Abstract
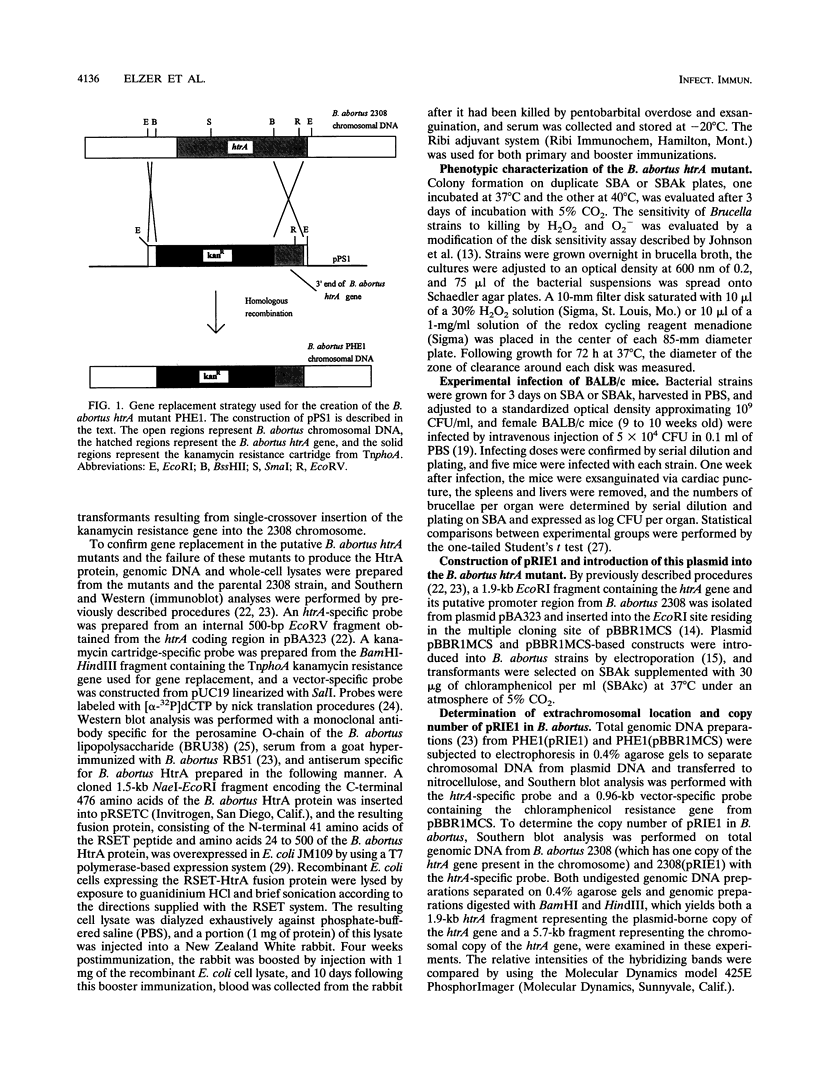
In order to evaluate the biological function of the Brucella abortus high-temperature-requirement A (HtrA) stress response protein homolog, the majority of the htrA gene was deleted from the chromosome of B. abortus 2308 via gene replacement. In contrast to the parental strain, the resulting htrA deletion mutant, designated PHE1, failed to grow on solid medium at 40 degrees C and demonstrated increased sensitivity to killing by H2O2 and O2- in disk sensitivity assays. BALB/c mice were infected with strains 2308 and PHE1 to assess the effect of the htrA mutation on virulence, and significantly fewer brucellae were recovered from the spleens of mice infected with PHE1 than from those of mice infected with 2308 at 1 week postinfection. Genetic complementation studies were performed to confirm the relationship between the htrA mutation and the phenotype observed for PHE1. Plasmid pRIE1 was constructed by inserting a 1.9-kb EcoRI fragment encoding the B. abortus htrA gene into the broad-host-range plasmid pBBR1MCS. Introduction of pRIE1 into PHE1 relieved the temperature- and H2O2-sensitive phenotypes of this mutant in vitro, and PHE1(pRIE1) colonized the spleens of BALB/c mice at levels equivalent to those of the parental 2308 strain at 1 week postinfection. These results support our previous proposal that the B. abortus htrA gene product functions as a stress response protein and further suggest that this protein contributes to virulence. These studies also demonstrate the utility of the broad-host-range plasmid pBBR1MCS for genetic complementation studies in Brucella spp., establishing a key reagent for more detailed genetic analysis of this important zoonotic pathogen.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu Kwaik Y., Eisenstein B. I., Engleberg N. C. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect Immun. 1993 Apr;61(4):1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin C. L., Winter A. J. Macrophages and Brucella. Immunol Ser. 1994;60:363–380. [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Bäumler A. J., Kusters J. G., Stojiljkovic I., Heffron F. Salmonella typhimurium loci involved in survival within macrophages. Infect Immun. 1994 May;62(5):1623–1630. doi: 10.1128/iai.62.5.1623-1630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield S. N., Strahan K., Pickard D., Charles I. G., Hormaeche C. E., Dougan G. Evaluation of Salmonella typhimurium strains harbouring defined mutations in htrA and aroA in the murine salmonellosis model. Microb Pathog. 1992 Feb;12(2):145–151. doi: 10.1016/0882-4010(92)90117-7. [DOI] [PubMed] [Google Scholar]
- Davies K. J., Lin S. W. Degradation of oxidatively denatured proteins in Escherichia coli. Free Radic Biol Med. 1988;5(4):215–223. doi: 10.1016/0891-5849(88)90015-9. [DOI] [PubMed] [Google Scholar]
- Davies K. J., Lin S. W. Oxidatively denatured proteins are degraded by an ATP-independent proteolytic pathway in Escherichia coli. Free Radic Biol Med. 1988;5(4):225–236. doi: 10.1016/0891-5849(88)90016-0. [DOI] [PubMed] [Google Scholar]
- Halling S. M., Detilleux P. G., Tatum F. M., Judge B. A., Mayfield J. E. Deletion of the BCSP31 gene of Brucella abortus by replacement. Infect Immun. 1991 Nov;59(11):3863–3868. doi: 10.1128/iai.59.11.3863-3868.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Leonard B., Benson R., Baldwin C. L. Macrophage control of Brucella abortus: role of reactive oxygen intermediates and nitric oxide. Cell Immunol. 1993 Oct 15;151(2):309–319. doi: 10.1006/cimm.1993.1241. [DOI] [PubMed] [Google Scholar]
- Johnson K., Charles I., Dougan G., Pickard D., O'Gaora P., Costa G., Ali T., Miller I., Hormaeche C. The role of a stress-response protein in Salmonella typhimurium virulence. Mol Microbiol. 1991 Feb;5(2):401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- Kovach M. E., Phillips R. W., Elzer P. H., Roop R. M., 2nd, Peterson K. M. pBBR1MCS: a broad-host-range cloning vector. Biotechniques. 1994 May;16(5):800–802. [PubMed] [Google Scholar]
- Lai F., Schurig G. G., Boyle S. M. Electroporation of a suicide plasmid bearing a transposon into Brucella abortus. Microb Pathog. 1990 Nov;9(5):363–368. doi: 10.1016/0882-4010(90)90070-7. [DOI] [PubMed] [Google Scholar]
- Lipinska B., Fayet O., Baird L., Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989 Mar;171(3):1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller I., Maskell D., Hormaeche C., Johnson K., Pickard D., Dougan G. Isolation of orally attenuated Salmonella typhimurium following TnphoA mutagenesis. Infect Immun. 1989 Sep;57(9):2758–2763. doi: 10.1128/iai.57.9.2758-2763.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect Immun. 1986 Aug;53(2):245–251. doi: 10.1128/iai.53.2.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby C. E., Fraser A. D. Plasmid transfer and plasmid-mediated genetic exchange in Brucella abortus. Can J Vet Res. 1989 Jul;53(3):326–330. [PMC free article] [PubMed] [Google Scholar]
- Roop R. M., 2nd, Fletcher T. W., Sriranganathan N. M., Boyle S. M., Schurig G. G. Identification of an immunoreactive Brucella abortus HtrA stress response protein homolog. Infect Immun. 1994 Mar;62(3):1000–1007. doi: 10.1128/iai.62.3.1000-1007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop R. M., 2nd, Price M. L., Dunn B. E., Boyle S. M., Sriranganathan N., Schurig G. G. Molecular cloning and nucleotide sequence analysis of the gene encoding the immunoreactive Brucella abortus Hsp60 protein, BA60K. Microb Pathog. 1992 Jan;12(1):47–62. doi: 10.1016/0882-4010(92)90065-v. [DOI] [PubMed] [Google Scholar]
- Schurig G. G., Hammerberg C., Finkler B. R. Monoclonal antibodies to Brucella surface antigens associated with the smooth lipopolysaccharide complex. Am J Vet Res. 1984 May;45(5):967–971. [PubMed] [Google Scholar]
- Seol J. H., Woo S. K., Jung E. M., Yoo S. J., Lee C. S., Kim K. J., Tanaka K., Ichihara A., Ha D. B., Chung C. H. Protease Do is essential for survival of Escherichia coli at high temperatures: its identity with the htrA gene product. Biochem Biophys Res Commun. 1991 Apr 30;176(2):730–736. doi: 10.1016/s0006-291x(05)80245-1. [DOI] [PubMed] [Google Scholar]
- Strauch K. L., Johnson K., Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989 May;171(5):2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Verger J. M., Grayon M., Chaslus-Dancla E., Meurisse M., Lafont J. P. Conjugative transfer and in vitro/in vivo stability of the broad-host-range IncP R751 plasmid in Brucella spp. Plasmid. 1993 Mar;29(2):142–146. doi: 10.1006/plas.1993.1016. [DOI] [PubMed] [Google Scholar]




