the islands of these lizards, cane toads were introduced [65]. As the varanid populations fell, Rattus exulans populations began to rise in the Marshall Islands, and in the Palau Islands the numbers of beetles known to harm coconuts also rose. It is evident from these examples that varanids have the potential to limit or regulate prey populations through predation, particularly if they themselves are not being regulated through competitive interactions or other factors.
4. Implications of mammalian carnivore management
‘Natural’ or manipulative experiments relevant to varanid and eutherian carnivores suggest real competitive interactions over proximate and evolutionary time scales. The distribution of varanid lizards through Asia and Australia suggests a competitive interaction with medium-sized mammalian carnivores such as cats, mustelids and viverrids [16,18]. Varanids share a similar niche with that of eutherian carnivores in the Old World and carnivorous marsupials in Australia [16,31,67], yet small varanids are only found east of Wallace's Line, primarily in Australia and the islands of southeast Asia, where eutherian carnivores have only recently arrived. This is suggestive of strong competitive interactions between these two groups, where small varanids are unable to persist in the presence of eutherian carnivores as they are vulnerable to predation throughout their ontogenies, whereas some larger species grow out of susceptibility [18,68], and although dietary overlap is lower than for larger varanids, they may still compete for resources. Smaller species of varanids also have smaller clutches than their larger counterparts [37] and therefore have a lower reproductive potential for escaping predator regulation. Most useful in this instance would be age-specific survival rates of varanids to determine how much more vulnerable juveniles and sub-adults are to predation by foxes and cats, though this is exceedingly difficult to estimate directly. Survival estimates calculated from life tables may be possible; however, life expectancy and longevity of varanids in the field, the age at which females reach reproductive maturity and a methodology for determining age of living individuals are yet to be determined. Determining age in deceased animals has been described using skeletochronology [69,70]. Sweet & Pianka [68] speculated that both large and small varanids have been able to coexist through evolutionary time with marsupial carnivores in Australia owing to varanids possessing a superior intellect. The introduction of foxes and cats to Australia is, in effect, a large on-going natural experiment assessing the extent of competition between varanids and eutherian carnivores.
Varanids have been demonstrated to respond to long-term fox control and to feral cat management. In response to a 10-year fox baiting programme in New South Wales, V. gouldii activity increased fivefold (as measured by track counts) compared with a similar area that was unbaited [59]. Diurnal reptiles were also reported to increase, but nocturnal reptiles declined in the baited area. The authors concluded that the reduction in fox numbers led to a shift in the dominant predator from foxes to varanid lizards. In turn, there was a shift in reptile community structure, reflecting the varanids' greater efficiency as a predator of nocturnal reptiles in their daytime refuges. Such an impact on prey may also be a consequence of increasing feral cat predation, but cats were not monitored in this study. A similar response by varanids and impact on nocturnal reptiles was conjectured in a predator exclosure experiment [71]. Gecko abundance declined in the absence of foxes and cats but presence of varanids; however, the numerical response of V. gouldii to feral animal exclusion could not be detected with the monitoring approach employed in that study. Varanus gouldii has a lower dietary niche overlap with foxes than several other varanid species (figure 1) yet exhibits a mesopredator release response, suggesting stronger responses are possible with other varanid species. Claridge et al. [72] reported that with decreasing detection rates of foxes and cats over 10 years at both baited and unbaited sites, detection of V. varius increased or remained constant. Following eradication of feral cats on Faure Island in Shark Bay, Western Australia, V. gouldii increased from an almost undetectable level to become readily sighted across the island and sightings of juveniles have become noticeably more frequent ([73]; J. Williams 2009, personal communication). Similarly on the Peron Peninsula in Shark Bay, the management of both foxes and cats has seen an increase in varanid tracks (C. Sims & N. Burrows 2009, personal communication).
In contrast, Edwards et al. [74] found that where fox and cat numbers declined in response to rabbit warren ripping, no consistent change in varanid lizard numbers was evident. It is unclear whether this lack of response is owing to an insufficient knockdown of foxes and cats to release varanids from competition, because insufficient time had passed to observe a population response by varanids, because competition between the species is weak, or because other factors masked a response. For example, the loss of rabbits as a prey resource may have offset any benefit to varanids of the reduced number of competitors.
The likelihood of competition between varanids and introduced foxes and feral cats suggests that effective predator management programmes could lead to mesopredator release of some varanids. Large-scale control of introduced foxes is primarily carried out using dried meat or sausage style baits with the toxin sodium monofluoroacetate (1080) [75], and as such, these toxic baits pose a minimal risk to varanids. The 1080 tolerance of three species of varanid (V. gouldii, V. rosenbergi and V. varius) is several hundred times greater than that of foxes or cats [76,77] and is several thousand times higher for V. rosenbergi where this species co-occurs with fluoroacetate-bearing plants in Western Australia [77]. Other control strategies such as shooting or trapping are even more target specific. Consequently, varanid populations would be expected to experience no adverse impact from such programmes and, as discussed above, have real potential to be released from competitive interactions, increase in numbers and then in turn exert a greater influence on prey community structure.
Despite similarities in their niches, we hypothesize varanids and introduced predators will impact the prey community in different ways. Seasonal differences in activity patterns will mean mammalian predators will exert a greater pressure on prey groups such as the small mammals that are also obliged to remain active in cooler or harsher conditions, whereas many reptilian and invertebrate prey species only emerge in the warmer or wetter months. A shift towards a varanid-dominated predator community would be expected to result in higher small mammal biomass, and a shift in reptile community composition favouring diurnally active species that can evade capture as was observed by Olsson et al. [59]. Many varanids are also ecosystem engineers by turning over and excavating soils as they forage and dig refuges, benefiting some species more than others [78,79]. Therefore, a shift in predator dominance towards varanids has the potential to significantly alter the composition of the small fauna community.
Effective management of foxes and cats may result in a shift in dominance towards varanids resulting in community structures resembling the historic condition, or may result in an entirely different system. Regime shifts resulting from large disturbances such as fox and cat invasions are being documented [80], the consequences of which are that systems do not always return to their former state when the disturbance is removed and management objectives must be reset [81–83]. The recovery of V. gouldii after fox management suggests that even without complete fox removal, varanids can resume their previously held influence [59], but the loss of elements of the biota such as key fossorial species or the influence of synergistic disturbances such as habitat fragmentation may mean this is impossible in other systems [80].
5. Future research
Much of the focus of mammalian predator control programmes and mesopredator release theory in general has been on the response of mammalian mesopredators, ignoring the roles and responses of large reptilian predators despite suggestions that non-mammalian predators may be important competitors (e.g. [11,15,18,48]). We advocate that non-mammalian predators such as varanids, which have the potential to exhibit mesopredator release and impact the faunal community structure, should also be considered when monitoring the impact of predator control programmes. A strategy for detecting mesopredator release of varanid lizards is outlined below.
Mesopredator release of varanids may be observed as numerical and/or behavioural changes which then have an impact on prey communities. Given a large-scale manipulation that reduces the numbers of either foxes or cats or both, along with suitable experimental controls, a series of predictions can be made for a number of key observations that may distinguish three alternative hypotheses outlined in table 1: (i) that varanids are not released from competition with introduced mammalian carnivores; (ii) that varanids are released from interference competition (aggressive encounters and behavioural avoidance); and (iii) that varanids are released from exploitation competition (competition for shared resources). Note that exploitation and interference competition are not mutually exclusive and may operate concurrently. However, we treat these separately here because interference competition can operate on much shorter temporal scales through behavioural avoidance, whereas exploitation competition will be evident only after sufficient time has passed for varanids to overcome demographic lags and the population density builds.
Table 1.
A prediction table of three alternative scenarios of interspecific competition and the predicted responses of varanid lizard populations to an effective experimental manipulation of either foxes or cats.
| observations | varanids released from exploitation competition | varanids released from interference competition | varanids not released from competition |
|---|---|---|---|
| varanid density | increase | increase | no change |
| varanid size structure | no difference in size classes between experimental treatments and controls | initial rise in smaller size class individuals | no change |
| varanid home range | shrink but do not shift | shift to include areas previously occupied by dominant predator | no change |
| varanid diet | varanids in treatment areas consume more prey normally favoured by dominant predator | no change, or varanids consume more carrion previously monopolized by dominant predator | varanid diet does not differ between experimental treatments and controls |
| varanid habitat use | no change | varanids avoid direct encounters with dominant predators | no change |
| varanid temporal activity | reduced activity across the day in experimental treatments | varanids emerging earlier and retreating later in the day when dominant predators were more active | no difference in activity times between experimental treatments and controls |
We include here five points on detecting mesopredator release involving varanids along with some recommendations.
— A release from competitive interactions with foxes or cats would result in an increase in varanid lizard densities. However, estimating the numerical responses of varanids is technically difficult as no validation has been provided for whether abundance indices such as track counts, number of sightings or burrow counts has a predictable relationship with density. A comparison of these techniques with a mark–recapture study is essential, though only possible for species that can be trapped and marked, or otherwise detected systematically. It is possible that several of the larger species of varanid that have a scavenging habit may be attracted with rotten meat lures to a trap or monitored location, providing an independent and systematic means of detection (as in [84]).
— In order to detect a dietary shift of the mesopredator in response to a knockdown in the dominant predator, the investigation will need to measure the diets of both the dominant and subordinate predators. Prey availability will also need to be measured so that comparisons between treatment and control sites are not confounded. This has the added advantage that any impacts on prey community structure in any taxon may be detected. Indeed impacts on prey taxa other than mammals are more likely in the instance that varanids become the dominant predator.
— A release of varanids from interference competition would be conclusively revealed by rapid shifts in the geographical areas of activity by varanids into areas previously occupied by foxes or cats. An example of a rapid spatial as well as foraging shift in subordinate insectivorous mammals has been shown with the controlled removal of dominant competitors [85]. Such shifts in spatial activity area of varanids associated with interference competition would require concurrent monitoring of spatial activity of foxes or cats. Temporal shifts may also be apparent as varanids use hours of the day previously monopolized by the dominant species.
— To further test whether varanids influence prey community structure, an experiment that excludes varanids but not their prey (as in [59]) is recommended, preferably across biota, which looks to detect changes in prey abundance and diversity when compared with experimental control areas.
— To estimate the rates of mortality at each stage of the life cycle of varanids, it would be most useful to construct an age-specific life table for each varanid species. This would require: individual growth rate, age at maturity, life expectancy, longevity, clutch size and frequency of clutches. The age of individual varanids has been reliably determined using skeletochronology, the technique of counting growth rings in bones [69,70]; however, this is a destructive approach and alternative methods are necessary for extant populations. These parameters would aid in determining the species of varanid most at risk from introduced predators and whether intraguild predation is a likely driving factor limiting varanid population growth.
We conclude that several medium to large varanid lizards are likely to experience exploitation competition with introduced foxes and feral cats, particularly V. varius that exhibits extensive niche overlap with both. Dietary overlap is greater with feral cats than with foxes for most varanid species. This may have important implications for varanids if fox control releases feral cats, potentially increasing competition pressure. Recent examples of population recovery of V. gouldii after fox and cat control, and consequent impacts on faunal communities, suggest that it and five other varanids with similar or greater niche overlap have the potential to exhibit mesopredator release as a consequence of effective mammalian carnivore management. Further research is urgently needed to determine if mesopredator release of varanid lizards is a widespread consequence of mammalian carnivore management and the implications this may have on faunal communities.
Acknowledgements
D.S. and A.G. were supported by Postdoctoral Fellowships from the Invasive Animals Cooperative Research Center. Brian Green, Peggy Rismiller, Mike McKelvey and three anonymous reviewers provided valuable comments on an earlier draft.
References
- 1.Glen A. S., Dickman C. R. 2005. Complex interactions among mammalian carnivores in Australia, and their implications for wildlife management. Biol. Rev. 80, 387–401 10.1017/S1464793105006718 (doi:10.1017/S1464793105006718) [DOI] [PubMed] [Google Scholar]
- 2.Johnson C. N., Isaac J. L., Fisher D. O. 2007. Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia. Proc. R. Soc. B 274, 341–346 10.1098/rspb.2006.3711 (doi:10.1098/rspb.2006.3711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courchamp F., Chapuis J. L., Pascal M. 2003. Mammal invaders on islands: impact, control and control impact. Biol. Rev. 78, 347–383 10.1017/S1464793102006061 (doi:10.1017/S1464793102006061) [DOI] [PubMed] [Google Scholar]
- 4.Courchamp F., Langlais M., Sugihara G. 1999. Cats protecting birds: modelling the mesopredator release effect. J. Anim. Ecol. 68, 282–292 10.1046/j.1365-2656.1999.00285.x (doi:10.1046/j.1365-2656.1999.00285.x) [DOI] [Google Scholar]
- 5.Crooks K. R., Soulé M. E. 1999. Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400, 563–566 10.1038/23028 (doi:10.1038/23028) [DOI] [Google Scholar]
- 6.Le Corre M. 2008. Conservation biology: cats, rats and seabirds. Nature 451, 134–135 10.1038/451134a (doi:10.1038/451134a) [DOI] [PubMed] [Google Scholar]
- 7.Prugh L. R., Stoner C. J., Epps C. W., Bean W. T., Ripple W. J., Laliberte A. S., Brashares J. S. 2009. The rise of the mesopredator. Bioscience 59, 779–791 10.1525/bio.2009.59.9.9 (doi:10.1525/bio.2009.59.9.9) [DOI] [Google Scholar]
- 8.Ritchie E. G., Johnson C. N. 2009. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982–998 10.1111/j.1461-0248.2009.01347.x (doi:10.1111/j.1461-0248.2009.01347.x) [DOI] [PubMed] [Google Scholar]
- 9.Rogers C. M., Caro M. J. 1998. Song sparrows, top carnivores and nest predation: a test of the mesopredator release hypothesis. Oecologia 116, 227–233 10.1007/s004420050583 (doi:10.1007/s004420050583) [DOI] [PubMed] [Google Scholar]
- 10.Trewby I. D., et al. 2008. Experimental evidence of competitive release in sympatric carnivores. Biol. Lett. 4, 170–172 10.1098/rsbl.2007.0516 (doi:10.1098/rsbl.2007.0516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barton B. T., Roth J. D. 2008. Implications of intraguild predation for sea turtle nest protection. Biol. Conserv. 141, 2139–2145 10.1016/j.biocon.2008.06.013 (doi:10.1016/j.biocon.2008.06.013) [DOI] [Google Scholar]
- 12.Baum J. K., Worm B. 2009. Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 78, 699–714 10.1111/j.1365-2656.2009.01531.x (doi:10.1111/j.1365-2656.2009.01531.x) [DOI] [PubMed] [Google Scholar]
- 13.Sergio F., Hiraldo F. 2008. Intraguild predation in raptor assemblages: a review. Ibis 150, 132–145 10.1111/j.1474-919X.2008.00786.x (doi:10.1111/j.1474-919X.2008.00786.x) [DOI] [Google Scholar]
- 14.Nowak E. M., Theimer T. C., Schuett G. W. 2008. Functional and numerical responses of predators: where do vipers fit in the traditional paradigms? Biol. Rev. 83, 601–620 10.1111/j.1469-185X.2008.00056.x (doi:10.1111/j.1469-185X.2008.00056.x) [DOI] [PubMed] [Google Scholar]
- 15.Ray J. C., Redford K. H., Steneck R., Berger J. Large carnivores and the conservation of biodiversity. Washington, DC: Island Press; 2005. [Google Scholar]
- 16.Pianka E. R. 1969. Habitat specificity, speciation, and species density in Australian desert lizards. Ecology 50, 498–502 10.2307/1933908 (doi:10.2307/1933908) [DOI] [Google Scholar]
- 17.Pough F. H. 1973. Lizard energetics and diet. Ecology 54, 837–844 10.2307/1935678 (doi:10.2307/1935678) [DOI] [Google Scholar]
- 18.Sweet S. S., Pianka E. R. 2007. Monitors, mammals, and Wallace's Line. Mertensiella 16, 79–99 [Google Scholar]
- 19.McKenzie N. L., et al. 2007. Analysis of factors implicated in the recent decline of Australia's mammal fauna. J. Biogeogr. 34, 597–611 10.1111/j.1365-2699.2006.01639.x (doi:10.1111/j.1365-2699.2006.01639.x) [DOI] [Google Scholar]
- 20.Abbott I. 2002. Origin and spread of the cat, Felis catus, on mainland Australia, with a discussion of the magnitude of its early impact on native fauna. Wildl. Res. 29, 51–74 10.1071/WR01011 (doi:10.1071/WR01011) [DOI] [Google Scholar]
- 21.Dickman C. R. 1996a. Impact of exotic generalist predators on the native fauna of Australia. Wildl. Biol. 2, 185–195 [Google Scholar]
- 22.Kinnear J. E., Sumner N. R., Onus M. L. 2002. The red fox in Australia: an exotic predator turned biocontrol agent. Biol. Conserv. 108, 335–359 10.1016/S0006-3207(02)00116-7 (doi:10.1016/S0006-3207(02)00116-7) [DOI] [Google Scholar]
- 23.Salo P., Korpimaki E., Banks P. B., Nordstrom M., Dickman C. R. 2007. Alien predators are more dangerous than native predators to prey populations. Proc. R. Soc. B 274, 1237–1243 10.1098/rspb.2006.0444 (doi:10.1098/rspb.2006.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith A. P., Quin D. G. 1996. Patterns and causes of extinction and decline in Australian conilurine rodents. Biol. Conserv. 77, 243–267 10.1016/0006-3207(96)00002-X (doi:10.1016/0006-3207(96)00002-X) [DOI] [Google Scholar]
- 25.Saunders G. R., Gentle M. N., Dickman C. R. 2010. The impacts and management of foxes Vulpes vulpes in Australia. Mamm. Rev. 40, 181–211 [Google Scholar]
- 26.Burrows N. D., Algar D., Robinson A. D., Sinagra J., Ward B., Liddelow G. 2003. Controlling introduced predators in the Gibson Desert of Western Australia. J. Arid Environ. 55, 691–713 10.1016/S0140-1963(02)00317-8 (doi:10.1016/S0140-1963(02)00317-8) [DOI] [Google Scholar]
- 27.Mac Nally R. C. 1983. On assessing the significance of interspecific competition to guild structure. Ecology 64, 1646–1652 10.2307/1937517 (doi:10.2307/1937517) [DOI] [Google Scholar]
- 28.Pianka E. R. 1974. Niche overlap and diffuse competition. Proc. Natl Acad. Sci. USA 71, 2141–2145 10.1073/pnas.71.5.2141 (doi:10.1073/pnas.71.5.2141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Losos J. B., Greene H. W. 1988. Ecological and evolutionary implications of diet in monitor lizards. Biol. J. Linn. Soc. 35, 379–407 10.1111/j.1095-8312.1988.tb00477.x (doi:10.1111/j.1095-8312.1988.tb00477.x) [DOI] [Google Scholar]
- 30.Shine R. 1986. Food habits, habitats and reproductive biology of four sympatric species of varanid lizards in tropical Australia. Herpetologica 42, 346–360 [Google Scholar]
- 31.Pianka E. R. 1973. The structure of lizard communities. Annu. Rev. Ecol. Syst. 4, 53–74 10.1146/annurev.es.04.110173.000413 (doi:10.1146/annurev.es.04.110173.000413) [DOI] [Google Scholar]
- 32.Lapidge S. J., Henshall S. 2001. Diet of foxes and cats, with evidence of predation in yellow-footed rock-wallabies (Petrogale xanthopus celeris) by foxes, in southwestern Queensland. Aust. Mammal. 23, 47–51 [Google Scholar]
- 33.Risbey D. A., Calver M. C., Short J. 1999. The impact of cats and foxes on the small vertebrate fauna of Heirisson Prong, Western Australia. I. Exploring potential impact using diet analysis. Wildl. Res. 26, 621–630 10.1071/WR98066 (doi:10.1071/WR98066) [DOI] [Google Scholar]
- 34.Triggs B., Brunner H., Cullen J. M. 1984. The food of fox, dog and cat in Croajingalong National Park, south-eastern Victoria. Aust. Wildl. Res. 11, 491–499 10.1071/WR9840491 (doi:10.1071/WR9840491) [DOI] [Google Scholar]
- 35.Pianka E. R. 1994. Comparative ecology of Varanus in the Great Victoria Desert. Aust. J. Ecol. 19, 395–408 10.1111/j.1442-9993.1994.tb00505.x (doi:10.1111/j.1442-9993.1994.tb00505.x) [DOI] [Google Scholar]
- 36.Sutherland D. R. In review Dietary niche overlap and size partitioning in sympatric varanid lizards. Herpetologica. [Google Scholar]
- 37.Pianka E. R., King D. R., King R. A. 2004. Varanoid lizards of the world. Bloomington, IN: Indiana University Press [Google Scholar]
- 38.Van Dyck S., Strahan R. (eds) 2008. The mammals of Australia. Sydney, Australia: Reed New Holland [Google Scholar]
- 39.Mayes P. J., Thompson G. G., Withers P. C. 2005. Diet and foraging behaviour of the semi-aquatic Varanus mertensi (Reptilia: Varanidae). Wildl. Res. 32, 67–74 10.1071/WR04040 (doi:10.1071/WR04040) [DOI] [Google Scholar]
- 40.Sweet S. S. 1999. Spatial ecology of Varanus glauerti and V. glebopalma in northern Australia. Mertensiella 11, 317–366 [Google Scholar]
- 41.Loveridge A. J., Macdonald D. W. 2007. Niche separation in sympatric jackals (Canis mesomelas and Canis adustus). J. Zool. (Lond.) 259, 143–153 10.1017/S0952836902003114 (doi:10.1017/S0952836902003114) [DOI] [Google Scholar]
- 42.Fedriani J. M., Fuller T. K., Sauvajot R. M., York E. C. 2000. Competition and intraguild predation among three sympatric carnivores. Oecologia 125, 258–270 10.1007/s004420000448 (doi:10.1007/s004420000448) [DOI] [PubMed] [Google Scholar]
- 43.Creel S. R., Spong G., Creel N. 2001. Interspecific competition and the population biology of extinction prone carnivores. In Carnivore conservation (eds Gittleman J. L., Funk S. M., Macdonald D. W., Wayne R. K.), pp. 35–60 Cambridge, UK: Cambridge University Press [Google Scholar]
- 44.Donadio E., Buskirk S. W. 2006. Diet, morphology, and interspecific killing in Carnivora. Am. Nat. 167, 524–536 10.1086/501033 (doi:10.1086/501033) [DOI] [PubMed] [Google Scholar]
- 45.Polis G., Myers C., Holt R. 1989. The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu. Rev. Ecol. Syst. 20, 297–330 10.1146/annurev.es.20.110189.001501 (doi:10.1146/annurev.es.20.110189.001501) [DOI] [Google Scholar]
- 46.Sweet S. S. 2007. Comparative spatial ecology of two small arboreal monitors in Northern Australia. Mertensiella 16, 378–402 [Google Scholar]
- 47.Catling P. C. 1988. Similarities and contrasts in the diets of foxes, Vulpes vulpes, and cats, Felis catus, relative to fluctuating prey populations and drought. Aust. Wildl. Res. 15, 307–317 10.1071/WR9880307 (doi:10.1071/WR9880307) [DOI] [Google Scholar]
- 48.Dickman C. R. 1996. Overview of the impacts of feral cats on Australian native fauna, pp. 1–92 Canberra, Australia: Australian Nature Conservation Agency [Google Scholar]
- 49.Molsher R. L. 1999. The ecology of feral cats, Felis catus, in open forest in New South Wales: interactions with food resources and foxes. PhD thesis, School of Biological Sciences, University of Sydney, Sydney, Australia, p. 257 [Google Scholar]
- 50.Rismiller P. D., McKelvey M. W. 2003. Twenty-seven years of wildcats and kittens, case history of a feral predator on the Pelican Lagoon Peninsula, Kangaroo Island. In Feral cat control, some new ideas for 2003, Kangaroo Island/Roxby Downs, pp. 9–16 Kingscote, Australia: Kangaroo Island Rotary Club [Google Scholar]
- 51.Weavers B. W. 1989. Diet of the Lace Monitor Lizard (Varanus varius) in south-eastern Australia. Aust. Zool. 25, 83–85 [Google Scholar]
- 52.Fleay D. 1950. Goannas: giant lizards of the Australian bush. Anim. Kingdom 53, 92–96 [Google Scholar]
- 53.Green B., Dryden G., Dryden K. 1991. Field energetics of a large carnivorous lizard, Varanus rosenbergi. Oecologia 88, 547–551 [DOI] [PubMed] [Google Scholar]
- 54.Guarino F. 2001. Diet of a large carnivorous lizard, Varanus varius. Wildl. Res. 28, 627–630 10.1071/WR01001 (doi:10.1071/WR01001) [DOI] [Google Scholar]
- 55.Christian K. A., Corbett L. K., Green B., Weavers B. W. 1995. Seasonal activity and energetics of two species of varanid lizards in tropical Australia. Oecologia 103, 349–357 10.1007/BF00328624 (doi:10.1007/BF00328624) [DOI] [PubMed] [Google Scholar]
- 56.Porter W. P., Munger J. C., Stewart W. C., Budaraju S., Jaeger J. 1994. Endotherm energetics: from a scalable individual-based model to ecological applications. Aust. J. Zool. 42, 125–162 10.1071/ZO9940125 (doi:10.1071/ZO9940125) [DOI] [Google Scholar]
- 57.Doody J. S., Green B., Sims R., Rhind D., West P., Steer D. 2006. Indirect impacts of invasive cane toads (Bufo marinus) on nest predation in pig-nosed turtles (Carettochelys insculpta). Wildl. Res. 33, 349–354 10.1071/WR05042 (doi:10.1071/WR05042) [DOI] [Google Scholar]
- 58.Blamires S. J. 2004. Habitat preferences of coastal goannas (Varanus panoptes): are they exploiters of sea turtle nests at Fog Bay, Australia? Copeia 2004, 370–377 10.1643/CH-03-016R1 (doi:10.1643/CH-03-016R1) [DOI] [Google Scholar]
- 59.Olsson M., Wapstra E., Swan G., Snaith E., Clarke R., Madsen T. 2005. Effects of long-term fox baiting on species composition and abundance in an Australian lizard community. Austral Ecol. 30, 907–913 10.1111/j.1442-9993.2005.01534.x (doi:10.1111/j.1442-9993.2005.01534.x) [DOI] [Google Scholar]
- 60.Russell B. G., Smith B., Augee M. L. 2003. Changes to a population of common ringtail possums (Pseudocheirus peregrinus) after bushfire. Wildl. Res. 30, 389–396 10.1071/WR01047 (doi:10.1071/WR01047) [DOI] [Google Scholar]
- 61.Mirtschin P. 1982. The Gould's goanna, an Australian native, alien to Reevesby Island. S Aust. Nat. 57, 18–19 [Google Scholar]
- 62.Robinson A. C., Mirtschin P. J., Copley P. D., Canty P. D., Jenkins R. B. 1985. The Reevesby Island goanna—a problem in conservation management. S. Aust. Nat. 59, 56–62 [Google Scholar]
- 63.Greer A. E. 1989. The biology and evolution of Australian lizards. Chipping Norton, UK: Surrey Beatty & Sons [Google Scholar]
- 64.Cota M. 2008. Varanus indicus and its presence on the Mariana Islands: natural geographic distribution vs. introduction. Biawak 2, 18–27 [Google Scholar]
- 65.Dryden G. 1965. The food and feeding habits of Varanus indicus on Guam. Micronesica 2, 73–76 [Google Scholar]
- 66.Uchida T. 1967. Observations on the monitor lizard, Varanus indicus (Daudin) as a rat control agent on Ifaluk, Western Caroline Islands. Micronesica 3, 17–18 [PMC free article] [PubMed] [Google Scholar]
- 67.Pianka E. R. 1981. Diversity and adaptive radiations of Australian desert lizards. In Ecological biodiversity of Australia (ed. Keast A.), pp. 1375–1392 The Hague, The Netherlands: Dr W. Junk bv Publishers [Google Scholar]
- 68.Sweet S. S., Pianka E. R. 2003. The lizard kings—small monitors roam to the east of an unseen frontier; mammals roam to the west. Nat. Hist. 112, 40–45 [Google Scholar]
- 69.Castanet J. 1994. Age estimation and longevity in reptiles. Gerontology 40, 174–192 10.1159/000213586 (doi:10.1159/000213586) [DOI] [PubMed] [Google Scholar]
- 70.de Buffrénil V., Castanet J. 2000. Age estimation by skeletochronology in the Nile monitor (Varanus niloticus), a highly exploited species. J. Herpetol. 34, 414–424 10.2307/1565365 (doi:10.2307/1565365) [DOI] [Google Scholar]
- 71.Moseby K. E., Hill B. M., Read J. L. 2009. Arid recovery—a comparison of reptile and small mammal populations inside and outside a large rabbit, cat and fox-proof exclosure in arid South Australia. Austral Ecol. 34, 156–169 10.1111/j.1442-9993.2008.01916.x (doi:10.1111/j.1442-9993.2008.01916.x) [DOI] [Google Scholar]
- 72.Claridge A. W., Cunningham R. B., Catling P. C., Reid A. M. 2010. Trends in the activity levels of forest-dwelling vertebrate fauna against a background of intensive baiting for foxes. Forest Ecol. Manag. 260, 822–832 10.1016/j.foreco.2010.05.041 (doi:10.1016/j.foreco.2010.05.041) [DOI] [Google Scholar]
- 73.Rowles C. 2008. The diet of the Gould's monitor Varanus gouldii and its potential impact on the translocated mammals of Faure Island, Shark Bay, Western Australia. Honours School of Environmental Biology, Curtin University, Perth, Australia, p. 76 [Google Scholar]
- 74.Edwards G. P., Dobbie W., Berman D. M. 2002. Warren ripping: its impacts on European rabbits and other wildlife of central Australia amid the establishment of rabbit haemorrhagic disease. Wildl. Res. 29, 567–575 10.1071/WR00098 (doi:10.1071/WR00098) [DOI] [Google Scholar]
- 75.Saunders G., Coman B. J., Kinnear J. E., Braysher M. 1995. Managing vertebrate pests: foxes. Canberra, Australia: Australian Government Publishing Service [Google Scholar]
- 76.McIlroy J. C., King D. R., Oliver A. J. 1985. The sensitivity of Australian animals to 1080 poison VIII. Amphibians and reptiles. Wildl. Res. 12, 113–118 10.1071/WR9850113 (doi:10.1071/WR9850113) [DOI] [Google Scholar]
- 77.Twigg L. E., King D. R. 1991. The impact of fluoroacetate-bearing vegetation on native Australian fauna: a review. Oikos 61, 412–430 10.2307/3545249 (doi:10.2307/3545249) [DOI] [Google Scholar]
- 78.James A. I., Eldridge D. J. 2007. Reintroduction of fossorial native mammals and potential impacts on ecosystem processes in an Australian desert landscape. Biol. Conserv. 138, 351–359 10.1016/j.biocon.2007.04.029 (doi:10.1016/j.biocon.2007.04.029) [DOI] [Google Scholar]
- 79.Whitford W. G. 1998. Contribution of pits dug by goannas (Varanus gouldii) to the dynamics of banded mulga landscapes in eastern Australia. J. Arid Environ. 40, 453–457 10.1006/jare.1998.0464 (doi:10.1006/jare.1998.0464) [DOI] [Google Scholar]
- 80.Hobbs R. J. 2001. Synergisms among habitat fragmentation, livestock grazing, and biotic invasions in southwestern Australia. Conserv. Biol. 15, 1522–1528 10.1046/j.1523-1739.2001.01092.x (doi:10.1046/j.1523-1739.2001.01092.x) [DOI] [Google Scholar]
- 81.Hobbs R. J., et al. 2006. Novel ecosystems: theoretical and management aspects of the new ecological world order. Global Ecol. Biogeogr. 15, 1–7 10.1111/j.1466-822X.2006.00212.x (doi:10.1111/j.1466-822X.2006.00212.x) [DOI] [Google Scholar]
- 82.Scheffer M., Carpenter S. R. 2003. Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol. Evol. 18, 648–656 10.1016/j.tree.2003.09.002 (doi:10.1016/j.tree.2003.09.002) [DOI] [Google Scholar]
- 83.Suding K. N., Hobbs R. J. 2009. Threshold models in restoration and conservation: a developing framework. Trends Ecol. Evol. 24, 271–279 10.1016/j.tree.2008.11.012 (doi:10.1016/j.tree.2008.11.012) [DOI] [PubMed] [Google Scholar]
- 84.Smith J. G. 2004. Fish and company smell after three days: increasing capture rates of carrion-eating varanid lizards. Herpetol. Rev. 35, 41–42 [Google Scholar]
- 85.Dickman C. R. 1988. Body size, prey size, and community structure in insectivorous mammals. Ecology 69, 569–580 10.2307/1941006 (doi:10.2307/1941006) [DOI] [Google Scholar]