Abstract
Anthropoid primates are distinguished from other mammals by having relatively large primary visual cortices (V1) and complex facial expressions. We present a comparative test of the hypothesis that facial expression processing coevolved with the expansion of V1 in anthropoids. Previously published data were analysed using phylogenetic comparative methods. The results of our study suggest a pattern of correlated evolution linking social group size, facial motor control and cortical visual processing in catarrhines, but not platyrrhines. Catarrhines that live in relatively large social groups tended to have relatively large facial motor nuclei, and relatively large primary visual cortices. We conclude that catarrhine brains are adapted for producing and processing complex facial displays.
Keywords: brain evolution, facial motor nucleus, neocortex, face perception
1. Introduction
Anthropoid primates exhibit a variety of adaptations in the visual system for high acuity that distinguish them from other mammals [1,2]. Anthropoids are also distinguished from other mammals by their use of complex facial expressions to facilitate social interactions [3]. Taken together, these observations have led some researchers to suggest a role for facial expression processing in the evolution of anthropoid visual systems, especially cortical visual areas [4,5].
Because the primary visual cortex (V1) is the earliest stage of visual processing in the cerebral cortex, it is important for basic analyses of spatial frequency, orientation and colour. Notably, V1 is relatively large in anthropoids when compared with other mammals. This is true whether V1 is scaled against body size, brain size or the size of other brain regions [6–8]. These scaling features represent possible neural adaptations for visual information processing in anthropoids that might be relevant to discriminating facial displays. Indeed, previous studies have noted a positive correlation between relative V1 volume and group size in anthropoids, indicating a possible social explanation for V1 expansion [5,9].
Evolutionary links between V1 and facial expression may be approached by examining the facial motor nucleus. The facial nucleus is located in the brainstem and contains motoneurons that directly innervate the muscles of facial expression via cranial nerve VII. Thus, facial nucleus volume can be used as a proxy for facial motor control in comparative studies. A previous study examined the relationship between relative facial nucleus volume and social group size in primates, but did not find a statistically significant correlation [10]. However, the relationship between facial nucleus volume and social group size has yet to be examined in anthropoids separately from other primates.
Our study provides a comparative test of the hypothesis that anthropoid primary visual cortices are adapted for processing complex facial expressions. This hypothesis makes two main predictions: (i) species that live in larger groups should have greater facial motor control and (ii) species with greater facial motor control should have enhanced cortical visual processing capabilities. To test these predictions, we examined the relationship between relative facial nucleus volume and social group size in anthropoids. Then, we examined the relationship between facial nucleus volume and V1 volume after controlling for the size of the rest of the brain.
2. Material and methods
Brain component volumes and group size data for 23 non-human anthropoid species were taken from previously published sources [6,10–12]. We examined trait correlations using multiple regression analyses. Two sets of analyses were carried out: (i) we examined the relative volume of the facial nucleus in relation to group size; and (ii) we examined the volume of V1 in relation to facial nucleus volume after controlling for brain size. Separate regression models were generated for platyrrhines and catarrhines. We used medulla volume to adjust for size in analyses relating facial nucleus volume to group size [10]. The volume of the rest of the brain (i.e. total brain volume–(neocortex volume + facial nucleus volume)) was used to control for size in analyses relating V1 volume to facial nucleus volume [5]. All data were log-transformed (natural) prior to analysis. Regression coefficients and associated standard errors were generated using a phylogenetic generalized least-squares (PGLS) approach [13]. We used Compare 4.6b [14] to perform PGLS regressions, based on a Bayesian estimate of phylogeny and associated branch lengths downloaded from the 10k Trees website (http://www.10ktrees.fas.harvard.edu/).
3. Results
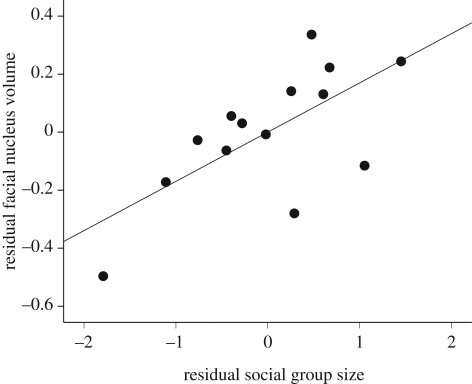
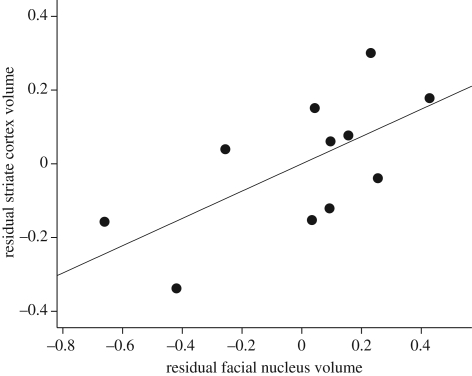
The amount of variance explained (R2) was greater than 90 per cent in all PGLS regression models (see electronic supplementary material). Medulla volume was strongly correlated with facial nucleus volume in catarrhines (b = 1.07; t11 = 11.89; p < 0.001) and platyrrhines (b = 0.93; t6 = 7.75; p < 0.001). Catarrhines exhibited a significant positive correlation (figure 1) between group size and facial nucleus volume after controlling for medulla volume (b = 0.17; t11 = 2.83; p = 0.016). However, this effect was absent from platyrrhines (b = −0.03; t6 = −0.30; p = 0.774). The size of the rest of the brain was significantly correlated with V1 volume in both platyrrhines (b = 0.73; t5 = 2.43; p = 0.059) and catarrhines (b = 0.40; t8 = 2.67; p = 0.028). Catarrhines exhibited a positive correlation (figure 2) between facial nucleus volume and V1 volume after controlling for the size of the rest the brain (b = 0.35; t8 = 2.33; p = 0.048). In contrast, facial nucleus volume and V1 volume were not correlated in platyrrhines after controlling for brain size (b = 0.11; t5 = 0.25; p = 0.813).
Figure 1.
Scatter plot depicting the positive correlation between facial nucleus volume and group size after controlling for medulla volume in catarrhines (n = 14). Values are unstandardized residuals from ordinary least squares (OLS) regression with medulla volume as the independent variable. Trend line is an OLS fit.
Figure 2.
Scatter plot depicting the positive correlation between striate cortex volume and facial nucleus volume after controlling for the rest of the brain in catarrhines (n = 11). Values are unstandardized residuals from ordinary least squares (OLS) regression with the volume of the rest of the brain as the independent variable. Trend line is an OLS fit.
4. Discussion
The results of our study suggest a pattern of correlated evolution linking group size, facial motor control and cortical visual processing in catarrhine primates. Species that live in relatively large social groups tended to have relatively large facial motor nuclei, and species with enlarged facial nuclei had relatively large primary visual cortices. These results mirror previous findings that species characterized by relatively large social groups also have enhanced facial mobility [15] and relatively large V1 volumes [5,9]. Thus, our results support the view that the primary visual cortex is adapted for processing facial signals in certain anthropoids [4]. Alternative explanations for these patterns are possible however. Furthermore, additional factors may be responsible for V1 expansion in anthropoids, including diet [5].
The idea that catarrhine brains are adapted for processing facial expressions is further suggested by the existence of functionally independent mechanisms for recognizing facial expressions versus facial identity. For example, some brain-damaged patients and autistic individuals have deficits in recognizing facial expressions even though they can easily identify individuals from their faces [16–18]. The opposite is true of patients with prosopagnosia, or ‘face blindness’ [19]. It has been demonstrated that several regions in the temporal lobe of macaques and humans are selectively activated when presented with images of facial displays [20].
Unlike catarrhines, platyrrhines do not exhibit signs of selection for facial expression processing. This implies a fundamental difference between infraorders with regard to facial expression. It has been suggested that platyrrhines in general have fewer and less-complex facial displays than catarrhines [21–23]. In particular, marmosets and tamarins (Callitrichinae) have been described as ‘poker-faced’ with regard to facial expression [21]. Similarly, the facial displays of squirrel monkeys (Saimiri sciureus) have been described as ‘subtle’ and ‘not sustained as in macaques’ [24]. On the other hand, some New World species do have complex facial displays [21]. Capuchins (genus Cebus), in particular, exhibit catarrhine-like patterns of facial expression [25]. It seems that while most catarrhines rely on facial displays to some degree, the same cannot be said for most platyrrhines, with the exception of a few taxa. Platyrrhines may rely more heavily on olfactory signals instead [26].
Taxonomic differences between catarrhines and platyrrhines may also exist in the organization of connections between regions of facial motor representation in the neocortex. Specifically, in Japanese macaques (Macaca fuscata), many neurons of the supplementary motor area (SMA) project to the orofacial region of primary motor cortex [27], while relatively few neurons in the SMA of owl monkeys (Aotus trivirgatus) make these projections [28]. Moreover, compared with the broad region of SMA that can evoke orofacial movements in macaques [29], electrical surface stimulation of the SMA in squirrel monkeys does not evoke orofacial movements [30]. Similarly, intracortical microstimulation of only a small region of SMA in owl monkeys evokes orofacial movement [31]. These data further support our conclusion that catarrhines, but not platyrrhines, have co-evolved visual and motor neural systems specifically relevant to the use of complex facial expressions.
References
- 1.Ross C. F. 2000. Into the light: the origin of Anthropoidea. Annu. Rev. Anthropol. 29, 147–194 10.1146/annurev.anthro.29.1.147 (doi:10.1146/annurev.anthro.29.1.147) [DOI] [Google Scholar]
- 2.Kirk E. C., Kay R. F. 2004. The evolution of high visual acuity in the Anthropoidea. In Anthropoid origins: new visions (eds Ross C. F., Kay R. F.), pp. 539–602 New York, NY: Kluwer Academic/Plenum Publishers [Google Scholar]
- 3.van Hooff J. A. R. A. M. 1967. The facial displays of the catarrhine monkeys and apes. In Primate ethology (ed. Morris D.), pp. 7–68 Chicago, IL: Aldine [Google Scholar]
- 4.Allman J. 1977. Evolution of the visual system in early primates. In Progress in psychobiology and physiological psychology (eds Sprague J. M., Epstein A. N.), pp. 1–53 New York, NY: Academic Press [Google Scholar]
- 5.Barton R. A. 1998. Visual specialization and brain evolution in primates. Proc. R. Soc. Lond. B 265, 1933–1937 10.1098/rspb.1998.0523 (doi:10.1098/rspb.1998.0523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frahm H. D., Stephan H., Baron G. 1984. Comparison of brain structure volumes in Insectivora and Primates. 5. Area striata (AS). J. Hirnforsch. 25, 537–557 [PubMed] [Google Scholar]
- 7.Bush E. C., Allman J. M. 2004. Three-dimensional structure and evolution of primate primary visual cortex. Anat. Rec. 281A, 1088–1094 10.1002/ar.a.20114 (doi:10.1002/ar.a.20114) [DOI] [PubMed] [Google Scholar]
- 8.Barton R. A. 2007. Evolutionary specialization in mammalian cortical structure. J. Evol. Biol. 20, 1504–1511 10.1111/j.1420-9101.2007.01330.x (doi:10.1111/j.1420-9101.2007.01330.x) [DOI] [PubMed] [Google Scholar]
- 9.Joffe T. H., Dunbar R. I. M. 1997. Visual and socio-cognitive information processing in primate brain evolution. Proc. R. Soc. Lond. B 264, 1303–1307 10.1098/rspb.1997.0180 (doi:10.1098/rspb.1997.0180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherwood C. C., Hof P. R., Holloway R. L., Semendeferi K., Gannon P. J., Frahm H. D., Zilles K. 2005. Evolution of the brainstem orofacial motor system in primates: a comparative study of trigeminal, facial, and hypoglossal nuclei. J. Hum. Evol. 48, 45–84 10.1146/annurev.anthro.29.1.147 (doi:10.1146/annurev.anthro.29.1.147) [DOI] [PubMed] [Google Scholar]
- 11.Nunn C. L., van Schaik C. P. 2001. A comparative approach to reconstructing the socioecology of extinct primates. In Reconstructing behavior in the fossil record (eds Plavcan J. M., Jungers W. L., Kay R. F., van Schaik C. P.), pp. 159–188 New York, NY: Kluwer Academic [Google Scholar]
- 12.de Sousa A. A., Sherwood C. C., Mohlberg H., Amunts K., Schleicher A., MacLeod C. E., Hof P. R., Frahm H., Zilles K. 2010. Hominoid visual brain structure volumes and the position of the lunate sulcus. J. Hum. Evol. 58, 281–292 10.1016/j.jhevol.2009.11.011 (doi:10.1016/j.jhevol.2009.11.011) [DOI] [PubMed] [Google Scholar]
- 13.Martins E. P., Hansen T. F. 1997. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667 10.1086/286013 (doi:10.1086/286013) [DOI] [Google Scholar]
- 14.Martins E. P. 2004. COMPARE, version 4.6b. Computer programs for the statistical analysis of comparative data. Bloomington, IN: Department of Biology, Indiana University; See http://compare.bio.indiana.edu/ [Google Scholar]
- 15.Dobson S. D. 2009. Socioecological correlates of facial mobility in nonhuman anthropoids. Am. J. Phys. Anthropol. 139, 413–420 10.1002/ajpa.21007 (doi:10.1002/ajpa.21007) [DOI] [PubMed] [Google Scholar]
- 16.Ellis H. D., Young A. W. 1998. Faces in the biological and social context. In Face and mind (ed. Young A. W.), pp. 67–95 New York, NY: Oxford University Press [Google Scholar]
- 17.Young A. W., Newcombe F., deHaan E. H. F., Small M. F., Hay D. C. 1998. Dissociable deficits after brain injury. In Face and mind (ed. Young A. W.), pp. 181–208 New York, NY: Oxford University Press [Google Scholar]
- 18.Celani G., Battacchi M. W., Arcidiacono L. 1999. The understanding of the emotional meaning of facial expressions in people with autism. J. Autism Dev. Disord. 29, 57–66 10.1023/A:1025970600181 (doi:10.1023/A:1025970600181) [DOI] [PubMed] [Google Scholar]
- 19.Damasio A. R., Tranel D., Damasio H. 1990. Face agnosia and the neural substrates of memory. Annu. Rev. Neurosci. 13, 89–109 10.1146/annurev.ne.13.030190.000513 (doi:10.1146/annurev.ne.13.030190.000513) [DOI] [PubMed] [Google Scholar]
- 20.Tsao D. Y., Livingstone M. S. 2008. Mechanisms of face perception. Ann. Rev. Neurosci. 31, 411–437 10.1146/annurev.neuro.30.051606.094238 (doi:10.1146/annurev.neuro.30.051606.094238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moynihan M. 1967. Comparative aspects of communication in New World primates. In Primate ethology (ed. Morris D.), pp. 236–266 Chicago, IL: Aldine [Google Scholar]
- 22.Eisenberg J. F. 1976. Communication mechanisms and social integration in the black spider monkey, Ateles fusciceps robustus, and related species. Smithson. Contrib. Zool. 213, 1–108 [Google Scholar]
- 23.Weigel R. M. 1979. The facial expressions of the brown capuchin monkey (Cebus apella). Behaviour 68, 250–276 10.1163/156853979X00331 (doi:10.1163/156853979X00331) [DOI] [Google Scholar]
- 24.Marriott B. M., Salzen E. A. 1978. Facial expressions in captive squirrel monkeys (Saimiri sciureus). Folia Primatol. 29, 1–18 10.1159/000155823 (doi:10.1159/000155823) [DOI] [PubMed] [Google Scholar]
- 25.De Marco A., Petit O., Visalberghi E. 2008. The repertoire and social function of facial displays in Cebus capucinus. Int. J. Primatol. 29, 469–486 10.1007/s10764-007-9174-0 (doi:10.1007/s10764-007-9174-0) [DOI] [Google Scholar]
- 26.Barton R. A. 2006. Olfactory evolution and behavioral ecology in primates. Am. J. Primatol. 68, 545–558 10.1002/ajp.20251 (doi:10.1002/ajp.20251) [DOI] [PubMed] [Google Scholar]
- 27.Tokuno H., Takada M., Nambu A., Inase M. 1997. Reevaluation of ipsilateral corticocortical inputs to the orofacial region of the primary motor cortex in the macaque monkey. J. Comp. Neurol. 389, 34–48 (doi:10.1002/(SICI)1096-9861(19971208)389:1<34::AID-CNE3>3.0.CO;2-F) [DOI] [PubMed] [Google Scholar]
- 28.Stepniewska I., Preuss T. M., Kaas J. H. 1993. Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (M1) of owl monkeys. J. Comp. Neurol. 330, 238–271 10.1002/cne.903300207 (doi:10.1002/cne.903300207) [DOI] [PubMed] [Google Scholar]
- 29.Godschalk M., Mitz A. R., van Duin B., van der Burg H. 1995. Somatotopy of monkey premotor cortex examined with microstimulation. Neurosci. Res. 23, 269–279 10.1016/0168-0102(95)00950-7 (doi:10.1016/0168-0102(95)00950-7) [DOI] [PubMed] [Google Scholar]
- 30.Welker W. I., Benjamin R. M., Miles R. C., Woolsey C. N. 1957. Motor effects of stimulation of cerebral cortex of squirrel monkey (Saimiri sciureus). J. Neurophysiol. 20, 347–364 [DOI] [PubMed] [Google Scholar]
- 31.Gould H. J. d., Cusick C. G., Pons T. P., Kaas J. H. 1986. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J. Comp. Neurol. 247, 297–325 10.1002/cne.902470303 (doi:10.1002/cne.902470303) [DOI] [PubMed] [Google Scholar]