Abstract
Nonlinear vocal phenomena are a ubiquitous feature of human and non-human animal vocalizations. Although we understand how these complex acoustic intrusions are generated, it is not clear whether they function adaptively for the animals producing them. One explanation is that nonlinearities make calls more unpredictable, increasing behavioural responses and ultimately reducing the chances of habituation to these call types. Meerkats (Suricata suricatta) exhibit nonlinear subharmonics in their predator alarm calls. We specifically tested the ‘unpredictability hypothesis’ by playing back naturally occurring nonlinear and linear medium-urgency alarm call bouts. Results indicate that subjects responded more strongly and foraged less after hearing nonlinear alarm calls. We argue that these findings support the unpredictability hypothesis and suggest this is the first study in animals or humans to show that nonlinear vocal phenomena function adaptively.
Keywords: meerkats, nonlinearities, adaptive function
1. Introduction
Nonlinear phenomena in vocalizations, complex intrusions into the normal spectral structure of calls, have received considerable recent interest [1]. Consequently, it is now obvious that these irregularities pervade the vocal systems of a huge range of animal species; from the screams of humans [2] to the ultrasonic calls of frogs [3]. Furthermore, by combining acoustic analysis [4], excised laryngeal experimentation [5] and computer modelling, we also better understand why nonlinearities (from here on ‘NLP’) arise and the various forms they can assume (see [1]). However, this is only part of the puzzle, as, it still remains unclear what function, if any, nonlinearities serve for the species producing them.
A number of theories exist to explain the potential adaptive significance of NLP. NLP could act as an honest signal, transferring information regarding vocal apparatus symmetry and hence, mate quality [4]. Alternatively, NLP may facilitate individual discrimination [6], provide cues regarding the movement direction of the caller [7] or confuse predators by mimicking the fundamental frequency of a larger animal during normal phonation [1]. Finally, the unpredictability generated by NLP may be difficult to ignore, inducing a heightened behavioural response, ultimately preventing receivers from habituating to certain call types (the ‘unpredictability hypothesis’, [1,8]). This adaptive function would be particularly plausible for calls that influence the survival of individuals, such as screams or alarm calls and indeed it has been shown that baby and animal screams containing NLP are more evocative to human listeners than vocalizations without [9,10].
However, no study in humans or animals has convincingly shown that, within the same call type, naturally occurring nonlinearities are meaningful for receivers. This approach is critical to elucidate whether such integral components of animal vocalizations function adaptively, and are not mere byproducts emanating from the physical properties of the sound production system [1].
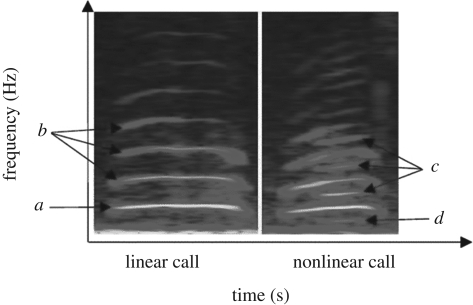
Here, we test the ‘unpredictability hypothesis’ of NLP using meerkat alarm calls. Meerkats (Suricata suricatta) exhibit predator-type and urgency-specific alarm calls [11] and further spectrographic analyses indicated the clear presence (and absence) of subharmonic nonlinear intrusions, within a single alarm call variant (S. W. Townsend & M. B. Manser 1995–ongoing, unpublished data; figure 1). We conducted playback experiments exposing meerkats to nonlinear (test) and linear (control) medium-urgency aerial alarm call bouts [11]. In line with the unpredictability hypothesis, we predicted that meerkats should be more aroused by alarm calls possessing subharmonic NLP than those without, and subsequently exhibit stronger behavioural responses.
Figure 1.
Spectrogram showing a linear and a nonlinear call from a medium-urgency aerial alarm call bout. (a) The fundamental frequency of the call, (b) harmonic overtones associated with this tonal call type, (c,d) nonlinear subharmonic intrusions into the call's spectral structure.
2. Material and methods
(a). Study site and subjects
Playback experiments were carried out on eight groups of meerkats at the Kalahari Meerkat Project (KMP), South Africa [11] from August to November 2009. As part of the KMP's long-term data collection, all animals were tagged with subcutaneous transponders and with dye markings for individual identification. All subjects were habituated to a level that allowed close observations within 1 m.
(b). Call selection
We selected nine medium-urgency aerial alarm call bout stimuli, four possessing subharmonic nonlinear intrusions (average proportion of nonlinear elements 46% (range 33–62%)) and five without, following the protocol outlined by Fitch et al. [1], from a data pool of 38 call bouts, using Cool Edit Pro 2000 (figure 1; electronic supplementary material). Only nine stimuli in total were pre-selected, because we wanted to use natural alarm call bouts, of the same urgency and predator-class, with good signal-to-noise ratio, which were also matched in temporal parameters (see electronic supplementary material). An acoustic analysis on the available calls further indicated that structurally, and statistically, calls of the control and test categories did not differ (see electronic supplementary material).
(c). Playback protocol
Playback experiments were conducted on dominant male meerkats in eight different groups. Because of the specificity of the stimuli needed, all four nonlinear stimuli and three of five linear stimuli were tested in two groups, while the remaining two linear stimuli were tested in only one group. Subjects were followed for at least 30 min (range +3 h) prior to playbacks; to control for sensitization effects, we recorded all occurrence data on alarm call frequency. Call bouts were played back from an iPod-touch connected to a JBL loud-speaker at a volume adjusted to match the amplitude measured for call bouts elicited during natural predator encounters (digital sound-pressure meter: Voltcraft SL-100). Playbacks were only conducted on foraging meerkats, at least 50 m from their sleeping burrow and 10 m from any boltholes, at a distance of 12–15 m from the focal individual. Experiments were only performed if there had been no disturbance (predator encounter, group encounters) during the previous 10 min of observation. Playback subjects were filmed for at least 30 s before and 1 min after using a JVC solid-state digital video camera (JVC-everio GZ-MG150). To control for differential responses to playback conditions owing to satiation, after playbacks, each male was weighed to estimate foraging success (Δ experimental weight − morning weight/foraging time). Finally, to avoid order-effects, we ensured that 50 per cent of the subjects received nonlinear playbacks first (n = 4) and 50 per cent received linear playbacks first (n = 4). Playbacks on the same male were separated by at least 7 days.
(d). Behavioural responses
We analysed videos frame-by-frame using Observer XT 7.0. We quantified the strongest behavioural response to the playbacks by grading the behaviours observed in the following minute into five response categories of increasing strength: (i) no response, (ii) vigilant, (iii) standing guard, (iv) running, and (v) running to a bolthole. We allocated each playback 1 response category, representing the strongest response exhibited in the 1 min after playback and this value was then used in analyses. Furthermore, we looked specifically at the strongest behavioural category ‘running to a bolthole’ to determine if this behaviour was employed differentially between playbacks. We made a binary distinction between running to a bolthole (1) and all other behaviours (0). Finally, we also wanted to obtain an estimate of time to relaxation. Unfortunately, we could not use the absolute time to relax, because this behaviour was automatically confounded by the running to a bolthole response category, given that once at the bolthole (the strongest response category) and safe, individuals would immediately relax. To circumvent this, we took foraging time in the minute after playback as a proxy measure of time to relax, as the decision to return to normal foraging suggests that the threat is no longer perceived. To ensure accurate coding of video tapes, a second observer blind-coded 75 per cent of trials (12 trials) (see electronic supplementary material).
(e). Statistical analyses
Owing to the non-normal distribution of the data, we employed exact non-parametric tests to analyse the categorical and continuous response variables. To determine if there were differences in the utilization of the strongest response category (Running to a bolthole), we used a generalized linear mixed effects model (GLMM) with a binomial error structure (see electronic supplementary material).
3. Results
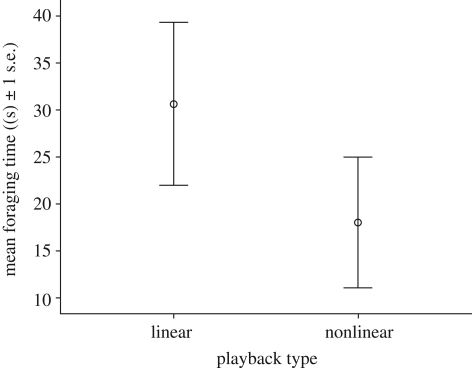
Meerkats responded differentially to the playback conditions. They responded more strongly after hearing nonlinear than linear playbacks (exact Wilcoxon signed-rank test, nnonlinear − nlinear = 8, Z =− 2.23, p = 0.031) and spent less time foraging (mean ± s.d.: linear = 30 ± 24 s, nonlinear = 18 ± 19 s; nnonlinear − nlinear = 8, Z =− 2.52, p = 0.008; figure 2). Meerkats employed the strongest response category, running to a bolt-hole, significantly more often after hearing nonlinear than linear playbacks (nonlinear = 62.5% of trials, linear = 0% of trials, GLMM, LR = 9.28, d.f. = 1, p = 0.017).
Figure 2.
The effect of playback type on foraging time (s) in the minute after playbacks.
There was no difference between experimental and control conditions for the additional factors analysed: alarm call number per hour before playback (mean ± s.d.: nonlinear = 0.48 ± 0.48, linear = 0.49 ± 0.36, exact Wilcoxon signed-rank test, nnonlinear − nlinear = 8, Z =− 0.420, p = 0.742) or foraging success (mean weight gain h−1: nonlinear = 2.16± 3 g, linear = 2.7 ± 2.34 g, nnonlinear − nlinear = 6, Z =− 0.674, p = 0.625).
4. Discussion
Nonlinear phenomena pervade the vocal systems of species across the animal kingdom. However, until now, the idea that such complex intrusions can function adaptively has not been clearly demonstrated. Previous attempts have been complicated by variation in call-type used [10], or the artificial synthesizing of nonlinear intrusions during playbacks [8].
In meerkats, we show that naturally occurring subharmonic NLP induce a stronger behavioural escape response than a control sequence without nonlinearities. Probably, as a result of this more intense behavioural response, we additionally found that, when exposed to nonlinear alarm call bouts, individuals generally needed longer to relax. Because the reaction exhibited by subjects could be driven by sensitization to alarm calls or satiation, we recorded prior alarm call exposure and foraging success. However, neither variable differed significantly between the two playback conditions, indicating that NLP were responsible for the observed behavioural changes.
We suggest that our results support the unpredictability hypothesis [1,8], which postulates that nonlinearities make calls less predictable and thus more evocative to listeners, preventing receivers from ignoring such calls. This makes particular sense with regards to meerkat alarm calls. Meerkats inhabit open desert environments, where the threat of predation is extremely high [11]. This, combined with the fact that their vision is compromised by their tendency to forage in the sand, means that alarm calls play a central role in the survival and ultimate fitness of meerkats. Experimental manipulations have shown that habituation to alarm calls can occur in meerkats [12]; given the detrimental effect such habituation could have on survival, meerkats may well have evolved NLP as a mechanism to temper this.
As animals exposed to danger may become highly aroused and temporarily lose control of their vocal membranes, it has additionally been suggested that receivers may perceive NLP as communicating risk or urgency [8,13,14]. However, in meerkats, this explanation seems less plausible, as exemplars were taken from within the same call-type and hence except for the subharmonic intrusion, were matched structurally in urgency levels (see [11]). Furthermore, preliminary data suggest that nonlinearities occur only at low rates (20%) in their medium-urgency aerial alarm calls (S. W. Townsend & M. B. Manser 1995–ongoing, unpublished data), a level that is unlikely to allow reliable perception of urgency or risk levels.
To our knowledge, this is the first study indicating that nonlinearities function adaptively, initiating a change in behavioural response. The comparative study of animal communication strives to elucidate whether facets of our own language system are unique or whether they have a more deeply rooted evolutionary history [15]. Our results support the notion that there is some form of underlying phylogenetic continuity to NLP in humans [9]. Whether nonlinearities generally function in contexts where unpredictability and ultimately habituation prevention, is of advantage still remains to be confirmed, though further systematic habituation–dishabituation studies within the same call type, with and without NLP, will help to clarify this. We hope our work will stimulate the much needed additional research into the function of animal and human nonlinearities.
Acknowledgements
Thanks to Tim Clutton-Brock, for KMP research permission, Zoe Peacock and Simone Birrer for help with experiments, Tecumseh Fitch and Roman Furrer for discussions and Klaus Zuberbuhler, Dan Rankin and two anonymous reviewers for manuscript comments. University of Zurich provided funding.
References
- 1.Fitch W. T., Neubauer J., Herzel H. 2002. Calls out of chaos: the adaptive significance of nonlinear phenomena in mammalian vocal production. Anim. Behav. 63, 407–418 10.1006/anbe.2001.1912 (doi:10.1006/anbe.2001.1912) [DOI] [Google Scholar]
- 2.Herzel H., Wendler J. 1991. Evidence of chaos in phonatory samples. In Eurospeach-1991: Second European Conference on Speech Communication and Technology (ed. ISCA), pp. 263–266 Genova, Italy: ISCA [Google Scholar]
- 3.Suthers R. A., Narins P. M., Lin W.-Y., Schnitzler H.-U., Denzinger A., Xu C.-H., Feng A. S. 2006. Voices of the dead: complex nonlinear vocal signals from the larynx of an ultrasonic frog. J. Exp. Biol. 209, 4984–4993 10.1242/jeb.02594 (doi:10.1242/jeb.02594) [DOI] [PubMed] [Google Scholar]
- 4.Riede T., Arcadi A. C., Owren M. J. 2007. Nonlinear acoustics in the pant hoots of common chimpanzees Pan troglodytes: vocalizing at the edge. J. Acoust. Soc. Am. 121, 1758–1767 10.1121/1.2427115 (doi:10.1121/1.2427115) [DOI] [PubMed] [Google Scholar]
- 5.Brown C., Alipour F., Berry D. A., Montequin D. 2003. Laryngeal biomechanics and vocal communication in the squirrel monkey (Saimiri boliviensis). J. Acoust. Soc. Am. 113, 2114–2126 10.1121/1.1528930 (doi:10.1121/1.1528930) [DOI] [PubMed] [Google Scholar]
- 6.Volodina E. V., Volodin I. A., Isaeva I. V., Unck C. 2006. Biphonation may function to enhance individual recognition in the dhole, Cuon alpinus. Ethology 112, 815–825 10.1111/j.1439-0310.2006.01231.x (doi:10.1111/j.1439-0310.2006.01231.x) [DOI] [Google Scholar]
- 7.Miller P. J. O. 2002. Mixed-directionality of killer wale stereotyped calls: a direction of movement cue? Behav. Ecol. Sociobiol. 52, 262–270 10.1007/s00265-002-0508-9 (doi:10.1007/s00265-002-0508-9) [DOI] [Google Scholar]
- 8.Blumstein D. T., Recapet C. 2009. The sound of arousal: the addition of novel non-linearities increases responsiveness in marmot alarm calls. Ethology 115, 1074–1081 10.1111/j.1439-0310.2009.01691.x (doi:10.1111/j.1439-0310.2009.01691.x) [DOI] [Google Scholar]
- 9.Belin P., Fecteau S., Charest I., Nicastro N., Hauser M. D., Armony J. L. 2008. Human cerebral responses to animal affective vocalizations. Proc. R. Soc. B 275, 473–481 10.1098/rspb.2007.1460 (doi:10.1098/rspb.2007.1460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mende W., Herzel H., Wermke K. 1990. Bifurcations and chaos in newborn infant cries. Phys. Lett. A 145, 418–424 10.1016/0375-9601(90)90305-8 (doi:10.1016/0375-9601(90)90305-8) [DOI] [Google Scholar]
- 11.Manser M. 2001. The acoustic structure of suricates' alarm calls varies with predator type and the level of response urgency. Proc. R. Soc. Lond. B 268, 2315–2324 10.1098/rspb.2001.1773 (doi:10.1098/rspb.2001.1773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schibler F., Manser M. B. 2007. The irrelevance of individual discrimination in meerkat alarm calls. Anim. Behav. 74, 1259–1268 10.1016/j.anbehav.2007.02.026 (doi:10.1016/j.anbehav.2007.02.026) [DOI] [Google Scholar]
- 13.Blumstein D. T., Richardson D. T., Cooley L., Winternitz J., Daniel J. C. 2008. The structure, meaning, and function of yellow-bellied marmot pup screams. Anim. Behav. 76, 1055–1064 10.1016/j.anbehav.2008.06.002 (doi:10.1016/j.anbehav.2008.06.002) [DOI] [Google Scholar]
- 14.Blumstein D. T., Davitian R., Kaye P. D. 2010. Do film soundtracks contain non-linear analogues to influence emotion? Biol. Lett. (doi:10.1098/rsbl.2010.0333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser M., Chomsky N., Fitch W. T. 2002. The faculty of language: what is it, who has it and how did it evolve? Science 298, 1569–1579 10.1126/science.298.5598.1569 (doi:10.1126/science.298.5598.1569) [DOI] [PubMed] [Google Scholar]