Abstract
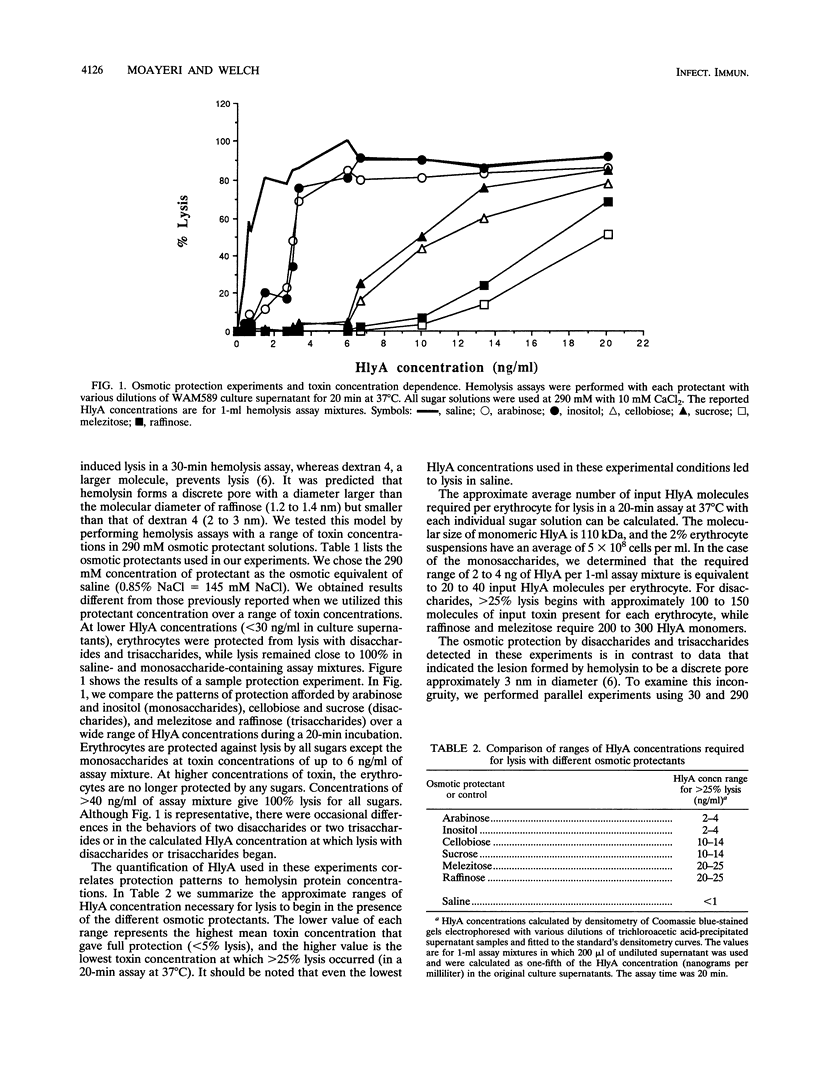
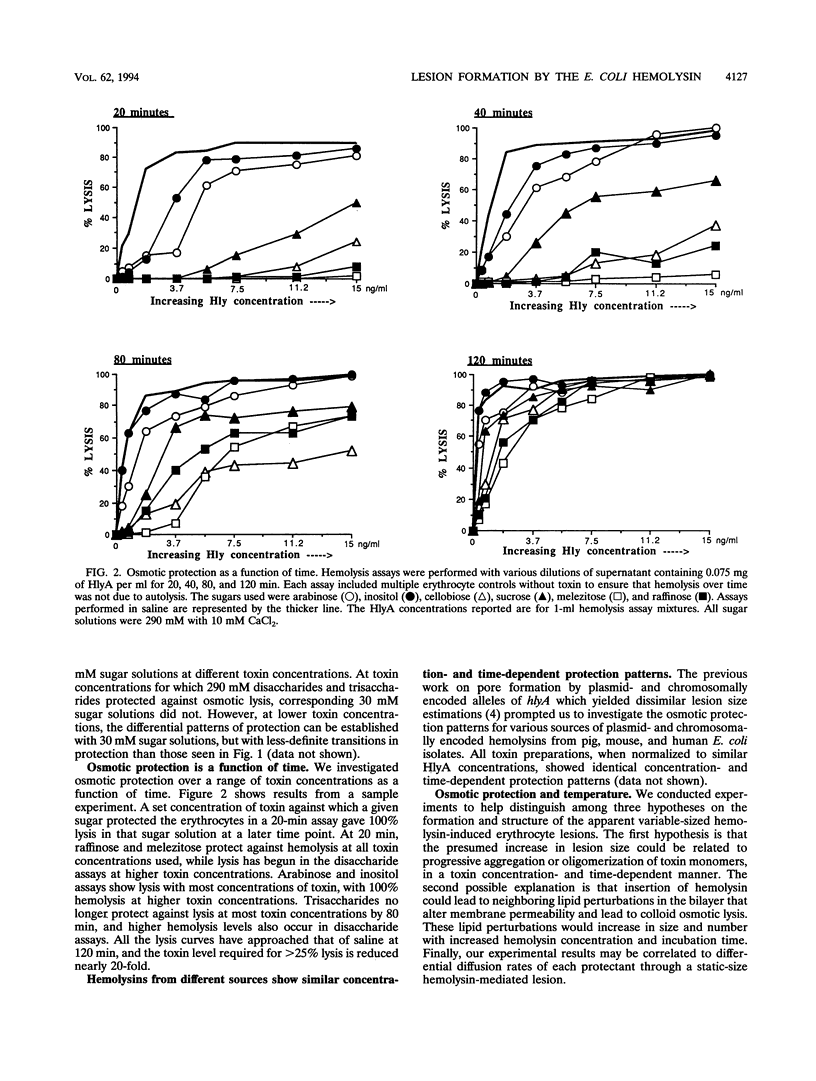
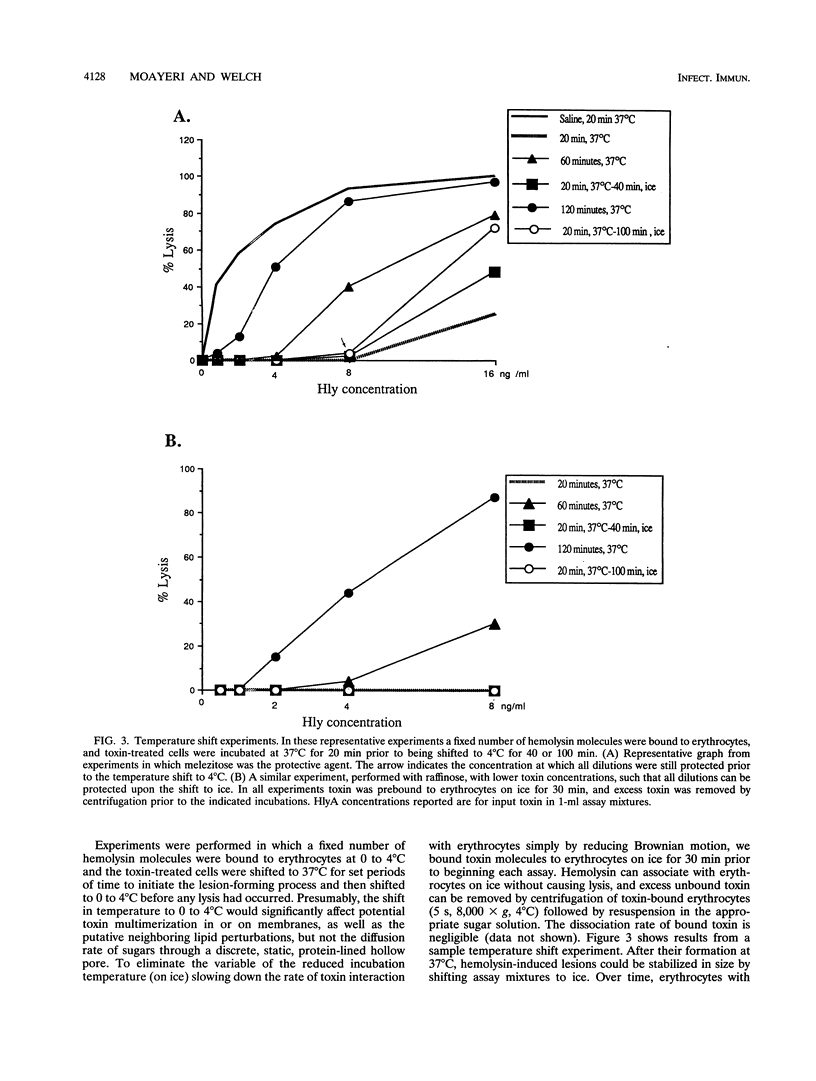
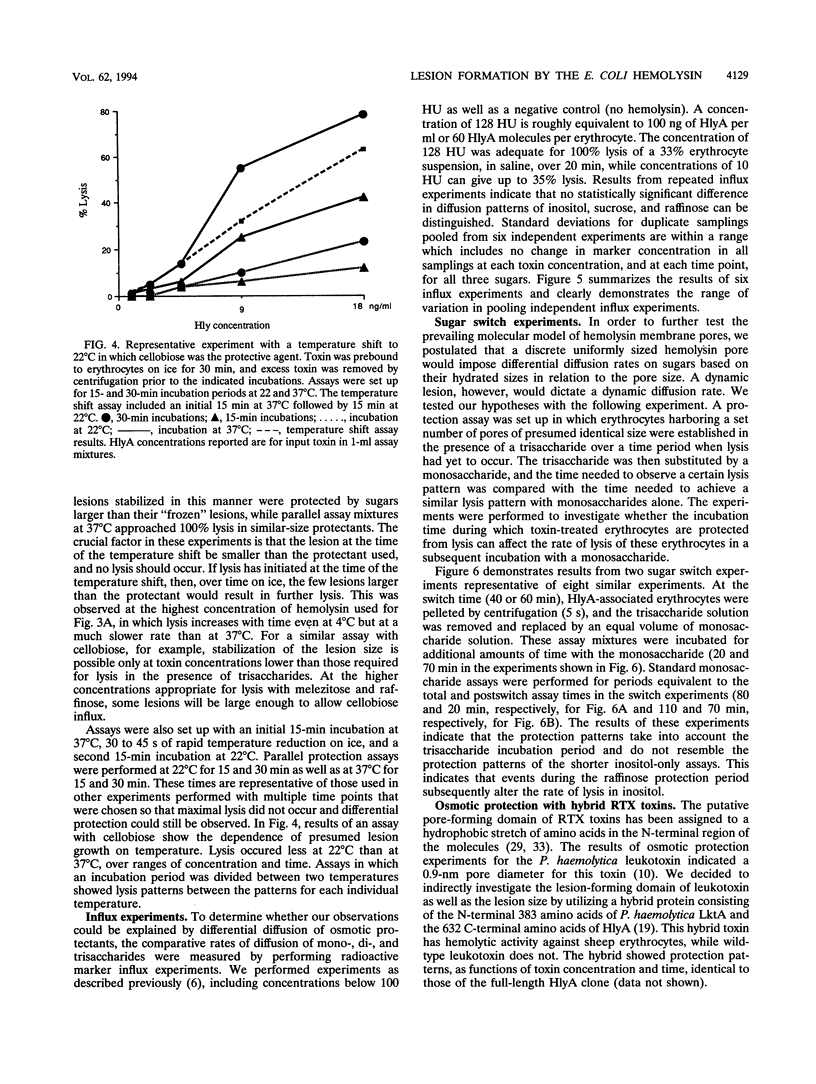
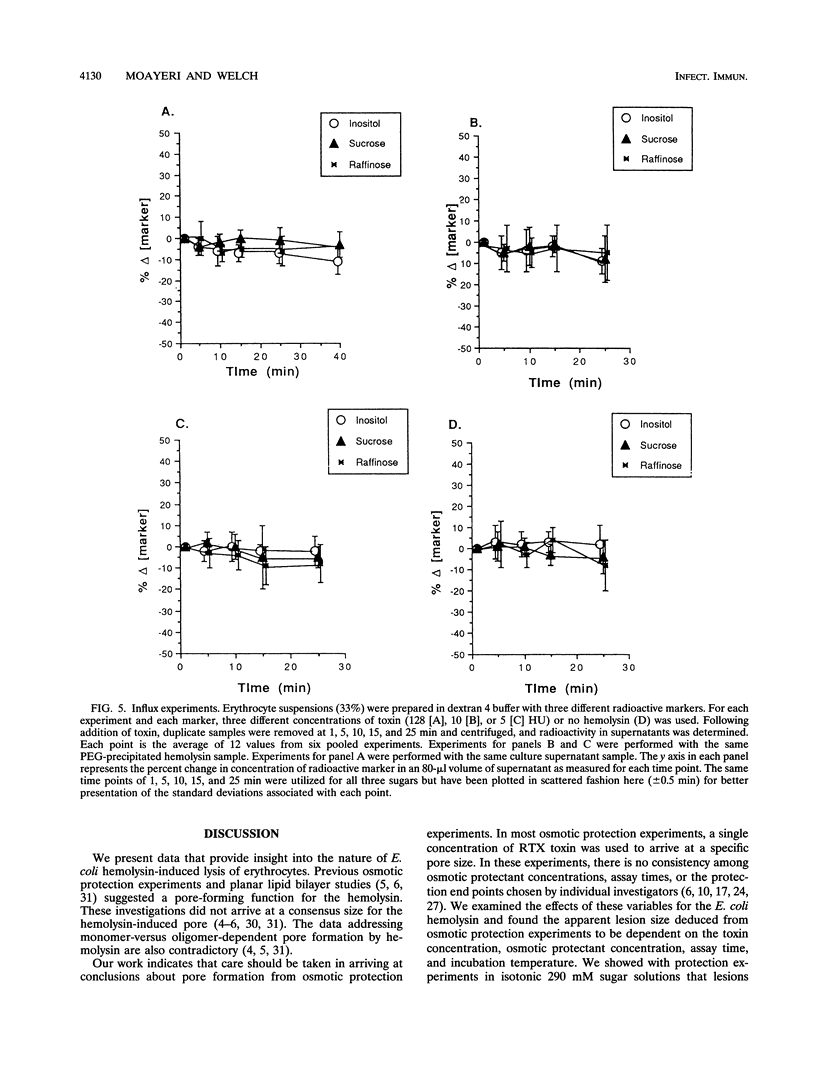
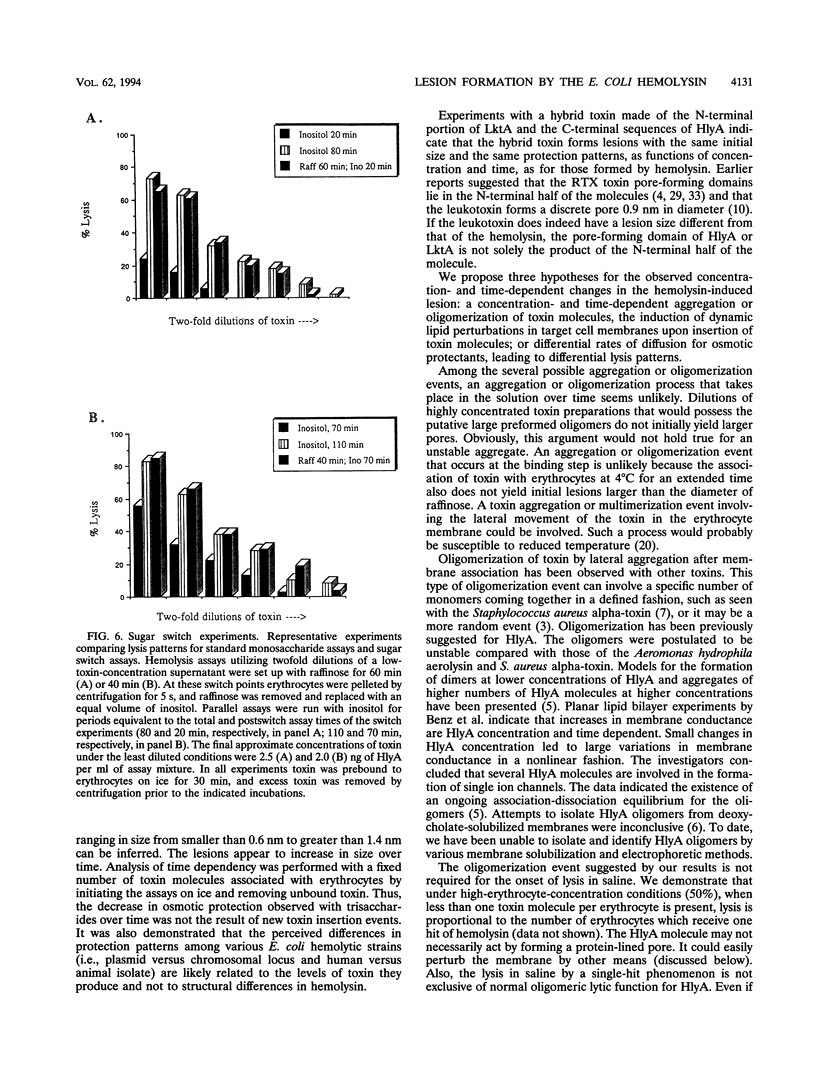
We performed osmotic protection experiments to test the hypothesis that the Escherichia coli hemolysin forms a discrete-size pore in erythrocyte membranes. The effects of toxin concentration, assay time, temperature, and protectant concentrations were examined. The results we present here raise doubts about the existing model of pore formation by hemolysin. We demonstrate that osmotic protection by various sugars of different sizes is a function of hemolysin concentration and assay time. The data indicate that under various conditions, lesion sizes with a diameter ranging from < 0.6 to > 1.2 nm can be inferred. Quantification of hemolysin permitted the estimation of the number of HlyA structural protein molecules required per erythrocyte for lysis in the presence of each protectant. It appears that hemolysin induces heterogeneous erythrocyte lesions which increase in size over time. Influx experiments utilizing radioactive sugar markers indicated that time-dependent osmotic protection patterns are independent of the diffusion rates of individual protectants. We demonstrate that the rate of the putative growth in the size of hemolysin-mediated lesions is temperature dependent. The erythrocyte membrane lesions formed at 37 degrees C can be stabilized in size when shifted to 4 degrees C. On the basis of these data, new models for the nature of the hemolysin-mediated erythrocyte membrane lesions are presented.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson J. J., Shamoo A. E. Anionic detergents as divalent cation ionophores across black lipid membranes. J Membr Biol. 1979 Nov 30;50(3-4):241–255. doi: 10.1007/BF01868891. [DOI] [PubMed] [Google Scholar]
- Alder G. M., Arnold W. M., Bashford C. L., Drake A. F., Pasternak C. A., Zimmermann U. Divalent cation-sensitive pores formed by natural and synthetic melittin and by Triton X-100. Biochim Biophys Acta. 1991 Jan 9;1061(1):111–120. doi: 10.1016/0005-2736(91)90275-d. [DOI] [PubMed] [Google Scholar]
- Belmonte G., Cescatti L., Ferrari B., Nicolussi T., Ropele M., Menestrina G. Pore formation by Staphylococcus aureus alpha-toxin in lipid bilayers. Dependence upon temperature and toxin concentration. Eur Biophys J. 1987;14(6):349–358. doi: 10.1007/BF00262320. [DOI] [PubMed] [Google Scholar]
- Benz R., Döbereiner A., Ludwig A., Goebel W. Haemolysin of Escherichia coli: comparison of pore-forming properties between chromosome and plasmid-encoded haemolysins. FEMS Microbiol Immunol. 1992 Sep;5(1-3):55–62. doi: 10.1111/j.1574-6968.1992.tb05887.x. [DOI] [PubMed] [Google Scholar]
- Benz R., Schmid A., Wagner W., Goebel W. Pore formation by the Escherichia coli hemolysin: evidence for an association-dissociation equilibrium of the pore-forming aggregates. Infect Immun. 1989 Mar;57(3):887–895. doi: 10.1128/iai.57.3.887-895.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991 Dec;55(4):733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. D., Borsos T. Studies on the terminal stages of immune hemolysis. V. Evidence that not all complement-produced transmembrane channels are equal. J Immunol. 1979 Jul;123(1):71–76. [PubMed] [Google Scholar]
- Boyle M. D., Gee A. P., Borsos T. Studies on the terminal stages of immune hemolysis. VI. Osmotic blockers of differing Stokes' radii detect complement-induced transmembrane channels of differing size. J Immunol. 1979 Jul;123(1):77–82. [PubMed] [Google Scholar]
- Clinkenbeard K. D., Mosier D. A., Confer A. W. Transmembrane pore size and role of cell swelling in cytotoxicity caused by Pasteurella haemolytica leukotoxin. Infect Immun. 1989 Feb;57(2):420–425. doi: 10.1128/iai.57.2.420-425.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote J. G. Structural and functional relationships among the RTX toxin determinants of gram-negative bacteria. FEMS Microbiol Rev. 1992 Feb;8(2):137–161. doi: 10.1111/j.1574-6968.1992.tb04961.x. [DOI] [PubMed] [Google Scholar]
- Dalmasso A. P., Benson B. A. Lesions of different functional size produced by human and guinea pig complement in sheep red cell membranes. J Immunol. 1981 Dec;127(6):2214–2218. [PubMed] [Google Scholar]
- Davies H. G., Marsden N. V., Ostling S. G., Zade-Oppen A. M. The effect of some neutral macromolecules on the pattern of hypotonic hemolysis. Acta Physiol Scand. 1968 Dec;74(4):577–593. doi: 10.1111/j.1748-1716.1968.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Eberspächer B., Hugo F., Pohl M., Bhakdi S. Functional similarity between the haemolysins of Escherichia coli and Morganella morganii. J Med Microbiol. 1990 Nov;33(3):165–170. doi: 10.1099/00222615-33-3-165. [DOI] [PubMed] [Google Scholar]
- Ehrmann I. E., Gray M. C., Gordon V. M., Gray L. S., Hewlett E. L. Hemolytic activity of adenylate cyclase toxin from Bordetella pertussis. FEBS Lett. 1991 Jan 14;278(1):79–83. doi: 10.1016/0014-5793(91)80088-k. [DOI] [PubMed] [Google Scholar]
- Esser A. F., Kolb W. P., Podack E. R., Müller-Eberhard H. J. Molecular reorganization of lipid bilayers by complement: a possible mechanism for membranolysis. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1410–1414. doi: 10.1073/pnas.76.3.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestier C., Welch R. A. Identification of RTX toxin target cell specificity domains by use of hybrid genes. Infect Immun. 1991 Nov;59(11):4212–4220. doi: 10.1128/iai.59.11.4212-4220.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler V., Branton D. Lateral mobility of human erythrocyte integral membrane proteins. Nature. 1977 Jul 7;268(5615):23–26. doi: 10.1038/268023a0. [DOI] [PubMed] [Google Scholar]
- GREEN H., BARROW P., GOLDBERG B. Effect of antibody and complement on permeability control in ascites tumor cells and erythrocytes. J Exp Med. 1959 Nov 1;110:699–713. doi: 10.1084/jem.110.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavedoni E. B., Chow Y. M., Dalmasso A. P. The functional size of the primary complement lesion in resealed erythrocyte membrane ghosts. J Immunol. 1979 Jan;122(1):240–245. [PubMed] [Google Scholar]
- Goebel W., Hedgpeth J. Cloning and functional characterization of the plasmid-encoded hemolysin determinant of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1290–1298. doi: 10.1128/jb.151.3.1290-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase M., Lally E. T., Berthold P., Korchak H. M., Taichman N. S. Effects of cations and osmotic protectants on cytolytic activity of Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun. 1990 Jun;58(6):1782–1788. doi: 10.1128/iai.58.6.1782-1788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen S. E., Mulcahy P. F., Wu G. K., Louis C. F. Calcium accumulation in human and sheep erythrocytes that is induced by Escherichia coli hemolysin. Toxicon. 1983;21(5):717–727. doi: 10.1016/0041-0101(83)90277-5. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Carney D. F., Papadimitriou J. C., Shin M. L. Effect of osmotic protection on nucleated cell killing by C5b-9: cell death is not affected by the prevention of cell swelling. Mol Immunol. 1989 Mar;26(3):323–331. doi: 10.1016/0161-5890(89)90087-4. [DOI] [PubMed] [Google Scholar]
- Lalonde G., McDonald T. V., Gardner P., O'Hanley P. D. Identification of a hemolysin from Actinobacillus pleuropneumoniae and characterization of its channel properties in planar phospholipid bilayers. J Biol Chem. 1989 Aug 15;264(23):13559–13564. [PubMed] [Google Scholar]
- Ludwig A., Benz R., Goebel W. Oligomerization of Escherichia coli haemolysin (HlyA) is involved in pore formation. Mol Gen Genet. 1993 Oct;241(1-2):89–96. doi: 10.1007/BF00280205. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Schmid A., Benz R., Goebel W. Mutations affecting pore formation by haemolysin from Escherichia coli. Mol Gen Genet. 1991 Apr;226(1-2):198–208. doi: 10.1007/BF00273604. [DOI] [PubMed] [Google Scholar]
- Menestrina G. Escherichia coli hemolysin permeabilizes small unilamellar vesicles loaded with calcein by a single-hit mechanism. FEBS Lett. 1988 May 9;232(1):217–220. doi: 10.1016/0014-5793(88)80420-4. [DOI] [PubMed] [Google Scholar]
- Menestrina G., Mackman N., Holland I. B., Bhakdi S. Escherichia coli haemolysin forms voltage-dependent ion channels in lipid membranes. Biochim Biophys Acta. 1987 Nov 27;905(1):109–117. doi: 10.1016/0005-2736(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Michaels D. W., Abramovitz A. S., Hammer C. H., Mayer M. M. Increased ion permeability of planar lipid bilayer membranes after treatment with the C5b-9 cytolytic attack mechanism of complement. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2852–2856. doi: 10.1073/pnas.73.8.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropeza-Wekerle R. L., Muller S., Briand J. P., Benz R., Schmid A., Goebel W. Haemolysin-derived synthetic peptides with pore-forming and haemolytic activity. Mol Microbiol. 1992 Jan;6(1):115–121. doi: 10.1111/j.1365-2958.1992.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Ostolaza H., Bartolomé B., Ortiz de Zárate I., de la Cruz F., Goñi F. M. Release of lipid vesicle contents by the bacterial protein toxin alpha-haemolysin. Biochim Biophys Acta. 1993 Apr 8;1147(1):81–88. doi: 10.1016/0005-2736(93)90318-t. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Biesecker G., Müller-Eberhard H. J. Membrane attack complex of complement: generation of high-affinity phospholipid binding sites by fusion of five hydrophilic plasma proteins. Proc Natl Acad Sci U S A. 1979 Feb;76(2):897–901. doi: 10.1073/pnas.76.2.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Mayer M. M. Life-span and size of the trans-membrane channel formed by large doses of complement. J Immunol. 1980 May;124(5):2281–2287. [PubMed] [Google Scholar]
- Ramm L. E., Whitlow M. B., Mayer M. M. Size of the transmembrane channels produced by complement proteins C5b-8. J Immunol. 1982 Sep;129(3):1143–1146. [PubMed] [Google Scholar]
- Ramm L. E., Whitlow M. B., Mayer M. M. Transmembrane channel formation by complement: functional analysis of the number of C5b6, C7, C8, and C9 molecules required for a single channel. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4751–4755. doi: 10.1073/pnas.79.15.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropele M., Menestrina G. Electrical properties and molecular architecture of the channel formed by Escherichia coli hemolysin in planar lipid membranes. Biochim Biophys Acta. 1989 Oct 2;985(1):9–18. doi: 10.1016/0005-2736(89)90096-5. [DOI] [PubMed] [Google Scholar]
- Seeman P. Macromolecules may inhibit diffusion of hemoglobin from lysing erythrocytes by exclusion of solvent. Can J Physiol Pharmacol. 1973 Mar;51(3):226–229. doi: 10.1139/y73-032. [DOI] [PubMed] [Google Scholar]
- Simone C. B., Henkart P. Inhibition of marker influx into complement-treated resealed erythrocyte ghosts by anti-C5. J Immunol. 1982 Mar;128(3):1168–1175. [PubMed] [Google Scholar]
- Sims P. J. Complement pores in erythrocyte membranes. Analysis of C8/C9 binding required for functional membrane damage. Biochim Biophys Acta. 1983 Aug 10;732(3):541–552. doi: 10.1016/0005-2736(83)90230-4. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Lauf P. K. Analysis of solute diffusion across the C5b-9 membrane lesion of complement: evidence that individual C5b-9 complexes do not function as discrete, uniform pores. J Immunol. 1980 Dec;125(6):2617–2625. [PubMed] [Google Scholar]
- Sims P. J., Lauf P. K. Steady-state analysis of tracer exchange across the C5b-9 complement lesion in a biological membrane. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5669–5673. doi: 10.1073/pnas.75.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A. Identification of two different hemolysin determinants in uropathogenic Proteus isolates. Infect Immun. 1987 Sep;55(9):2183–2190. doi: 10.1128/iai.55.9.2183-2190.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Pellett S. Transcriptional organization of the Escherichia coli hemolysin genes. J Bacteriol. 1988 Apr;170(4):1622–1630. doi: 10.1128/jb.170.4.1622-1630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A. Pore-forming cytolysins of gram-negative bacteria. Mol Microbiol. 1991 Mar;5(3):521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Zalman L. S., Müller-Eberhard H. J. Comparison of channels formed by poly C9, C5b-8 and the membrane attack complex of complement. Mol Immunol. 1990 Jun;27(6):533–537. doi: 10.1016/0161-5890(90)90072-8. [DOI] [PubMed] [Google Scholar]
- de la Cruz F., Müller D., Ortiz J. M., Goebel W. Hemolysis determinant common to Escherichia coli hemolytic plasmids of different incompatibility groups. J Bacteriol. 1980 Aug;143(2):825–833. doi: 10.1128/jb.143.2.825-833.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz F., Zabala J. C., Ortiz J. M. Incompatibility among alpha-hemolytic plasmids studied after inactivation of the alpha-hemolysin gene by transposition of Tn802. Plasmid. 1979 Oct;2(4):507–519. doi: 10.1016/0147-619x(79)90050-7. [DOI] [PubMed] [Google Scholar]



