Abstract
Semple et al. (Semple et al. in press, Biol. Lett. (doi:10.1098/rsbl.2009.1062)) argued that the ‘law of brevity’ (an inverse relationship between word length and frequency of use) applies not only to human language but also to vocal signalling in non-human primates, because coding efficiency is paramount in both situations. We analysed the frequency of use of signals of different duration in the vocal repertoires of two Neotropical primate species studied in the wild—the common marmoset (Callithrix jacchus) and the golden-backed uakari (Cacajao melanocephalus). The key prediction of the law of brevity was not supported in either species: although the most frequently emitted calls were relatively brief, they were not the shortest signals in the repertoire. The costs and benefits associated with signals of different duration must be appreciated to understand properly their frequency of use. Although relatively brief vocal signals may be favoured by natural selection in order to minimize energetic costs, the very briefest signals may be ambiguous, contain reduced information or be difficult to detect or locate, and may therefore be selected against. Analogies between human language and vocal communication in animals can be misleading as a basis for understanding frequency of use, because coding efficiency is not the only factor of importance in animal communication, and the costs and benefits associated with different signal durations will vary in a species-specific manner.
Keywords: law of brevity, Neotropical primates, vocal repertoire, signalling
1. Introduction
I strive to be brief, and I become obscure.
(Horace)
Are animal signals that are produced most frequently the briefest in duration? Such a prediction was made by Semple et al. [1] who argued that an inverse relationship between signal length and frequency of use is expected, given that long signals can be costly to produce, and because the frequent use of brief signals (as in human language) maximizes coding efficiency according to the ‘law of brevity’ [2].
We hypothesize that coding efficiency alone is insufficient to explain the relationship between frequency of use and signal duration in animal communication, and so tested whether the law of brevity is upheld in two species of Neotropical primates studied in the wild: the common marmoset (Callithrix jacchus) and the golden-backed uakari (Cacajao melanocephalus). Common marmosets are small and diurnal, live in groups of up to 15 individuals and usually have well-defined home ranges in the Atlantic rainforest of Brazil [3]. They have 13 different signals in their vocal repertoire, increasing to 17 if the different types of phee calls are considered as different signals [4]. Golden-backed uakaris are medium sized and diurnal, live in groups of up to 200 individuals, have a complex fission–fusion social organization and typically live in habitats with dense vegetation, such as the igapó and terra firme forests in the Brazilian and Colombian Amazon [5,6]. They have nine call types in their vocal repertoire [7].
2. Material and methods
For the vocal repertoire of common marmosets [4] and golden-backed uakaris [7], we related call duration to frequency of use, and describe call contexts in electronic supplementary material, tables S1 and S2. Animals from three social groups contributed to the final sample size in both species. Data for call use in common marmosets came from 493 focal observations (5 min duration each) distributed unevenly across 17 adults during 338 h of fieldwork between December 2004 and April 2005. Vocalizations are readily distinguishable (see spectrograms in electronic supplementary material, figure S1) and were categorized by BMB on a Panasonic RN-305 dictaphone. Data for golden-backed uakaris came from spectrogram analysis of 850 min of sound recordings made during ad libitum observation of the animals in 575 h of fieldwork in January–June 2008.
Our analyses only included data from adults; therefore, we used 12 out of 17 call types for common marmosets (predator-specific alarm calls were not included owing to their very infrequent use; only heard four times), and seven out of nine vocalizations in the repertoire of golden-backed uakaris (the ‘áhh’ call [7] was only heard regularly from a captive individual). To investigate the relationship between ‘signal duration’ and ‘frequency of signal use’ we first performed Spearman's rank correlations (rs) as the data were not normally distributed and we wanted to make our analyses comparable with that of Semple et al. [1]. Tests were two-tailed and conducted in SPSS for Windows v. 15 (SPSS Inc., Chicago, IL, USA).
For marmosets, we also controlled for repeated measures from the same individual by conducting a mixed model, which allows the inclusion of both random and fixed terms (see the electronic supplementary material for details); calls could not be assigned to individuals for the uakaris, so a similar analysis was not possible. To assess whether call duration influences frequency of use, we used a linear mixed model (LMM) based on a total of 1046 calls of 12 different types from 10 individuals (each were observed in more than 20 focal observation sessions; total of 417 sessions). Call types were ranked according to the mean duration (figure 1) and frequency of use was calculated as the proportion of all calls given by a particular individual. To control for variation in the number of calls recorded from different individuals, we included the total number of calls as a term in the model. The LMM was conducted in GenStat (10th edn, Lawes Agricultural Trust, Rothamsted, Harpenden, UK).
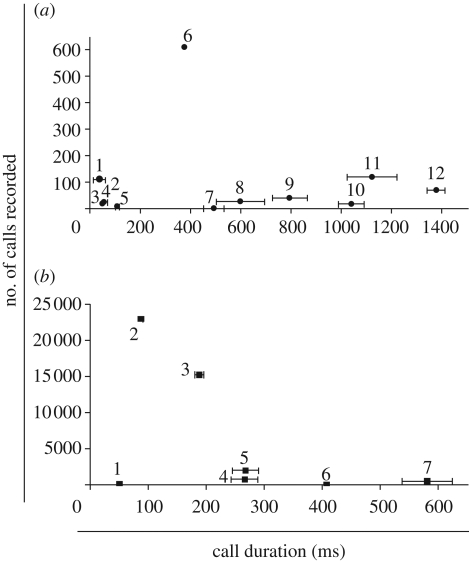
Figure 1.
Relationship between signal duration (mean ± s.e.m. for the population) and frequency of use of call type (summed for all individuals) in the vocal repertoire of adult (a) common marmosets and (b) golden-backed uakaris. For the common marmosets, data on frequency of use of the different call types came from focal observations of 17 individually identified and habituated adults (eight males and nine females; focal observations were not evenly distributed across individuals: mean number of sessions ± s.e.m. = 29 ± 6.14; range 4–102 focal sessions). For the golden-backed uakaris, data on frequency of use of the different call types came from three social groups (ranging from 5 to 26 individuals), but there was no individual identification of the adults. Numbers refer to the different call types defined in electronic supplementary material, tables S1 and S2).
3. Results
Call duration and frequency of use were not significantly correlated with each other in either common marmosets (Spearman's rank correlation: rs = 0.056, n = 12, p = 0.863) or golden-backed uakaris (rs = −0.357, n = 7, p = 0.432; figure 1). Rather, the distribution of frequency of use was skewed strongly to relatively short signals, with under-representation of the very briefest signals. Indeed, in the marmosets, there were five call types of shorter duration than the most frequently emitted call. If the outlying call type 6 (‘trill’) is removed from the common marmoset analysis, the relationship between duration and frequency of use is still virtually flat (rs = 0.091, n = 11, p = 0.790).
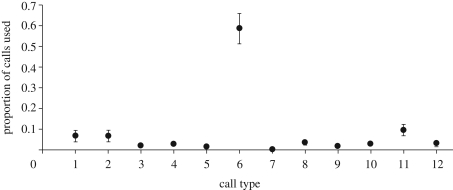
The data shown by Semple et al. [1] and in figure 1 here are potentially influenced by pseudoreplication—if, for example, one aberrant individual contributed most calls to the dataset, spurious conclusions may be reached. However, a mixed model controlling for repeated measures from the same common marmoset individuals also demonstrated no significant relationship between call duration and frequency of use (LMM: Wald statistic = 0.17, d.f. = 1, p = 0.681; figure 2 and electronic supplementary material, table S3).
Figure 2.
Relationship between ranked signal duration (1 is shortest, 12 longest) and proportional frequency of use (mean ± s.e.m. for 10 common marmosets where calls could be assigned to individuals). Mean number of sessions ± s.e.m. = 41.7 ± 8.29; range = 21–102 focal sessions.
In the marmosets, the longest calls were emitted by distant individuals isolated from their social group and the shortest calls were associated with vigilance behaviour (electronic supplementary material, table S1). In the uakaris, the longest duration calls were again associated with long-distance communication, while the shortest calls were emitted during play (electronic supplementary material, table S2).
4. Discussion
Our results indicate that the vocal repertoires of adult common marmosets and golden-backed uakaris do not follow the pattern predicted by the law of brevity [2], unlike the situation in Formosan macaques (Macaca cyclopis) [1]. For the latter, an inverse relationship existed between signal duration and frequency of use [1]. However, even in Semple et al.'s analysis, the macaques used the two briefest signals less frequently than the third briefest signal.
That the briefest signals are not the most frequently used is perhaps not surprising, given the different costs and benefits associated with signals of different durations. Very short calls may contain limited information, such as cues that identify the signaller. Although long calls may be costly to produce, they may nonetheless be honest signals of quality and hence will be favoured by sexual selection in some cases [8]. Signal duration will also depend on factors such as home-range size: for example, animals that defend large territories tend to use long duration calls to advertise presence and location of signallers [9].
In marmosets, the shortest signals are associated with vigilance (e.g. at territorial boundaries, when crossing between forest patches, when encountering novel observers), where rapid and clear signalling of potential danger is paramount. Vigilance behaviour is relatively uncommon, however, and so these signals are produced less frequently than other signals used in close-range contact communication among members of a social group. In the latter case, there is perhaps a greater need for encoding details about signaller identity and context, so there is selection for longer signals. In both species, the longest signals were produced for long-range communication, perhaps to maximize the likelihood of the signaller being detected.
Frequency of use will also depend on other factors that affect the costs of calling. For example, call amplitude—if brief, high-amplitude calls are more energetically expensive to produce, they may be selected against in comparison with longer duration signals of lower amplitude. Moreover, costs associated with increased conspicuousness to predators or prey [1] are only applicable in certain species. In both our study species (and indeed in the Formosan macaques; [1]), relatively brief signals were the most frequently used, perhaps suggesting that brevity is favoured to a certain extent, but that costs associated with very brief signals limit their use in vocal repertoires (i.e. there is stabilizing selection acting on signal duration).
Analogies between frequency of use in human language and animal communication may be misleading. Language involves extensive use of syntax whereby a large vocabulary can be arranged into sentences that convey a vast number of meanings to convey complex messages. Although Campbell's monkeys (Cercopithecus campbelli) use ‘proto-syntax’ by producing combinatorial signal sequences associated with specific meanings [10], the extent to which syntax is used is very limited in non-human animals. Language can convey complex messages of a virtually unlimited variety often about abstract situations via combinations of words, and the use of brief components in a vocabulary can be an efficient means of transmitting information in syntax-rich language.
Although human vocabularies can be very large, speech is broken up into a relatively small number of brief phonemes [11,12] that facilitate the learning of a vocabulary, and here brevity may be important. Brevity is likely to be vital in memorizing the components of a large vocabulary, especially given the important role of vocal imitation in human language [12]. Brevity brings benefits to both signallers and receivers in language [13]. Conversely, vocalizations of non-human animals must be subject to a wider range of constraints, and single signals might need to convey accurate information about situation-specific contexts regarding, for example, risk, urgency, referential specificity and signaller identity [14]. Such information is not necessarily best encoded in brief signals, and coding efficiency alone is insufficient to explain frequency of use in animal vocal communication. Semple et al. [1] encouraged a wider range of studies to test the generality of the law of brevity. Our study of two additional species does not support the hypothesis that the law of brevity is widely applicable in animal communication—we argue that relationships between signal duration and frequency of use are best considered on a species-specific basis, and must take account of the costs and benefits of different signal durations in a wide range of contexts.
Acknowledgements
The studies were non-invasive and complied with Brazilian law.
The Brazilian Council of Technological and Scientific Development (CNPq) supported B.M.B. (grant no. 130770/2005) during the marmoset study. Throughout the uakari study, Bezerra was supported by Programme Alban (the European Union Programme of High Level Scholarships for Latin America); ORS award (Overseas Research Students Award Scheme); University of Bristol; a Rufford Small Conservation Grant; an IDEA WILD grant, Amazon Ecopark Lodge and Living RainForest Foundation.
References
- 1.Semple S., Hsu M. J., Agoramoorthy G. In press Efficiency of coding in macaque vocal communication. Biol. Lett. 6, 469–471 10.1098/rsbl.2009.1062 (doi:10.1098/rsbl.2009.1062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zipf G. 1936. The psycho-biology of language: an introduction to dynamic philology. London, UK: George Routledge and Sons Ltd [Google Scholar]
- 3.Hershkovitz P. 1977. Living new world monkeys (Platyrrhini): with an introduction to primates, vol. 1 Chicago, IL: The University of Chicago Press [Google Scholar]
- 4.Bezerra B. M., Souto A. S. 2008. The structure and usage of the vocal repertoire of common marmosets. Int. J. Primatol. 29, 671–701 10.1007/s10764-008-9250-0 (doi:10.1007/s10764-008-9250-0) [DOI] [Google Scholar]
- 5.Defler T. R. 2003. Primates de Colombia. Bogotá, Colombia: Panamericana Formas e Impresos S.A [Google Scholar]
- 6.Bezerra B. M., Barnett A., Souto A. S., Jones G. In press Ethogram and natural history of golden-backed uakaris, Cacajao melanocephalus. Int. J. Primatol. [Google Scholar]
- 7.Bezerra B. M., Souto A. S., Jones G. In press Vocal repertoire of golden-backed uakaris (Cacajao melanocephalus): call structure and context. Int. J. Primatol. [DOI] [PubMed] [Google Scholar]
- 8.Maynard Smith J., Harper D. 2003. Animal signals. Oxford, NY: Oxford University Press [Google Scholar]
- 9.Bradbury J. W., Vehrencamp S. L. 1998. Principles of animal communication. Sunderland, MA: Sinauer [Google Scholar]
- 10.Ouattara K., Lemasson A., Zuberbühler K. 2009. Campbell's monkeys concatenate vocalizations into context-specific call sequences. Proc. Natl Acad. Sci. USA 106, 22 026–22 031 10.1073/pnas.0908118106 (doi:10.1073/pnas.0908118106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomsky N., Halle M. 1968. The sound pattern of English. New York, NY: Harper and Row [Google Scholar]
- 12.Hauser M. D., Chomsky N., Fitch W. T. 2002. The faculty of language: what is it, who has it, and how did it evolve? Science 298, 1569–1579 10.1126/science.298.5598.1569 (doi:10.1126/science.298.5598.1569) [DOI] [PubMed] [Google Scholar]
- 13.Ferrer i Cancho R., Solé R. V. 2003. Least effort and the origins of scaling in human language. Proc. Natl Acad. Sci. USA 100, 788–791 10.1073/pnas.0335980100 (doi:10.1073/pnas.0335980100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seyfarth R. M., Cheney D. L. 2003. Signalers and receivers in animal communication. Annu. Rev. Psychol. 54, 145–173 10.1146/annurev.psych.54.101601.145121 (doi:10.1146/annurev.psych.54.101601.145121) [DOI] [PubMed] [Google Scholar]