Abstract
In social insects, group behaviour can increase disease resistance among nest-mates and generate social prophylaxis. Stomodeal trophallaxis, or mutual feeding through regurgitation, may boost colony-level immunocompetence. We provide evidence for increased trophallactic behaviour among immunized workers of the carpenter ant Camponotus pennsylvanicus, which, together with increased antimicrobial activity of the regurgitate droplet, help explain the improved survival of droplet recipient ants relative to controls following an immune challenge. We have identified a protein related to cathepsin D, a lysosomal protease, as a potential contributor to the antimicrobial activity. The combined behavioural and immunological responses to infection in these ants probably represent an effective mechanism underlying the social facilitation of disease resistance, which could potentially produce socially mediated colony-wide prophylaxis. The externalization and sharing of an individual's immune responses via trophallaxis could be an important component of social immunity, allowing insect colonies to thrive under high pathogenic pressures.
Keywords: social immunity, trophallaxis, prophylaxis, disease resistance
1. Introduction
Social insects, because they exploit microbe-rich environments and live in densely populated colonies of highly related individuals, are especially susceptible to infection and rapid disease transmission [1]. These insects have evolved a variety of adaptations to cope with pathogenic pressures, including behavioural, biochemical and immunological responses [2,3]. While individual immune responses involve cellular immunity and the humoral production of effector molecules, group behaviour in social insects can also prevent infection and disease transmission [4,5] and achieve adaptive social immunity [6].
The social facilitation of disease resistance in termites and ants [7,8] was attributed to the transfer of immune factors or elicitors from exposed individuals to naive nest-mates during grooming or mutual feeding, resulting in social prophylaxis. The mechanisms underlying this phenomenon were not determined, however. We investigated one mechanism through which the transfer of immune factors could occur. Stomodeal trophallaxis, i.e. the sharing of liquid nutrients through mutual feeding, is common in social insects. Nutrients stored in the crop, or ‘social stomach’, are transferred throughout a colony during trophallaxis [9], along with hydrocarbons important in nest-mate recognition [10]. In this study, we examine the effects of an immune challenge on trophallactic behaviour, the antimicrobial activity of the regurgitate droplet and disease resistance in the ant Camponotus pennsylvanicus. This species exhibits frequent trophallaxis and lacks a metapleural gland [11], an important source of antimicrobial compounds in most ants [3]. We propose that the externalization and sharing of an individual's immunological factors through behavioural responses to infection are important in achieving social immunity in insects.
2. Material and methods
(a). Ant maintenance and injection treatments
Mature colonies of C. pennsylvanicus were collected from Billerica and Newton, MA, in August 2008 and 2009 and maintained in the laboratory using standard methods [9]. Purified lipopolysaccharide (LPS, Sigma), an immunogenic component of Gram-negative bacteria [12], and Serratia marcescens, a Gram-negative entomopathogen [1], were used to stimulate immune responses. Serratia marcescens grown in tryptic soy broth to 108 cells ml−1 were washed and diluted in sterile Ringer solution, and 1 ml aliquots were heat-killed in boiling water for 10 min. For behavioural, antimicrobial and protein analyses, median workers were randomly divided into four treatments: (i) naive: cold-immobilized only; (ii) Ringer: injected with sterile Ringer solution; (iii) immunized: injected with 108 cells ml−1 of heat-killed S. marcescens; and (iv) LPS: injected with 5 mg ml−1 of LPS in sterile Ringer. Workers were injected with 0.5 µl according to treatment as described by de Souza et al. [13]. Ants used for behavioural observations were immediately isolated, while ants used for antimicrobial activity and protein analyses were placed in respective treatment groups of 30. All ants were provided with 10 per cent sucrose.
(b). Behavioural observations
Dyadic encounters between workers were observed following the protocols of de Souza et al. [13]. Treatment pairings included: naive/naive (N/N); naive/Ringer (N/R); naive/immunized (N/I); naive/LPS (N/L); Ringer/Ringer (R/R); immunized/immunized (I/I); and LPS/LPS (L/L). Three replicates from four colonies were observed for each pairing. After 24 h of isolation, pairs were placed in test tubes (16 × 100 mm) stoppered with cotton. Three minutes post-pairing, the presence or absence of trophallaxis was recorded every 30 s for 30 min. To determine whether treated ants acted as donors during trophallaxis, three additional replicates per colony for N/R, N/I and N/L dyads were established with individuals marked with nail polish according to treatment.
(c). Antimicrobial activity and protein analyses
Two days post-treatment, light pressure was applied to the gaster, and 1 µl of the resulting regurgitate per ant was used for antimicrobial activity and protein analyses (electronic supplementary material, Note 1 and figure S1). A zone-of-inhibition assay adapted from Moret & Schmid-Hempel [12] was used to measure relative antimicrobial activity of droplets. Samples from four colonies were diluted in 1 μl of sodium cacodilate/CaCl2. Samples from naive (n = 37), Ringer (n = 38), immunized (n = 30) and LPS ants (n = 40) were analysed using tryptic soy agar plates (5% agar) seeded with 105 cells ml−1 of Arthrobacter VS10. SDS–PAGE of droplets from each treatment was performed using standard methods (Bio-Rad). An enhanced protein (approx. 35 kD) in samples from immunized and LPS-injected ants was selected as a possible antibacterial protein, and was blotted onto polyvinylidene difluoride membranes (Bio-Rad) and N-terminally sequenced at the Tufts University Core Facility, Boston, MA.
(d). In vivo survivorship
To test whether trophallaxis plays a role in social prophylaxis, 45 workers per replicate (two replicates per colony) from seven colonies were colour coded as donors (n = 30) and recipients (n = 15) of trophallactic exchanges. Donors were fed food and water while recipients received water only for 3 days to promote trophallactic exchanges. Subsequently, donor ants were separated into Ringer and immunized treatments as described. Five hungry recipients were placed with 10 normally fed donor nest-mates in dishes lined with moist filter paper, and allowed to interact for 3 days without food to induce trophallaxis. All ants were then pricked between the second and third tergites with sterile pulled capillaries dipped in active 5 × 103 cells ml−1 of S. marcescens, separated into groups according to treatment and donor/recipient and then provided food and water ad libitum. Survival of all ants (n = 495) was tracked for 21 days post-challenge. Dead ants were surface-sterilized and placed on tryptic soy agar to confirm death by S. marcescens (red bacterial growth).
(e). Statistical analysis
Data were analysed in SPSS 17. Behavioural and antimicrobial data were angular and logarithmically transformed, respectively, to improve normality, then fitted to general linear mixed models (treatment = fixed effect, colony = random effect), followed by Tukey's HSD for multiple comparisons. Trophallactic donor frequencies were analysed with a χ2-test. In vivo data were tested with a Cox proportional regression.
3. Results
(a). Trophallactic behaviour
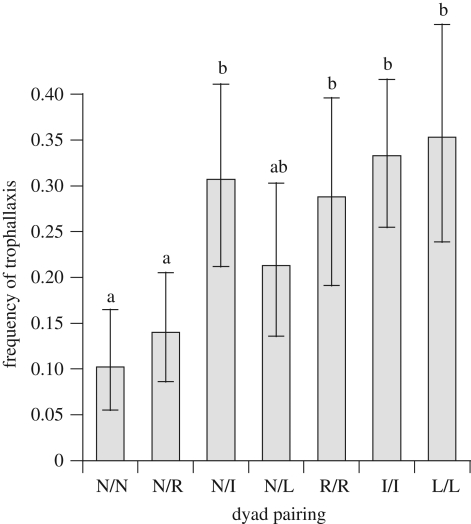
The frequency of trophallaxis differed significantly among dyad treatments (F6,18 = 4.802, p = 0.004), with significant increases in the N/I, R/R, I/I and L/L pairs relative to N/N pairs (p < 0.05, figure 1). There was a significant interaction effect between colony and dyad paring (F18,56 = 2.005, p = 0.025), and colony effect was borderline significant (F3,18 = 3.106, p = 0.052). Colonies showed similar trends of increased trophallaxis in dyads with at least one injected ant relative to N/N dyads (electronic supplementary material, figure S2). Individuals from the immunized, LPS and Ringer treatments acted disproportionately as donors to naive nest-mates during trophallaxis in 73.5 (n = 68), 72.6 (n = 73) and 64.3 per cent (n = 84) of observed encounters, respectively (χ2 = 25.3, d.f. = 2, p < 0.001), relative to a 50 per cent expected frequency.
Figure 1.
Back-transformed mean frequency of trophallactic exchanges and 95% CI pooled from four colonies after 24 h of isolation. Pairs (three per colony for each pairing) include naive workers (N), workers injected with Ringer solution (R), workers injected with heat-killed S. marcescens (I) and workers injected with LPS (L). Different letters indicate significant pairwise differences (Tukey's HSD, p < 0.05).
(b). Antimicrobial activity and protein content
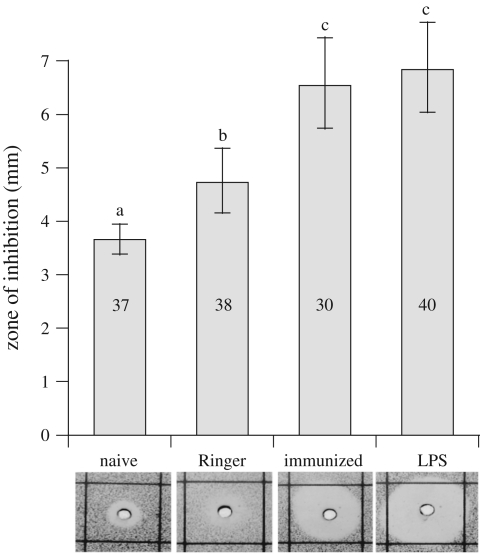
Antimicrobial activity of regurgitate droplets differed across treatments (F3,9 = 4.762, p = 0.029), with significant and progressive increases in Ringer (p = 0.001), immunized and LPS treatments (p < 0.001) relative to the naive treatment (figure 2). There was a significant interaction effect between colony and treatment (F9,129 = 9.797, p < 0.001), but colony effect was not independently significant (F3,9 = 0.351, p = 0.790). Colonies showed similar trends of increased antimicrobial activity in immune-challenged individuals relative to naive ants (electronic supplementary material, figure S3).
Figure 2.
Back-transformed antibacterial activity (mean diameter of zone of inhibition) of droplets and 95% CI for naive, Ringer, immunized and LPS individuals pooled from four colonies. Different letters indicate significant pairwise differences (Tukey's HSD, p < 0.05), and numbers indicate sample size. Example zones of inhibition are shown below corresponding treatments.
SDS–PAGE and band densitometry analysis in ImageJ [14] (http://rsb.info.nih.gov/ij/) revealed enhanced protein bands (relative to total sample protein) approximately 35 kD in droplets from immunized and LPS treatments compared with naive and Ringer treatments (electronic supplementary material, figures S4 and S5). Edman degradation revealed the N-terminal amino acid sequence to be: aeqgseplsnyydaqyyggi.
Cathepsin D from the mosquito Aedes aegypti is the closest sequence match (BLAST E-value = 0.003).
(c). In vivo survivorship
Ants receiving regurgitate droplets from immunized nest-mates exhibited the highest survival following a challenge relative to all other treatments. After controlling for the effect of colony of origin, which was a significant and independent predictor of worker mortality (Wald = 39.2, d.f. = 6, p < 0.001), the relative hazard ratios of death were 2.1 (for the control donor treatment, Wald = 9, d.f. = 1, p = 0.003), 1.8 (for the control recipient treatment, Wald = 4.7, d.f. = 1, p = 0.03) and 1.6 times (for the immunized donor treatment, Wald = 3.3, d.f. = 1, p = 0.08) higher than ants receiving regurgitate droplets from vaccinated nest-mates (electronic supplementary material, figure S6). Greater than 99 per cent of dead ants confirmed for infection by S. marcescens.
4. Discussion
In immune-challenged C. pennsylvanicus, increased trophallactic behaviour is coupled with increased antimicrobial activity of the donated regurgitate droplet. Although a similar induction of trophallactic exchanges was reported in Camponotus fellah [13], no connection between trophallaxis and the social spread of disease resistance was tested.
Collectively, our results strongly support the hypothesis that trophallaxis in C. pennsylvanicus is one mechanism by which social immunization and enhancement of disease resistance at the colony level may be achieved. Individual-level immune responses (i.e. upregulated effector molecules) can be externalized and redistributed throughout a colony via social interactions, thus reducing disease transmission and widespread infection. Unlike previous studies describing social immunity [5], we show the sharing of a physiological immune response among individuals facilitating enhanced disease resistance. Trophallactic exchanges from reproductives and/or workers to brood may also contribute to trans-generational immune priming in social insects [15]. Few studies have investigated the externalization of such immune factors in social insects [4]. Bulmer et al. [16] suggested that effector molecules incorporated in termite nest material may break down pathogens, releasing elicitors that prime colony immune defences. Similarly, the transfer of immune factors during stomodeal or proctodeal trophallaxis may be critical in coping with disease across the social insects (R. B. Rosengaus 2010, unpublished data).
The nature and mode of action of the immune-elicitors and/or antibacterial factors being transferred remain unclear. It is possible that increased antimicrobial activity may break down pathogens, releasing immune elicitors that could then be transported from the gut lumen to the haemolymph upon ingestion, priming the recipient's systemic innate immunity, an occurrence reported in insects and mammals [17,18]. Constitutive antimicrobial factors in the crop probably prevent microbial growth in the stored nutrients. The upregulation of such factors during an immune response could avert further growth of pathogens, reducing the risk of disease transmission during trophallaxis. The enhanced protein in C. pennsylvanicus is related to cathepsin D, a lysosomal aspartic protease involved in a range of functions in insects and vertebrates [19–21] that can exhibit antibacterial effector activity [22] and the proteolytic production of antimicrobial peptides. This protein could potentially be important in immune defences in C. pennsylvanicus by any one of these mechanisms. Current research testing whether cathepsin D contributes to the antibacterial activity observed in this study is underway.
Research focusing on the processes of molecular immune defences within the ecological contexts of ants and other social insects is vital to understanding the mechanisms and evolution of social insect immune responses [2,3]. This study indicates a link between behavioural interactions and molecular defences that have important implications for social immunity. Our results suggest an additional and novel role for trophallaxis in facilitating social prophylaxis and ultimately reducing colony-wide disease susceptibility.
Social insects are remarkably successful and ecologically dominant taxa in spite of the intense pathogenic pressures they encounter [1,2]. The evolutionary linkage between behavioural and immunological protection against disease could result in colony-wide immune competence, and may have contributed greatly to the success of social insects.
Acknowledgements
We thank Drs M. S. Bulmer, E. Gavrish, V. G. Godoy and T. R. Hartke for assistance with this study. This research was funded by an NSF CAREER award (DEB 0447316) to R.B.R. and a Junior Senior Honors Grant from the Northeastern University Honors Program to C.H.
References
- 1.Schmid-Hempel P. 1998. Parasites in social insects. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Rosengaus R. B., Traniello J. F. A., Bulmer M. In press Ecology, behavior and evolution of disease resistance in termites. In Termites: evolution, sociality, symbioses, ecology (eds Bignell D., Roisin Y.). New York, NY: Springer. [Google Scholar]
- 3.Schlüns H., Crozier R. H. 2009. Molecular and chemical immune defenses in ants (Hymentoptera: Formicidae). Myrmecol. News 12, 237–249 [Google Scholar]
- 4.Cremer S., Sixt M. 2009. Analogies in the evolution of individual and social immunity. Phil. Trans. R. Soc. B 364, 129–142 10.1098/rstb.2008.0166 (doi:10.1098/rstb.2008.0166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cremer S., Armitage S. A. O., Schmid-Hempel P. 2007. Social immunity. Curr. Biol. 17, R693–R702 10.1016/j.cub.2007.06.008 (doi:10.1016/j.cub.2007.06.008) [DOI] [PubMed] [Google Scholar]
- 6.Walker T., Hughes W. 2009. Adaptive social immunity in leaf-cutting ants. Biol. Lett. 5, 446–448 10.1098/rsbl.2009.0107 (doi:10.1098/rsbl.2009.0107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Traniello J. F. A., Rosengaus R. B., Savoie K. 2002. The development of immunity in a social insect: evidence for the group facilitation of disease resistance. Proc. Natl Acad. Sci. USA 99, 6838–6842 10.1073/pnas.102176599 (doi:10.1073/pnas.102176599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ugelvig L. V., Cremer S. 2007. Social prophylaxis: group interaction promotes collective immunity in ant colonies. Curr. Biol. 17, 1–5 10.1016/j.cub.2007.10.029 (doi:10.1016/j.cub.2007.10.029) [DOI] [PubMed] [Google Scholar]
- 9.Hölldobler B., Wilson E. O. 1990. The ants. Cambridge, MA: Belknap Press of Harvard University Press [Google Scholar]
- 10.Dahbi A., Hefetz A., Cerdá X., Lenoir A. 1999. Trophallaxis mediates uniformity of colony odor in Cataglyphis iberica ants (Hymenoptera, Formicidae). J. Insect Behav. 12, 559–567 10.1023/A:1020975009450 (doi:10.1023/A:1020975009450) [DOI] [Google Scholar]
- 11.Hölldobler B., Engel-Siegel H. 1984. On the metapleural gland of ants. Psyche 91, 201–224 10.1155/1984/70141 (doi:10.1155/1984/70141) [DOI] [Google Scholar]
- 12.Moret Y., Schmid-Hempel P. 2000. Survival for immunity: the price of immune system activation for bumblebee workers. Science 290, 1166–1168 10.1126/science.290.5494.1166 (doi:10.1126/science.290.5494.1166) [DOI] [PubMed] [Google Scholar]
- 13.de Souza D. J., Vlaenderen J. V., Moret Y., Lenoir A. 2008. Immune response affects ant trophallactic behavior. J. Insect Physiol. 54, 828–832 10.1016/j.jinsphys.2008.03.001 (doi:10.1016/j.jinsphys.2008.03.001) [DOI] [PubMed] [Google Scholar]
- 14.Rasband W. S. 1997–2004. ImageJ. Bethesda, MD: National Institutes of Health; See http://rsb.info.nih.gov/ij/ [Google Scholar]
- 15.Moret Y., Schmid-Hempel P. 2001. Entomology: immune defence in bumble-bee offspring. Nature 414, 506. 10.1038/35107138 (doi:10.1038/35107138) [DOI] [PubMed] [Google Scholar]
- 16.Bulmer M. S., Bachelet I., Raman R., Rosengaus R. B., Sasisekharan R. 2009. Targeting an antimicrobial effector function in insect immunity as a pest control strategy. Proc. Natl Acad. Sci. USA 106, 12 652–12 657 10.1073/pnas.0904063106 (doi:10.1073/pnas.0904063106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke T., Davis K., Lysenko E., Zhou A., Yu Y., Weiser J. 2010. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 16, 228–232 10.1038/nm.2087 (doi:10.1038/nm.2087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nehme N., Liégeois S., Kele B., Giammarinaro P., Pradel E., Hoffmann J., Ewbank J., Ferrandon D. 2007. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 3, 1694–1709 10.1371/journal.ppat.0030173 (doi:10.1371/journal.ppat.0030173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn J.-E., Zhu-Salzman K. 2009. CmCatD, a cathepsin D-like protease has a potential role in insect defense against a phytocystatin. J. Insect Physiol. 55, 678–685 10.1016/j.jinsphys.2009.04.016 (doi:10.1016/j.jinsphys.2009.04.016) [DOI] [PubMed] [Google Scholar]
- 20.Baechle D., et al. 2006. Cathepsin D is present in human eccrine sweat and involved in the postsecretory processing of the antimicrobial peptide DCD-1L. J. Biol. Chem. 281, 5406–5415 10.1074/jbc.M504670200 (doi:10.1074/jbc.M504670200) [DOI] [PubMed] [Google Scholar]
- 21.Cho J. H., Park I. Y., Kim H. S., Lee W. T., Kim M. S., Kim S. C. 2002. Cathepsin D produces antimicrobial peptide parasin I from histone H2A in the skin mucosa of fish. FASEB J. 16, 429–431 [DOI] [PubMed] [Google Scholar]
- 22.Thorne K. J., Oliver R. C., Barrett A. J. 1976. Lysis and killing of bacteria by lysosomal proteinases. Infect. Immun. 14, 555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]