Abstract
Soft-bodied taxa comprise an important component of the extant lophophorate fauna, but convincing fossils of soft-bodied lophophorates are extremely rare. A small fossil lophophorate, attached to a brachiopod dorsal valve, is described from the Silurian (Wenlock Series) Herefordshire Lagerstätte of England. This unmineralized organism was bilaterally symmetrical and comprised a subconical body attached basally to the host and partially enclosed by a broad ‘hood’; the body bore a small, coiled lophophore. Where the hood attached laterally, there is a series of transverse ridges and furrows. The affinities of this organism probably lie with Brachiopoda; the hood is interpreted as the homologue of a dorsal valve/mantle lobe, and the attachment as the homologue of the ventral valve and/or pedicle. The ridges are arranged in a manner that suggests constructional serial repetition, indicating that they are unlikely to represent mantle canals. Extant brachiopods are not serially structured, but morphological and molecular evidence suggests that their ancestors were. The new organism may belong to the brachiopod stem group, and might also represent a significant element of the Palaeozoic lophophorate fauna.
Keywords: Brachiopoda, exceptional preservation, serial repetition
1. Introduction
Lophophorate animals are lophotrochozoans characterized by a paired tentacular feeding organ (lophophore) and a sessile or near-sessile filter-feeding ecology. The most familiar are the biomineralized bryozoans and brachiopods; the former are colonial, the latter bivalved solitary forms. The recent lophophorate fauna also includes the soft-bodied Phoronida (‘horseshoe worms’; [1]) and Entoprocta (=Kamptozoa, ‘goblet worms’; [2]). The evolutionary history of mineralized lophophorates is well documented palaeontologically, but soft-bodied lophophorate fossils are extremely rare. Todd & Taylor [3] described a Jurassic entoproct, and Taylor [4] reviewed occurrences of Jurassic/Cretaceous ctenostomes (unmineralized bryozoans); all other candidate lophophorate body fossils are of Cambrian age. Dinomischus was initially described as entoproct-like [5], but subsequent opinion has treated its entoproct affinities as tentative at best [3]. Odontogriphus, initially described as a lophophorate [6], has been convincingly reinterpreted as molluscan [7]. More recently, several ‘soft-shelled’ brachiopods have been described [8–10]. These may bear on the origins of siphonotretid lingulates [9] and phoronids [8]; they are morphologically similar to mineralized lingulates.
We describe here a fossil of a new unmineralized lophophore-bearing organism, which falls outside the morphological range of any of the groups discussed above, from the Silurian (Wenlock Series, approx. 425 Ma) Herefordshire Lagerstätte of England [11]. This deposit preserves soft tissues of various invertebrates in high fidelity and three dimensions. Briggs et al. [11] provide a summary of the fauna. It includes the brachiopod Bethia serraticulma [12], the holotype of which bears an epifauna on both dorsal and ventral valves comprising three brachiopods, a tube-like structure and the specimen described here (E1 of Sutton et al. [12]; Oxford University Museum of Natural History number OUMNH C.29592).
2. Material and methods
The B. serraticulma specimen (OUMNH C.29586) and associated epifauna (OUMNH C.29589-93) are preserved as calcitic void fills in an early diagenetic carbonate concretion within volcaniclastic sediments. Specimens were serially ground at 20 µm intervals and digitally photographed, then digitally reconstructed and studied using the custom SPIERS software suite, implementing the methods of Sutton et al. [13,14]. A three-way split of the original concretion passes through the specimen described here, which has been reassembled digitally from three separately reconstructed pieces; the cracks that separate them are labelled in figure 1. Images (figure 1) were rendered as ray-traced virtual photographs. Datasets are housed in the Oxford University Museum of Natural History.
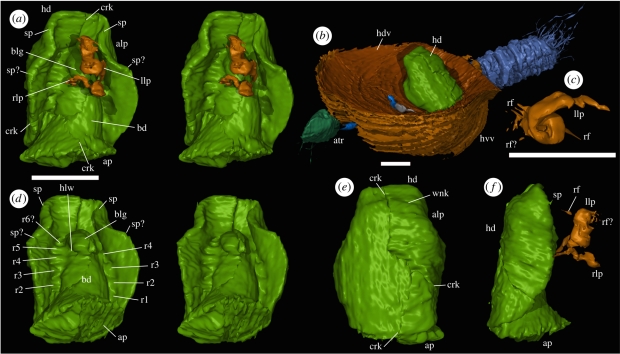
Figure 1.
Virtual reconstructions: (a,c–f) Drakozoon kalumon OUMNH C.29592. (a) Ventral view (stereo-pair); (c) posterior view (dorsal to right) of the left lophophore arm; (d) postero-ventral view (stereo-pair) with lophophore removed; (e) dorsal view; (f) lateral view. (b) Bethia serraticulma OUMNH C.29586, oblique view showing D. kalumon attachment position and attached fauna (OUMNH C.20589–C.29593). Scale bars, 1 mm. alp, antero-lateral pinching; ap, attachment plane; atr, ?atrypid OUMNH C.29589; bd, body; blg, bulge in body; crk, crack representing original split of host concretion; hd, hood (=dorsal valve/mantle); hdv, host dorsal valve; hlw, hollow beneath bulge in body; hvv, host ventral valve; llp, left arm of lophophore; r1–r5, r6?, ridges; rf, radiating filaments; rlp, right arm of lophophore; sp, sp?, spines; wkl, wrinkle in hood.
For descriptive purposes, the attachment point of OUMNH C.29592 is assumed to be posterior and the ‘hood’ dorsal.
3. Systematic palaeontology
Phylum: Brachiopoda?
Genus: Drakozoon gen. nov.
Derivation of name: Greek, drakôn (coiled) + zoon (animal), alluding to the coiled lophophore.
Diagnosis: Dorsal ‘valve’ small, unmineralized; discrete ventral valve absent. Attachment structure short, postero-terminally flared. Lophophore small, spirolophous. Lateral junction between body and dorsal valve with six? transverse ridges and furrows.
Species: Drakozoon kalumon sp. nov.
Derivation of name: Greek, kalumma (hood), alluding to the dorsal hood-like structure. The name is masculine.
Diagnosis: as for the genus (monotypic).
Holotype: OUMNH C.29592 (figure 1a,c–f), attached submedially to the dorsal valve of B. serraticulma holotype, OUMNH C.29586. No other material known.
Description: The specimen is bilaterally symmetrical, and consists of a subconical body with a partially enclosing dorsal hood and paired ‘appendages’ ventrally (figure 1a,f). The body is 1.7 mm long and suboval in transverse section; it flares posteriorly to form a subplanar surface, 1.4 mm wide and 1.2 mm deep, attached to the dorsal valve of the host brachiopod. The body tapers gently anteriorly, terminating in a rounded tip (figure 1a,d). Near the anterior, it is produced into a ventral bulge (figure 1a,d), the posterior slope of which is steeper than the anterior. The bulge is not sharply delineated from the rest of the body except postero-laterally, where a hollow (figure 1d) occurs.
At the postero-lateral corners of the bulge, immediately ventral to the posterior of the hollow, paired appendages interpreted as lophophore arms arise, their bases 0.3 mm apart. These structures are ribbon-like, flattened antero-posteriorly at their bases and initially extend ventrally and slightly laterally. The (incomplete?) right-hand arm continues ventrally in a weak spiral (figure 1a,f), flaring but remaining flattened terminally. The left arm also flares, but the flared region is longer and extends into a tight dorsally directed helix (figure 1a,c,f). Fine linear structures radiate distally (figure 1c,f); these are poorly preserved, but may represent lophophore filaments.
The dorsal side of the animal is enclosed by a hood, 2.2 mm in length and 1.7 mm in maximum width, interpreted as a valve or mantle-lobe homologue. This structure is subsymmetrical, suggesting some flexibility. It takes the form of a subcircular disc, approximately 0.15 mm thick at its margins, extending to the posterior attachment and weakly folded around the body. In the transverse section, the dorsal surface is near-straight medially and inflected into near-ventrally directed lateral folds (figure 1e). In the longitudinal section, the surface is straight except for the anteriormost 25 per cent, which is inflected into a straight antero-ventrally directed portion (figure 1f). Antero-laterally, the hood is pinched (figure 1a,d,e). Anteriorly, and possibly posteriorly to this pinching, the margin bears a small spine (figure 1a,d,f).
The body is separated from the hood laterally and anteriorly by a recessed region bearing weak subtransverse ridges and furrows. There are at least five to six pairs of ridges (r1–r5, r6?; figure 1d); those on opposite sides of the body apparently correspond closely in position. The internal surface of the hood is otherwise smooth; apparent wrinkles on the left side of figure 1d are artefacts of visualization. Externally, at least one weak margin-parallel ‘wrinkle’ (figure 1e) is evident, together with low ‘lumps’ distributed over the surface.
4. Discussion
Our interpretation of the appendages as a lophophore rests on the observations that they are: (i) paired and arise close to the axis, (ii) clearly flexible (compare left and right), (iii) coiled, and (iv) possibly filament bearing. They are smaller relative to body size than typical extant (and larger) examples, but this may simply reflect allometric lophophore growth.
Drakozoon kalumon does not closely resemble the entoprocts, bryozoans or adult phoronids among lophophorates, nor the ‘lophophore’-bearing pterobranch hemichordates. It is superficially similar to phoronid larvae (‘actinotrochs’, e.g. [1]), which have a ‘preoral lobe’ recalling the Drakozoon hood, and reach comparable sizes [15]. However, actinotrochs are not sessile and possess a very different form of lophophore. There are much more compelling similarities between D. kalumon and the Brachiopoda. The hood is potentially homologous with a dorsal valve (or dorsal mantle) in its disposition around the body, presumably with a similar function in providing protection and at least partially enclosing a filtration cavity. The solitary sessile ecology is also brachiopod-like, and the attachment pedicle-like. Brachiopod pedicles/cementation structures are not all homologous [16]; detailed comparison with all variants is beyond the scope of this paper, although we note the similarity to the attachment style inferred for colleplax-bearing taxa (see below).
Unlike a typical brachiopod, D. kalumon lacks a true ventral valve and fully enclosed mantle cavity, although the hood may have ‘pulled down’ against the surface of the host. Marginal setae are also absent; this is unlikely to be a preservational loss (OUM C.29589, an ?atrypide brachiopod attached to the same host, preserves setae). While the nature of the preservation prevents a determination of the original composition, the evidence of flexibility of the hood and its continuity with the body suggests that it was not biomineralized. There is no evidence for a brachiopod-like musculature.
The ridge and furrow structures probably do not represent mantle canals (the ridges) arising from the body; brachiopod mantle canals arise from one or two pairs of primary canals connecting to the body, while here at least five subparallel ridges are present, and do not branch or taper. The regularity and transverse orientation of these structures suggest serial organization. Extant lophophorates are not externally serialized as adults, but studies of craniid larvae [17] and brachiopod lateral mesenteries [18] suggest serialized ancestry. Evolutionary-development studies (e.g. [19]) suggest that serialization is homologous within Bilateria, and therefore plesiomorphic for lophotrochozoans (which include the metameric annelids). Precise phylogenetic placement of D. kalumon is hampered by a lack of consensus in lophotrochozoan molecular phylogenies, lophophorates resolving as paraphyletic [20] or less plausibly polyphyletic [21]. Nonetheless, external serialization and the partially brachiopod-like character suite (e.g. partially closed filter chamber) are suggestive of a position within the stem group of a brachiopod/phoronid clade. This clade probably nests within the sclerite-bearing tommotiids (e.g. [22]), which also lacked a closed filter chamber; additionally, the pad-like attachments inferred for colleplax-bearing stem-group brachiopods [23] are similar in size and position to that of D. kalumon. Tommotiids were biomineralizers, hence the absence of mineralization in Drakozoon is probably secondary.
This fossil broadens the directly documented morphological and ecological range of the Palaeozoic lophophorates. Small soft-bodied forms such as Drakozoon undoubtedly had a very low preservation potential. Their absence elsewhere in the record probably reflects taphonomic bias; they may have been widespread.
Acknowledgements
We thank the Natural Environment Research Council (grant NE/F017227/1) for support, and R. Fenn, T. Hall and J. Sinclair for general assistance.
References
- 1.Emig C. C. 1982. The biology of Phoronida. Adv. mar. Biol. 19, 1–89 10.1016/S0065-2881(08)60086-3 (doi:10.1016/S0065-2881(08)60086-3) [DOI] [Google Scholar]
- 2.Nielsen C. 2002. Entoprocta. In Encyclopedia of life sciences. Chichester, UK: John Wiley & Sons Ltd [Google Scholar]
- 3.Todd J. A., Taylor P. D. 1992. The first fossil entoproct. Naturwissenschaften 79, 311–314 10.1007/BF01138708 (doi:10.1007/BF01138708) [DOI] [Google Scholar]
- 4.Taylor P. D. 1990. Bioimmured ctenostomes from the Jurassic and the origin of the cheilostome Bryozoa. Palaeontology 33, 19–34 [Google Scholar]
- 5.Conway Morris S. C. 1977. A new entoproct-like organism from the Burgess Shale of British Columbia. Palaeontology 20, 833–845 [Google Scholar]
- 6.Conway Morris S. C. 1976. A new Cambrian lophophorate from the Burgess Shale of British Columbia. Palaeontology 19, 199–222 [Google Scholar]
- 7.Caron J. B., Scheltema A., Schander C., Rudkin D. 2006. A soft-bodied mollusc with radula from the Middle Cambrian Burgess Shale. Nature 442, 159–163 10.1038/nature04894 (doi:10.1038/nature04894) [DOI] [PubMed] [Google Scholar]
- 8.Balthasar U., Butterfield N. J. 2009. Early Cambrian ‘soft-shelled’ brachiopods as possible stem-group phoronids. Acta Palaeontol. Pol. 54, 307–314 [Google Scholar]
- 9.Holmer L. E., Caron J. B. 2006. A spinose stem group brachiopod with pedicle from the Middle Cambrian Burgess Shale. Acta Zool. 87, 273–290 10.1111/j.1463-6395.2006.00241.x (doi:10.1111/j.1463-6395.2006.00241.x) [DOI] [Google Scholar]
- 10.Zhang Z., Han J., Zhang X., Liu J., Guo J., Shu D. G. 2007. Note on the gut preserved in the Lower Cambrian Lingulellotreta (Lingulata, Brachiopoda) from southern China. Acta Zool. 88, 65–70 10.1111/j.1463-6395.2007.00252.x (doi:10.1111/j.1463-6395.2007.00252.x) [DOI] [Google Scholar]
- 11.Briggs D. E. G., Siveter D. J., Siveter D. J., Sutton M. D. 2008. Virtual fossils from a 425 million-year-old volcanic ash. Am. Sci. 96, 474–481 10.1511/2008.75.474 (doi:10.1511/2008.75.474) [DOI] [Google Scholar]
- 12.Sutton M. D., Briggs D. E. G., Siveter D. J., Siveter D. J. 2005. Silurian brachiopods with soft-tissue preservation. Nature 436, 1013–1015 10.1038/nature03846 (doi:10.1038/nature03846) [DOI] [PubMed] [Google Scholar]
- 13.Sutton M. D., Briggs D. E. G., Siveter D. J., Siveter D. J. 2001. Methodologies for the visualization and reconstruction of three-dimensional fossils from the Silurian Herefordshire Lagerstätte. Paleontol. Elec. 4, art. 2. See http://palaeo-electronica.org/2001_1/s2/issue1_01.htm [Google Scholar]
- 14.Sutton M. D., Briggs D. E. G., Siveter D. J., Siveter D. J., Orr P. J. 2002. The arthropod Offacolus kingi (Chelicerata) from the Silurian of Herefordshire, England: computer based morphological reconstructions and phylogenetic affinities. Proc. R. Soc. Lond. B 269, 1195–1203 10.1098/rspb.2002.1986 (doi:10.1098/rspb.2002.1986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Temereva E. N., Malakhov V. V., Chernyshev A. V. 2006. Giant actinotroch, a larva of phoronida from the South China Sea: the giant larva phenomenon. Dokl. Biol. Sci. 410, 410–413 10.1134/S001249660605019X (doi:10.1134/S001249660605019X) [DOI] [PubMed] [Google Scholar]
- 16.Williams A., Rowell B. 1997. Brachiopod anatomy. In Treatise on invertebrate paleontology, vol. 1 (ed. Kaesler R. L.), Part H, revised, pp. 6–56 Boulder, CO: Geological Society of America; Lawrence, KS: The University of Kansas [Google Scholar]
- 17.Nielsen C. 1991. The development of the brachiopod Crania (Neocrania) anomala (O. F. Müller) and its phylogenetic significance. Acta Zool. 72, 1–23 [Google Scholar]
- 18.Malakhov V. V., Kuzmina T. V. 2006. Metameric origin of lateral mesenteries in Brachiopoda. Dokl. Biol. Sci. 409, 340–342 10.1134/S0012496606040223 (doi:10.1134/S0012496606040223) [DOI] [Google Scholar]
- 19.De Robertis E. M. 2008. The molecular ancestry of segmentation mechanisms. Proc. Natl Acad. Sci. USA 105, 16 411–16 412 10.1073/pnas.0808774105 (doi:10.1073/pnas.0808774105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helmkampf M., Bruchhaus I., Hausdorf B. 2008. Phylogenomic analyses of lophophorates (brachiopods, phoronids and bryozoans) confirm the Lophotrochozoa concept. Proc. R. Soc. B 275, 1927–1933 10.1098/rspb.2008.0372 (doi:10.1098/rspb.2008.0372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passamaneck Y., Halanych K. M. 2006. Lophotrochozoan phylogeny assessed with LSU and SSU data: evidence of lophophorate polyphyly. Mol. Phylogenet. Evol. 40, 20–28 10.1016/j.ympev.2006.02.001 (doi:10.1016/j.ympev.2006.02.001) [DOI] [PubMed] [Google Scholar]
- 22.Skovsted C. B., Holmer L. E., Larsson C. M., Hogstrom A. E. S., Brock G. A., Topper T. P., Balthasar U., Stolk S. P., Paterson J. R. 2009. The scleritome of Paterimitra: an Early Cambrian stem group brachiopod from South Australia. Proc. R. Soc. B 276, 1651–1656 10.1098/rspb.2008.1655 (doi:10.1098/rspb.2008.1655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmer L. E., Stolk S. P., Skovsted C. B., Balthasar U., Popov L. E. 2009. The enigmatic early Cambrian Salanygolina—a stem group of rhynchonelliform chileate brachiopods? Palaeontology 52, 1–10 10.1111/j.1475-4983.2008.00831.x (doi:10.1111/j.1475-4983.2008.00831.x) [DOI] [Google Scholar]